Study on Establishing Reference Safe Concentrations of MRI Contrast Agents for Optimized Images: Paramagnetic Gd-DTPA-BMEA and Superparamagnetic Ferucarbotran
Abstract
1. Introduction
2. Materials and Methods
2.1. Contrast Agents
2.2. Dilution of Contrast Agents
2.3. Deployment
2.4. MRI Instrument
2.5. Pulse Sequence
2.6. In Vivo Experiment (IRB: 106-008)
2.7. Statistical Analysis
3. Results
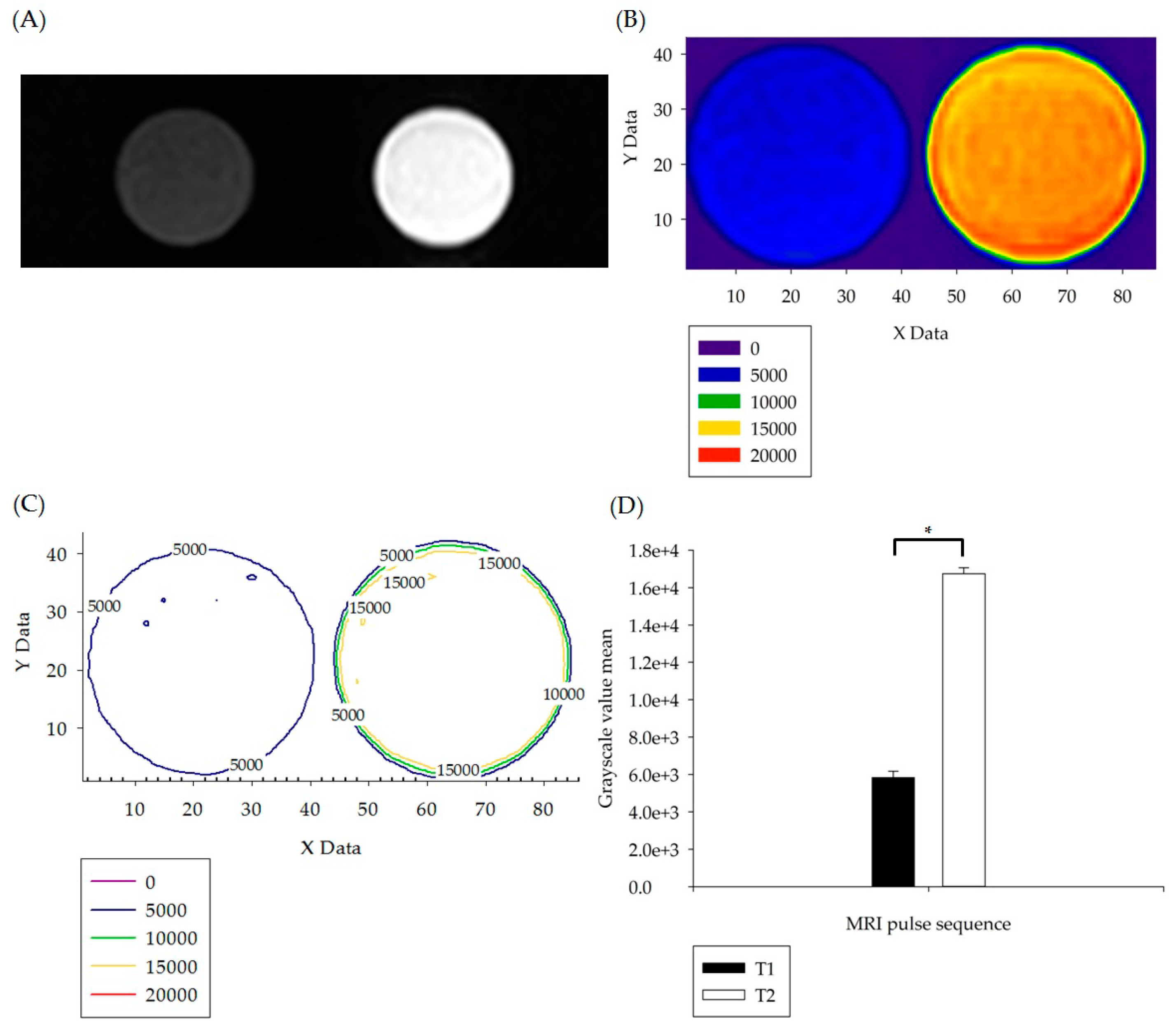
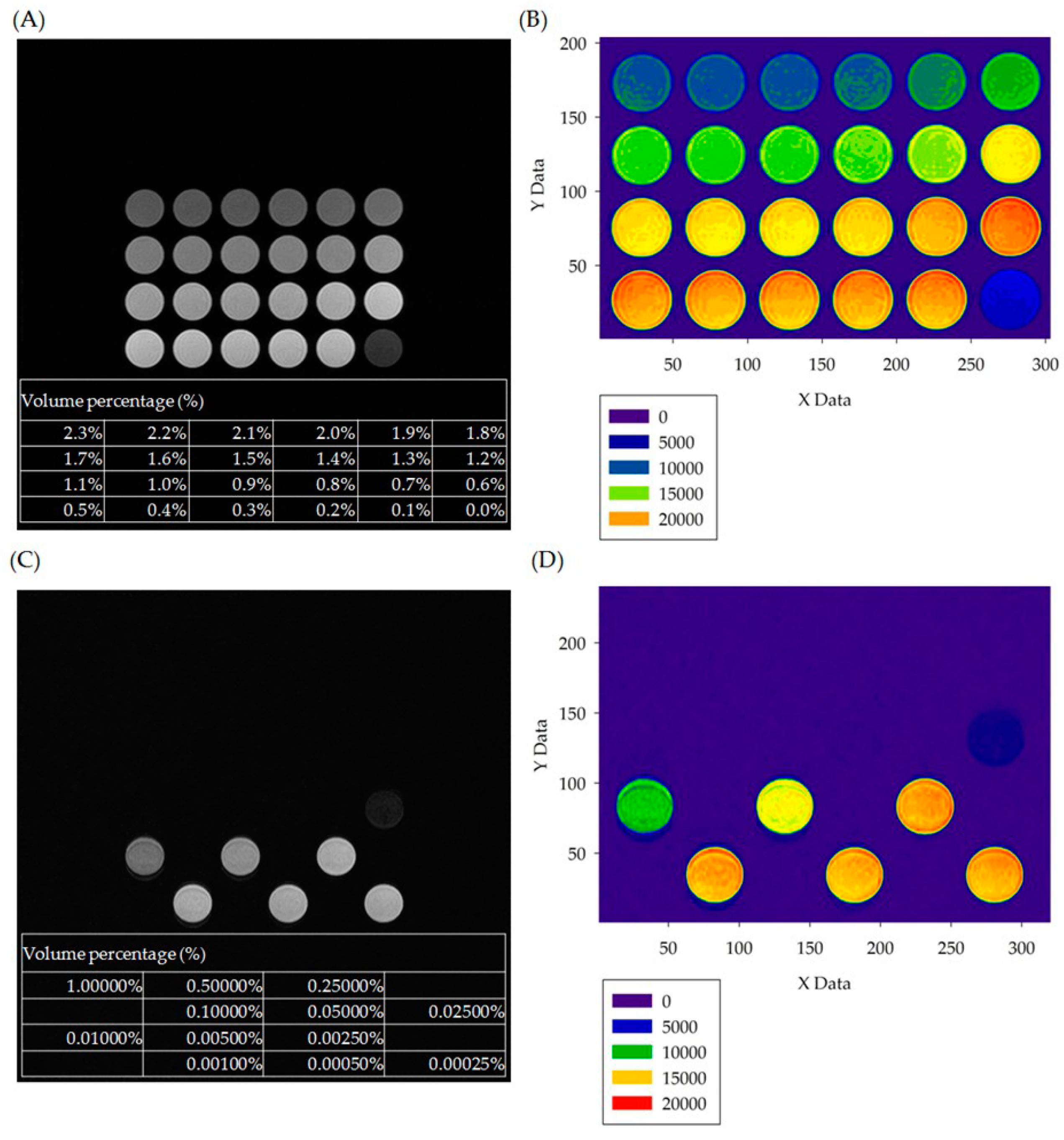
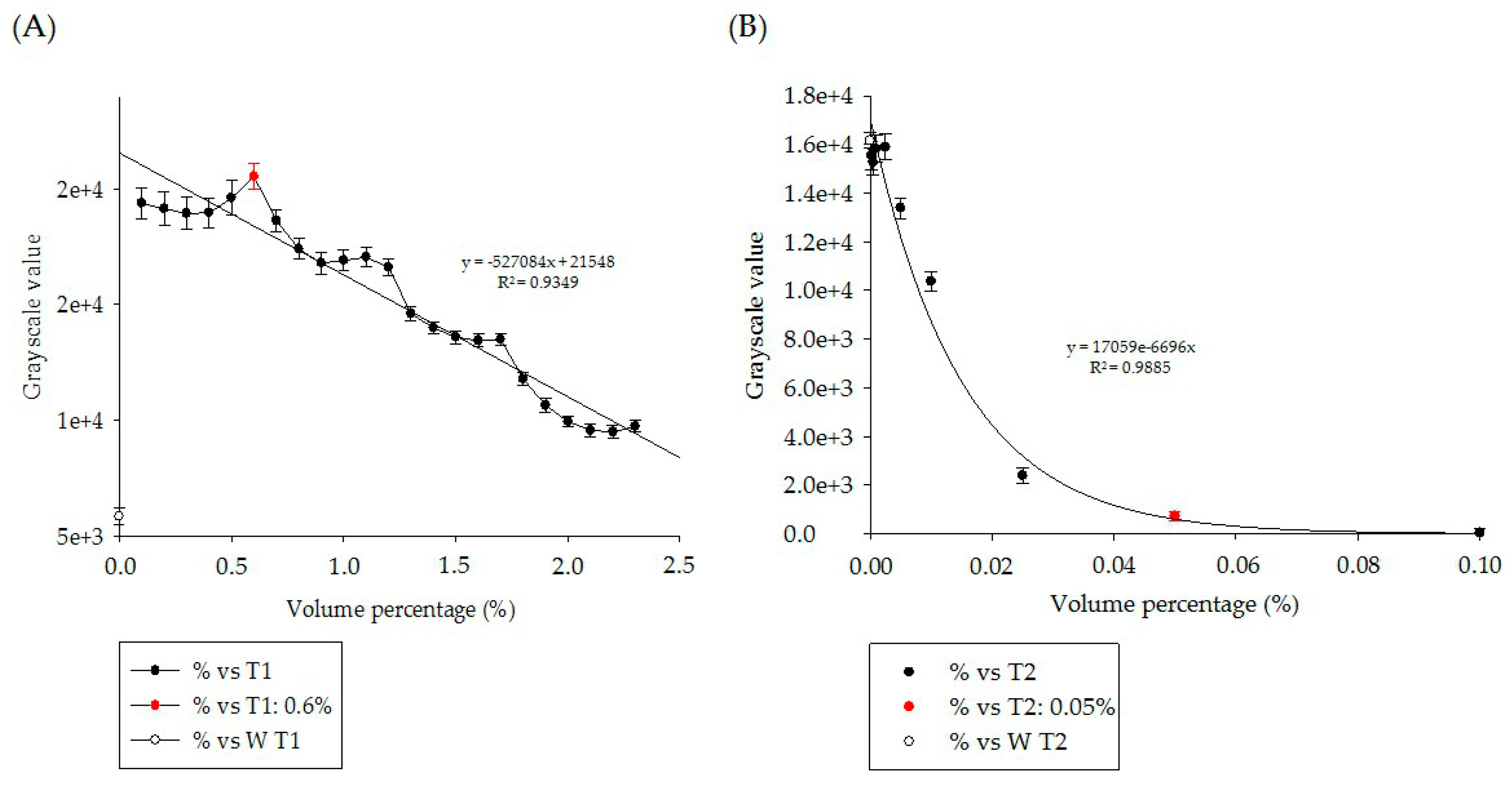
3.1. In Vitro Experiment Results
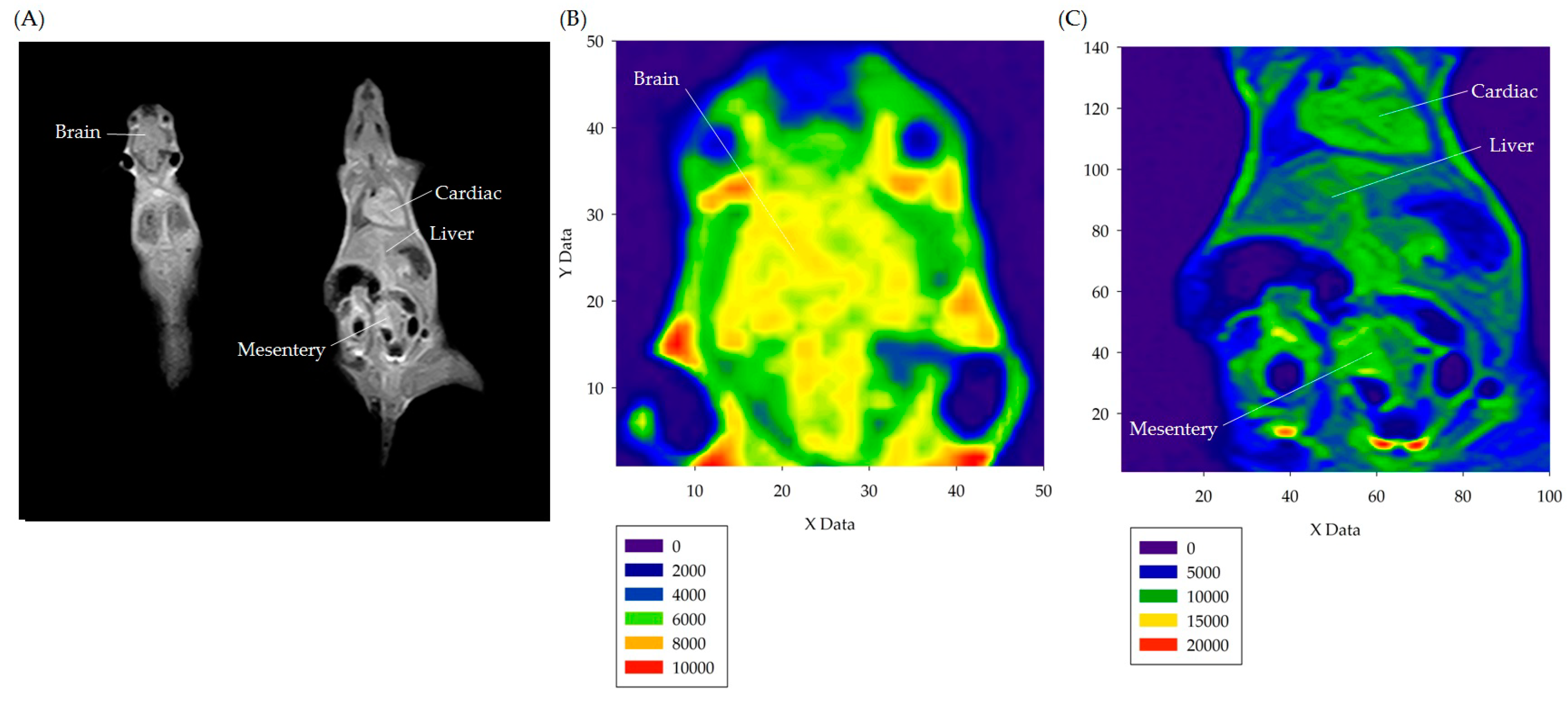
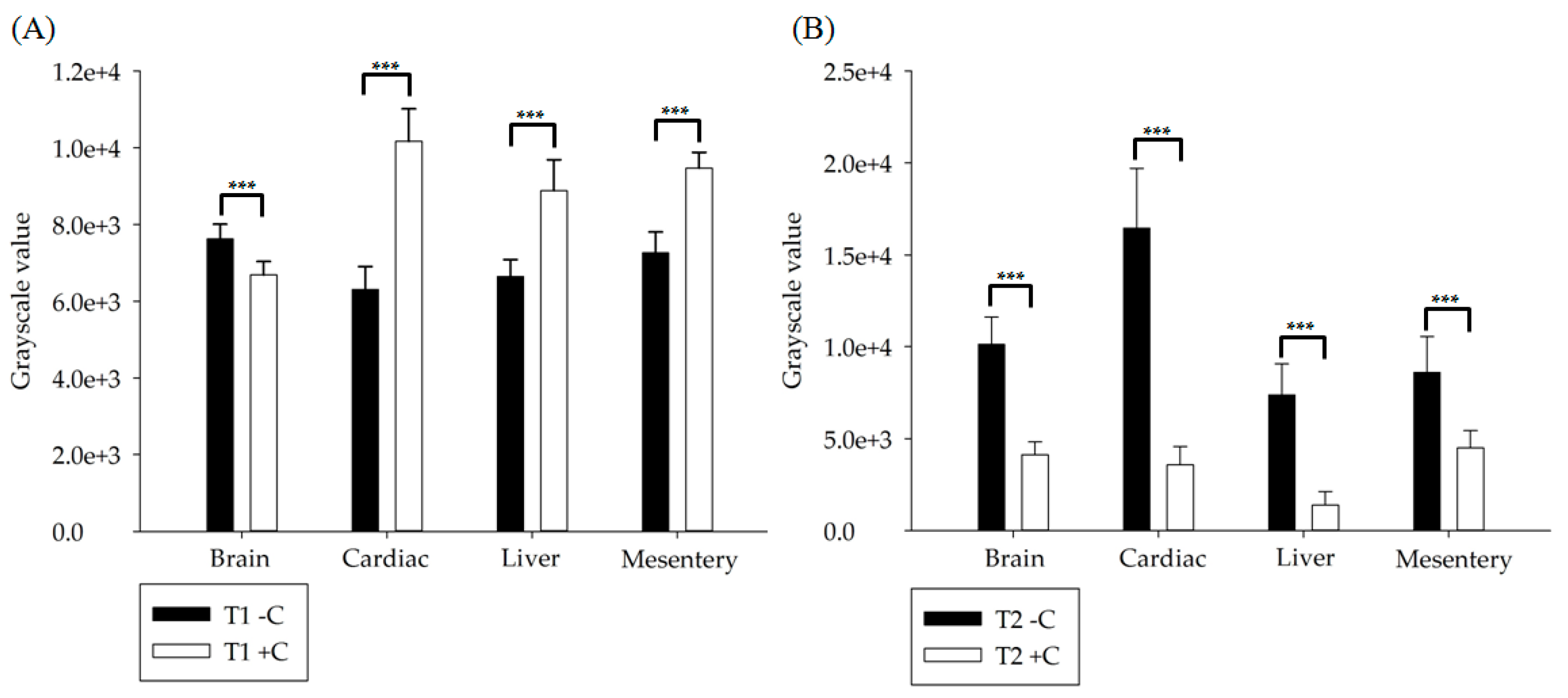
3.2. In Vivo Experiment Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauterbur, P.C. Image formation by induced local interactions: Examples employing