Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights
Abstract
1. Introduction
2. Molecular Insights
3. Polyphenols Effects on VOCs Release
3.1. Effects on Terpenoids
3.2. Effects on Esters
3.3. Effects on Alcohols
3.4. Effects on Volatile Phenols
3.5. Effects on Acids
3.6. Effects on Ketones
3.7. Effects on Oxygen Heterocycles (Furans/Lactones)
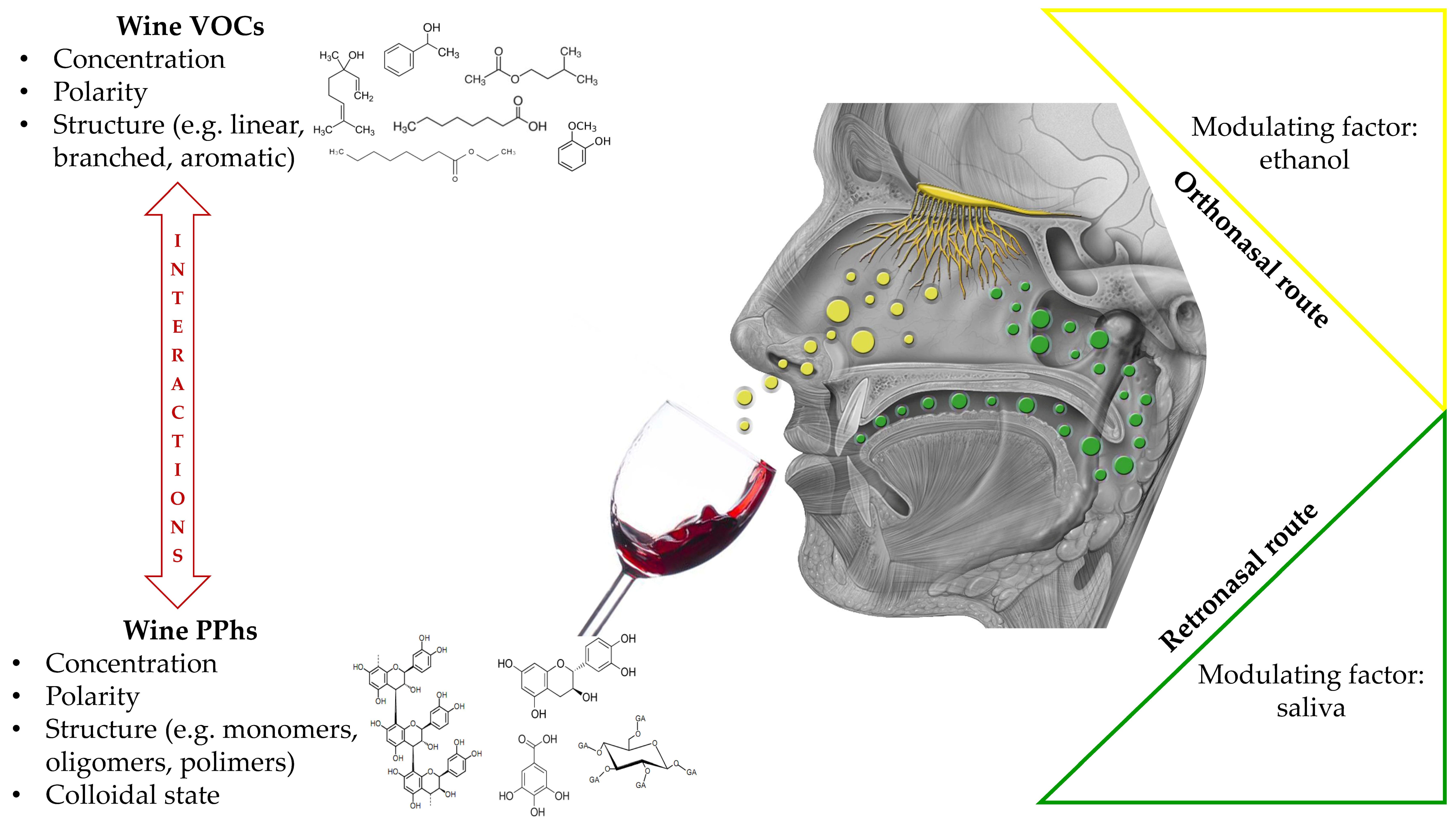
4. Polyphenols Effects on Aromas Release in Oral Conditions: The Role of Saliva
Impact of Saliva on Aroma through the Modulation of Polyphenols–VOCs Interactions
5. Polyphenols Effects on Aromas Sensory Perception
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Peynaud, E. The Taste of Wine: The Art and Science of Wine Appreciation, 1st ed.; Macdonald Orbis: London, UK, 1987. [Google Scholar]
- Green, B.G. Oral astringency: A tactile component of flavor. Acta Psychol. 1993, 84, 119–125. [Google Scholar] [CrossRef]
- Jover, A.J.V.; Montes, F.J.L.; Fuentes, M.d.M.F. Measuring perceptions of quality in food products: The case of red wine. Food Qual. Prefer. 2004, 15, 453–469. [Google Scholar] [CrossRef]
- Charters, S.; Pettigrew, S. The dimensions of wine quality. Food Qual. Prefer. 2007, 18, 997–1007. [Google Scholar] [CrossRef]
- King, E.S.; Kievit, R.L.; Curtin, C.; Swiegers, J.H.; Pretorius, I.S.; Bastian, S.E.P.; Francis, I.L. The effect of multiple yeasts coinoculations on Sauvignon Blanc wine aroma composition, sensory properties and consumer preference. Food Chem. 2010, 122, 618–626. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Avizcuri, J.M.; Echávarri, J.F.; Ferreira, V.; Fernández-Zurbano, P.; Valentin, D. Understanding quality judgements of red wines by experts: Effect of evaluation condition. Food Qual. Pref. 2016, 48, 216–227. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Canon, F. The role of saliva in odour release and perception. Food Chem. 2017, 226, 212–220. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Flavan-3-ols and Condensed tannins. In Understanding Wine Chemistry, 1st ed.; Waterhouse, A.L., Sacks, G.L., Jeffery, D.W., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2016; pp. 117–125. [Google Scholar]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H. Natural astringency in foodstuffs a molecular interpretation. Crit. Rev. Food Sci. Nutr. 1988, 27, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Cai, Y.; Martin, R.; Gaffney, R.H.; Goulding, P.N.; Magnolato, D.; Lilley, T.H.; Haslam, E. Polyphenol complexation some thoughts and observations. Phytochemistry 1988, 27, 2397–2409. [Google Scholar] [CrossRef]
- Hatano, T.; Hemingway, R.W. Association of (+)-catechin and catechin-(4Rf8)-catechin with oligopeptides. Chem. Commun. 1996, 2537–2538. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Astringency in foods. Food Process. Packag. 1954, 23, 124–127. [Google Scholar]
- Bate-Smith, E.C. Haemanalysis of tannins: The concept of relative astringency. Phytochemistry 1973, 12, 907–912. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Gilmore, M.M.; Beauchamp, G.K.; Green, B.G. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evidence that salivary proteins are involved in astringency. J. Sens. Stud. 1998, 13, 29–43. [Google Scholar] [CrossRef]
- Jöbstl, E.; O’Connell, J.; Fairclough, P.A.; Williamson, M.P. Astringency—A molecular model for polyphenol/protein binding. Fibre Diffr. Rev. 2004, 12, 66–69. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and perception. Crit. Rev. Food Sci. Nutr. 2008, 48, 858–875. [Google Scholar] [CrossRef]
- McRae, J.M.; Kennedy, J.A. Wine and grape tannin interactions with salivary proteins and their impact on astringency: A review of current research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef]
- Soares, S.; Vitorino, R.; Osório, H.; Fernandes, A.; Venâncio, A.; Mateus, N.; Amado, F.; de Freitas, V. Reactivity of human salivary proteins families toward food polyphenols. J. Agric. Food Chem. 2011, 59, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Engelen, L. Food Oral Processing: Fundamentals of Eating and Sensory Perception; Chen, J., Engelen, L., Eds.; Wiley-Blackwell, John Wiley & Sons Ltd.: Chichester, UK, 2012. [Google Scholar]
- Jiang, Y.; Gong, N.N.; Matsunami, H. Astringency: A more stringent definition. Chem. Senses 2014, 39, 467–469. [Google Scholar] [CrossRef]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef]
- Robichaud, J.L.; Noble, A.C. Astringency and bitterness of selected phenolics in wine. J. Sci. Food Agric. 1990, 52, 343–353. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Hufnagel, J.C.; Hofmann, T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; de Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; García-Estévez, I.; Gromann, P.; Brás, N.F.; Brandão, E.; Mateus, N.; De Freitas, V.; Behrens, M.; Meyerhof, W.; et al. Human bitter taste receptors are activated by different classes of polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Varela, P.; Gámbaro, A. Sensory descriptive analysis of Uruguayan Tannat wine: Correlation to quality assessment. J. Sens. Stud. 2006, 21, 203–217. [Google Scholar] [CrossRef]
- Li, H. Wine Tasting; China Science Press: Beijing, China, 2006. [Google Scholar]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Culleré, L.; Cacho, J.; Ferreira, V. Analysis for wine C5-C8 aldehydes through the determination of their O-(2,3,4,5,6-pentafluorobenzyl) oximes formed directly in the solid phase extraction cartridge. Anal. Chim. Acta 2004, 524, 201–206. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Q.; Duan, C.; Qu, W.; Wu, Y. Comparative study of aromatic compounds in young red wines from Cabernet Sauvignon, Cabernet Franc, and Cabernet Gernischet varieties in China. J. Food Sci. 2007, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tao, Y.S.; Wang, H. Impact odorants of Chardonnay dry white wine. Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
- Ferreira, V. Volatile aroma compounds and wine sensory attributes. In Managing Wine Quality, 2nd ed.; Volume Viticulture and Wine, Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 3–28. [Google Scholar]