nuclear magnetic resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Fox, N.C.; Freeborough, P.A. Brain atrophy progression measured from registered serial MRI: Validation and application to Alzheimer’s disease. J. Magn. Reson. Imaging 1997, 7, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Jaramillo, D.; Siegel, M.J. Whole-body MRI in children: Current status and future applications. Eur. J. Radiol. 2008, 68, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.A.; Wollstein, G.; Schuman, J.S. Clinical application of MRI in ophthalmology. NMR Biomed 2008, 21, 997–1002. [Google Scholar] [CrossRef]
- Atlas, S.W. Magnetic Resonance Imaging of the Brain and Spine, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Cadotte, D.W.; Wilson, J.R.; Mikulis, D.; Stroman, P.W.; Brady, S.; Fehlings, M.G. Conventional MRI as a diagnostic and prognostic tool in spinal cord injury: A systemic review of its application to date and an overview on emerging MRI methods. Expert Opin. Med. Diagn. 2011, 5, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.I.; Delattre, B.M.A.; Boto, J.; Gariani, J.; Dhouib, A.; Fitsiori, A.; Dietemann, J.L. Advanced magnetic resonance imaging (MRI) techniques of the spine and spinal cord in children and adults. Insights Imaging 2018, 9, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, R.H.; Bradley, W.G.; Lisanti, C.J. MRI: The Basics, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Murray, R.C. Equine MRI, 1st ed.; John Wiley & Sons: West Sussex, UK, 2010. [Google Scholar]