- Cameleyre, M.; Lytra, G.; Barbe, J.C. Static headspace analysis using low-pressure gas chromatography and mass spectrometry, application to determining multiple partition coefficients: A practical tool for understanding red wine fruity volatile perception and the sensory impact of higher alcohols. Anal. Chem. 2018, 90, 10812–10818. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C.; Di Leo Lira, P.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Robinson, A.L.; Ebeler, S.E.; Heymann, H.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. [Google Scholar] [CrossRef]
- Paravisini, L.; Guichard, E. Interactions between aroma compounds and food matrix. In Flavour: From Food to Perception, 1st ed.; Guichard, E., Salles, C., Morzel, M., Le Bon, A.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 208–234. [Google Scholar]
- Piombino, P.; Moio, L.; Genovese, A. Orthonasal vs. retronasal: Studying how volatiles’ hydrophobicity and matrix composition modulate the release of wine odorants in simulated conditions. Food Res. Int. 2019, 116, 548–558. [Google Scholar] [CrossRef]
- Sereni, A.; Osborne, J.; Tomasino, E. Exploring retro-nasal aroma’s influence on mouthfeel perception of Chardonnay wines. Beverages 2016, 2, 7. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Arapitsas, P.; Curioni, A.; Gerbi, V.; Parpinello, G.P.; Ugliano, M.; Piombino, P. Exploring Olfactory–Oral Cross-Modal Interactions through Sensory and Chemical Characteristics of Italian Red Wines. Foods 2020, 9, 1530. [Google Scholar] [CrossRef]
- Genovese, A.; Moio, L.; Sacchi, R.; Piombino, P. Sip volume affects oral release of wine volatiles. Food Res. Int. 2015, 77, 426–431. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smih, D.P.; Owens, S.M.; et al. Saliva from obese individuals suppresses the release of aroma compounds from wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef] [PubMed]
- Villamor, R.R.; Ross, C.R. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Bayonove, C.L. Interactions between wine polyphenols and aroma substances. An insight at the molecular level. J. Agric. Food Chem. 1999, 47, 678–684. [Google Scholar] [CrossRef]
- Jung, D.M.; de Ropp, J.S.; Ebeler, S.E. Study of interactions between food phenolics and aromatic flavors using one- and two-dimensional 1 H NMR spectroscopy. J. Agric. Food Chem. 2000, 48, 407–412. [Google Scholar] [CrossRef]
- Aronson, J.; Ebeler, S.E. Effect of polyphenol compounds on the headspace volatility of flavors. Am. J. Enol. Vitic. 2004, 55, 13–21. [Google Scholar]
- Poncet-Legrand, C.; Cartalade, D.; Putaux, J.L.; Cheynier, V.; Vernhet, A. Flavan-3-ol aggregation in model ethanolic solutions: Incidence of polyphenol structure, concentration, ethanol content, and ionic strength. Langmuir 2003, 19, 10563–10572. [Google Scholar] [CrossRef]
- Zanchi, D.; Vernhet, A.; Poncet-Legrand, C.; Cartalade, D.; Tribet, C.; Schweins, R.; Cabane, B. Colloidal dispersions of tannins in water–ethanol solutions. Langmuir 2007, 23, 9949–9959. [Google Scholar] [CrossRef]
- Ickes, C.M.; Cadwallader, K.R. Effects of Ethanol on Flavor Perception in Alcoholic Beverages. Chem. Percept. 2017, 10, 119–134. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Dambergs, R.G.; Kassara, S.; Parker, M.; Jeffery, D.W.; Herderich, M.J.; Smith, P.A. Phenolic compositions of 50 and 30 year sequences of Australian red wines: The impact of wine age. J. Agric. Food Chem. 2012, 60, 10093–10102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Hatzidimitriou, E.; Paraskevopoulou, A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides, and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. [Google Scholar] [CrossRef]
- Del Álamo, S.M.; Fernandez Escudero, J.A.; De Castro Torio, R. Changes in phenolic compounds and colour parameters of red wine aged with oak chips and in oak barrels. Food Sci. Technol. Int. 2004, 10, 233–241. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.K.; Song, H.C.; Tao, Y.S.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation—Part I: Phenolic acids. Food Chem. 2021, 341, 128288. [Google Scholar] [CrossRef]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; de Revel, G.; Teissedre, P.L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.P.; Campo, E.; Cullere, L.; Fernandez-Zurbano, P.; Valentin, D.; Ferreira, V. Effects of the nonvolatile matrix on the aroma perception of wine. J. Agric. Food Chem. 2010, 58, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Etievant, P.X. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; CRC Press: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Villamor, R.R.; Evans, M.A.; Mattinsonc, D.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in a model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Pons, M. The origin of ethylphenols in wines. J. Sci. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Perez-Coello, M.S.; Diaz-Maroto, M.C. Volatile compounds and wine aging. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 295–311. [Google Scholar]
- Ristic, R.; Boss, P.; Wilkinson, K. Influence of fruit maturity at harvest on the intensity of smoke taint in wine. Molecules 2015, 20, 8913. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, M.; Asproudi, A.; Guaita, M.; Borsa, D.; Motta, S.; Panero, L.; Bosso, A. Influence of the matrix composition on the volatility and sensory perception of 4-ethylphenol and 4-ethylguaiacol in model wine solutions. Food Chem. 2014, 149, 197–202. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. 2006, 62, 191–202. [Google Scholar] [CrossRef]
- Silva, P.; Cardoso, H.; Gerós, H. Studies on the wine spoilage capacity of Brettanomyces/Dekkera spp. Am. J. Enol. Viticult. 2004, 55, 65–72. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma-a review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Pons, M.; Dauphin, B.; La Guerche, S.; Pons, A.; Lavigne-Cruege, V.; Shinkaruk, S.; Bunner, D.; Richard, T.; Monti, J.-P.; Darriet, P. Identification of Impact Odorants Contributing to Fresh Mushroom Off-Flavor in Wines: Incidence of Their Reactivity with Nitrogen Compounds on the Decrease of the Olfactory Defect. J. Agric. Food Chem. 2011, 59, 3264–3272. [Google Scholar] [CrossRef]
- Cravero, M.C. Musty and moldy taint in wines: A review. Beverages 2020, 6, 41. [Google Scholar] [CrossRef]
- Oliveira e Silva, H.; Guedes de Pinho, P.; Machado, B.P.; Hogg, T.; Marques, J.C.; Câmara, J.S.; Albuquerque, F.; Silva Ferreira, A.C. Impact of forced-aging process on Madeira wine flavor. J. Agric. Food Chem. 2008, 56, 11989–11996. [Google Scholar] [CrossRef] [PubMed]