- Brown, M.A.; Semelka, R.C. MRI: Basic Principles and Applications, 4th ed.; John Wiley & Sons: Philadelphia, PA, USA, 2011. [Google Scholar]
- Weishaupt, D.; Köchli, V.D.; Marincek, B. How Does MRI Work? An Introduction to the Physics and Function of Magnetic Resonance Imaging, 2nd ed.; Springer Science & Business Media: Philadelphia, PA, USA, 2008. [Google Scholar]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Cordero, J.M. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Strijkers, G.J.; Mulder, W.J.M.; van Tilborg, G.A.F.; Nicolay, K. MRI contrast agents: Current status and future perspectives. Anticancer Agents Med. Chem. 2007, 7, 291–305. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar]
- Santra, S.; Jativa, S.D.; Kaittanis, C.; Normand, G.; Grimm, J.; Perez, J.M. Gadolinium-encapsulating iron oxide nanoprobe as activatable NMR/MRI contrast agent. ACS Nano 2012, 6, 7281–7294. [Google Scholar] [CrossRef]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood–brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M.; Schoerner, W.; Niendorf, H.P.; Laniado, M.; Koehler, D.; Claussen, C.; James, A.E., Jr. Initial clinical evaluation of gadolinium DTPA for contrast-enhanced magnetic resonance imaging. Magn. Reson. Imaging 1985, 3, 27–35. [Google Scholar] [CrossRef]
- Greenberger, P.A.; Patterson, R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J. Allergy Clin. Immunol. 1991, 87, 867–872. [Google Scholar] [CrossRef]
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur. Radiol. 2003, 13, 1266–1276. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef]
- Tesileanu, T.; Conte, M.M.; Briguglio, J.J.; Hermundstad, A.M.; Victor, J.D.; Balasubramanian, V. Efficient coding of natural scene statistics predicts discrimination thresholds for grayscale textures. Elife 2020, 9, e54347. [Google Scholar] [CrossRef]
- Misaki, M.; Savitz, J.; Zotev, V.; Phillips, R.; Yuan, H.; Young, K.D.; Bodurka, J. Contrast enhancement by combining T1-and T 2-weighted structural brain MR Images. Magn. Reson. Med. 2015, 74, 1609–1620. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Identifies No Harmful Effects to Date with Brain Retention of Gadolinium-Based Contrast Agents for MRIs; Review to Continue. 2017. Available online: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-identifies-no-harmful-effects-date-brain-retention-gadolinium-based (accessed on 26 January 2021).