- Salles, C.; Chagnon, M.C.; Feron, G.; Guichard, E.; Laboure, H.; Morzel, M.; Semon, E.; Tarrega, A.; Yven, C. In-mouth mechanisms leading to flavor release and perception. Crit. Rev. Food Sci. Nutr. 2011, 51, 67–90. [Google Scholar] [CrossRef]
- Noble, A.C. Taste–aroma interactions. Trends Food Sci. Technol. 1996, 7, 439–443. [Google Scholar] [CrossRef]
- Buettner, A.; Beauchamp, J. Chemical input–sensory output: Diverse modes of physiology–flavour interaction. Food Qual. Prefer. 2010, 21, 915–924. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Impact of the nonvolatile wine matrix composition on the in vivo aroma release from wines. J. Agric. Food Chem. 2014, 62, 66–73. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Brulé, M.; Feron, G.; Canon, F. Does interindividual variability of saliva affect the release and metabolization of aroma compounds ex vivo? The particular case of elderly suffering or not from hyposalivation. J. Texture Stud. 2019, 50, 36–44. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Feron, G.; Canon, F. Physiological and oral parameters contribute prediction of retronasal aroma release in an elderly cohort. Food Chem. 2020, 128355. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 73–95. [Google Scholar] [CrossRef]
- Guichard, E. Flavour retention and release from protein solutions. Biotechnol. Adv. 2006, 24, 226–229. [Google Scholar] [CrossRef]
- Cheynier, V.; Sarni-Manchado, P. Wine taste and mouthfeel. In Managing Wine Quality: Viticulture and Wine Quality, 1st ed.; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 29–72. [Google Scholar]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel perception of wine: Oral physiology, components and instrumental characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Roberts, D.D.; Acree, T.E. Simulation of retronasal aroma using a modified headspace technique: Investigating the effect of saliva, temperature, shearing, and oil on flavour release. J. Agric. Food. Chem. 1995, 43, 2179–2186. [Google Scholar] [CrossRef]
- van Ruth, S.; Roozen, J. Influence of mastication and saliva on aroma release in a model mouth system. Food Chem. 2000, 71, 339–345. [Google Scholar] [CrossRef]
- Friel, E.N.; Taylor, A.J. Effect of salivary components on volatile partitioning from solution. J. Agric. Food. Chem. 2001, 49, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A. Influence of human saliva on odorant concentrations. 2. Aldehydes, alcohols, 3-alkyl-2-methoxypyrazines, methoxyphenols, and 3-hydroxy-4, 5-dimethyl-2(5H)-furanone. J. Agric. Food. Chem. 2002, 50, 7105–7110. [Google Scholar] [CrossRef]
- Buettner, A. Influence of human salivary enzymes on odorant concentration changes occurring in vivo. 1. Ester and thiols. J. Agric. Food. Chem. 2002, 50, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Pagès-Hélary, S.; Andriot, I.; Guichard, E.; Canon, F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and a-amylase. Food Res. Int. 2014, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Ployon, S.; Brulé, M.; Andriot, I.; Morzel, M.; Canon, F. Understanding retention and metabolization of aroma compounds using an in vitro model of oral mucosa. Food Chem. 2020, 318, 126468. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.E. Abilities of peroxidases to catalyse peroxidase-oxidase oxidation of thiols. Biochem. J. 1988, 256, 757–762. [Google Scholar] [CrossRef]