- Ali, M.M.; Liu, G.; Shah, T.; Flask, C.A.; Pagel, M.D. Using two chemical exchange saturation transfer magnetic resonance imaging contrast agents for molecular imaging studies. Acc. Chem. Res. 2009, 42, 915–924. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Z.; Liu, G. Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives. Pharmaceuticals 2021, 14, 11. [Google Scholar] [CrossRef]
- Burton, M.E. Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Dieckhoff, J.; Kaul, M.G.; Mummert, T.; Jung, C.; Salamon, J.; Adam, G.; Ittrich, H. In vivo liver visualizations with magnetic particle imaging based on the calibration measurement approach. Phys. Med. Biol. 2017, 62, 3470. [Google Scholar] [CrossRef]
- Mørkenborg, J.; Pedersen, M.; Jensen, F.T.; Stødkilde-Jørgensen, H.; Djurhuus, J.C.; Frøkiær, J. Quantitative assessment of Gd-DTPA contrast agent from signal enhancement: An in vitro study. Magn. Reson. Imaging 2003, 21, 637–643. [Google Scholar] [CrossRef]
- Runge, V.M. Safety of approved MR contrast media for intravenous injection. J. Magn. Reson. Imaging JMRI 2000, 12, 205–213. [Google Scholar] [CrossRef]
- Runge, V.M.; Clanton, J.A.; Price, A.C.; Wehr, C.J.; Herzer, W.A.; Partain, C.L.; James, A.E., Jr. The use of Gd DTPA as a perfusion agent and marker of blood-brain barrier disruption. Magn. Reson. Imaging 1985, 3, 43–55. [Google Scholar] [CrossRef]
- Kopp, A.F.; Laniado, M.; Dammann, F.; Stern, W.; Grönewäller, E.; Balzer, T.; Claussen, C.D. MR imaging of the liver with Resovist: Safety, efficacy, and pharmacodynamic properties. Radiology 1997, 204, 749–756. [Google Scholar] [CrossRef]
- Lee, J.S.; Goo, E.H.; Park, C.S.; Lee, S.Y.; Choi, Y.S. A Study on Usefulness of Specific Agents with Liver Disease at MRI Imaging: Comparison with Ferucarbotran and Gd-EOB-DTPA Contrast Agents. Korean J. Med. Phys. 2009, 20, 235–243. [Google Scholar]
- Bulte, J.W. In vivo MRI cell tracking: Clinical studies. Am. J. Roentgenol. 2009, 193, 314–325. [Google Scholar] [CrossRef]
- Cho, E.S.; Yu, J.S.; Kim, M.J.; Kim, J.H.; Chung, J.J.; Kim, K.W. Focal Eosinophilic Necrosis on Superparamagnetic Iron Oxide–Enhanced MRI. Am. J. Roentgenol. 2010, 194, 1296–1302. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Kang, H.Y.; Baek, S.Y.; Kim, S.M.; Shin, M.J. Assessment of musculoskeletal infection in rats to determine usefulness of SPIO-enhanced MRI. Am. J. Roentgenol. 2007, 189, 542–548. [Google Scholar] [CrossRef]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.A.; Mlady, G.; Eberhardt, S.C.; Sillerud, L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. Am. J. Roentgenol. 2015, 204, 302–313. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, W.-T.; Chou, Y.-H.; Tu, J.-W.; Wang, A.-Y.; Lai, L.-H. Study on Establishing Reference Safe Concentrations of MRI Contrast Agents for Optimized Images: Paramagnetic Gd-DTPA-BMEA and Superparamagnetic Ferucarbotran. Appl. Sci. 2021, 11, 1165. https://doi.org/10.3390/app11031165
Hsiao W-T, Chou Y-H, Tu J-W, Wang A-Y, Lai L-H. Study on Establishing Reference Safe Concentrations of MRI Contrast Agents for Optimized Images: Paramagnetic Gd-DTPA-BMEA and Superparamagnetic Ferucarbotran. Applied Sciences. 2021; 11(3):1165. https://doi.org/10.3390/app11031165
Chicago/Turabian StyleHsiao, Wen-Tien, Yi-Hong Chou, Jhong-Wei Tu, Ai-Yih Wang, and Lu-Han Lai. 2021. "Study on Establishing Reference Safe Concentrations of MRI Contrast Agents for Optimized Images: Paramagnetic Gd-DTPA-BMEA and Superparamagnetic Ferucarbotran" Applied Sciences 11, no. 3: 1165. https://doi.org/10.3390/app11031165
APA StyleHsiao, W.-T., Chou, Y.-H., Tu, J.-W., Wang, A.-Y., & Lai, L.-H. (2021). Study on Establishing Reference Safe Concentrations of MRI Contrast Agents for Optimized Images: Paramagnetic Gd-DTPA-BMEA and Superparamagnetic Ferucarbotran. Applied Sciences, 11(3), 1165. https://doi.org/10.3390/app11031165