- Bohren, K.M.; Bullock, B.; Wermuth, B.; Gabbay, K.H. The aldo-keto reductase superfamily—cDNA and deduced amino-acid sequences of human aldehyde and aldose reductases. J. Biol. Chem. 1989, 264, 9547–9551. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Muñoz-González, C.; Jiménez-Girón, A.; Perez-Jiménez, M.; Pozo-Bayón, M.Á. Aroma release in the oral cavity after wine intake is influenced by wine matrix composition. Food Chem. 2018, 243, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jiménez, M.; Chaya, C.; Pozo-Bayón, M.Á. Individual differences and effect of phenolic compounds in the immediate and prolonged in-mouth aroma release and retronasal aroma intensity during wine tasting. Food Chem. 2019, 285, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Perez-Jiménez, M.; Pozo-Bayón, M.Á. Oral persistence of esters is affected by wine matrix composition. Food Res. Int. 2020, 135, 109286. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Canon, F.; Feron, G.; Guichard, E.; Pozo-Bayón, M.Á. Assessment wine aroma persistence by using an in vivo PTR-ToF-MS approach and its relationship with salivary parameters. Molecules 2019, 24, 1277. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Slots, J. Salivary enzymes. Origin and relationship to periodontal disease. J. Periodontal. Res. 1983, 18, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Ihalin, R.; Loimaranta, L.; Tenovuo, J. Origin, structure, and biological activities of peroxidases in human saliva. Arch. Biochem. Biophys. 2006, 445, 261–268. [Google Scholar] [CrossRef]
- Hemingway, K.M.; Alston, M.J.; Chappell, C.G.; Taylor, A.J. Carbohydrate-flavour conjugates in wine. Carbohydr. Polym. 1999, 38, 283–286. [Google Scholar] [CrossRef]
- Starkenmann, C.; Le Calvé, B.; Niclass, Y.; Cayeux, I.; Beccucci, S.; Troccaz, M. Olfactory perception of cysteine-S-conjugates from fruits and vegetables. J. Agric. Food Chem. 2008, 56, 9575–9580. [Google Scholar] [CrossRef]
- Juntheikki, M.R.; Julkunen-Tiitto, R. Inhibition of b-glucosidase and esterase by tannins from Betula, Salix, and Pinus species. J. Chem. Ecol. 2000, 26, 1151–1165. [Google Scholar] [CrossRef]
- Weng, Z.M.; Ge, G.B.; Dou, T.Y.; Wang, P.; Liu, P.K.; Tian, X.H.; Qiao, N.; Yü, Y.; Zou, L.W.; Zhou, Q.; et al. Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2. Bioorganic Chem. 2018, 77, 320–329. [Google Scholar] [CrossRef]
- Linforth, R.; Taylor, A.J. Persistence of volatile compounds in the breath after their consumption in aqueous solutions. J. Agric. Food Chem. 2000, 48, 5419–5423. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A.; Beer, A.; Hannig, C.; Settles, M. Observation of the swallowing process by application of videofluoroscopy and real-time magnetic resonance imaging-consequences for retronasal aroma stimulation. Chem. Senses 2001, 26, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A. Investigation of potent odorants and afterodor development in two Chardonnay wines using the buccal odor screening system (BOSS). J. Agric. Food Chem. 2004, 52, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Buffo, R.; Rapp, J.; Krick, T.; Reineccius, G. Persistence of aroma compounds in human breath after consuming an aqueous model aroma mixture. Food Chem. 2005, 89, 103–108. [Google Scholar] [CrossRef]
- Lubbers, S.; Landy, P.; Voilley, A. Retention and release of aroma compounds in foods containing proteins. Food Technol. 1998, 52, 68. [Google Scholar]
- Asquit, T.N.; Uhlig, J.; Mehansho, H.; Putman, L.; Carlson, D.M.; Butler, L. Binding of condensed tannins to salivary proline-rich glycoproteins: The role of carbohydrate. J. Agric. Food. Chem. 1987, 35, 331–334. [Google Scholar] [CrossRef]
- Etaio, I.; Meillon, S.; Pérez-Elortondo, F.J.; Schlich, P. Dynamic sensory description of Rioja Alavesa red wines made by different winemaking practices by using Temporal Dominance of Sensations. J. Sci. Food Agric. 2016, 96, 3492–3499. [Google Scholar] [CrossRef]
- Lund, C.M.; Nicolau, L.; Gardenr, R.C.; Kilmartin, P.A. Effect of polyphenols on the perception of key aroma compounds from Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2009, 15, 18–26. [Google Scholar] [CrossRef]
- Goldner, M.C.; di Leo, L.P.; van Baren, C.; Bandoni, A. Influence of polyphenol levels on the perception of aroma in Vitis vinifera cv. Malbec wines. S. Afr. J. Enol. Vitic. 2011, 32, 21–27. [Google Scholar] [CrossRef]
- Baker, A.K.; Ross, C.F. Sensory evaluation of impact of wine matrix on red wine finish: A preliminary study. J. Sens. Stud. 2014, 29, 139–148. [Google Scholar] [CrossRef]
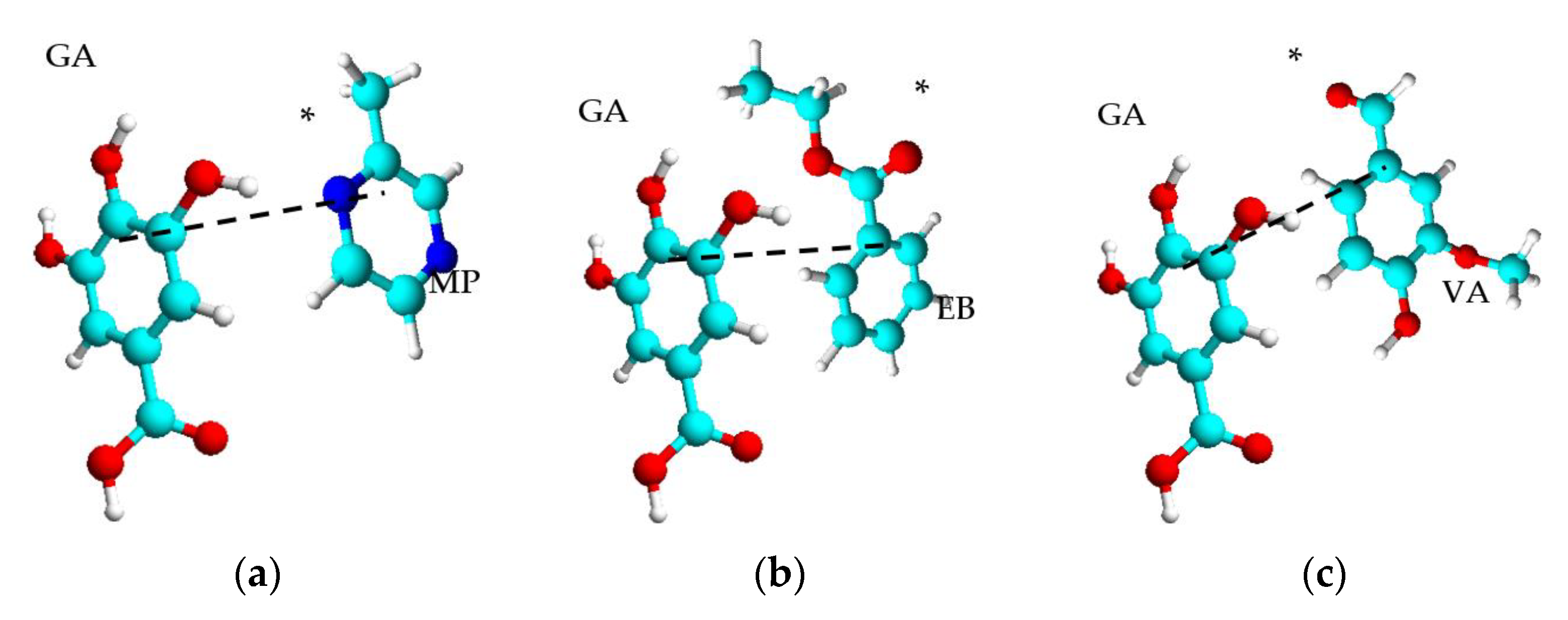
| Aromas Characteristics | Matrix Characteristics | Effects | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Descriptors * | Concentration | logP (o/w) * | Type of Matrix | %Ethanol (v/v) | Added Tannins | Tannin Content | Effects on Release | Effects on Orthonasal Perception | |
| MONOTERPENOIDS | ||||||||||
| α-Terpineol | Pine, terpenic, lilac, citrus, woody, floral | 0–0.433 mg/L | 2.67 | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ (ns) | ||||||||
| Linalool | Citrus, floral, sweet, bois de rose, woody, green blueberry | 1 mg/L | 2.97 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↓ | ||||||||
| 0–0.498 mg/L | White wine | 12 | TPC = 230 | ↑ (ns) | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ (ns) | ||||||||
| Terpinen-4-ol | Peppery, woody, earthy, musty, sweet | 0–0.665 mg/L | 3.26 | White wine | 12 | TPC = 230 | ↑ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| β-Citronellol | Floral, leathery, waxy, rose, citrus | 0–1.563 mg/L | 3.30 | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| Nerol | Sweet, natural, citrus, magnolia | 0–7.838 mg/L | 3.47 | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| ESTERS | ||||||||||
| Diethyl succinate | Fruity, apple, cooked apple, ylang | 20 mg/L | 1.26 | Model wine solution | 10 | Skin tannins extract | 0.5–3 g/L | ↓ | [63] | |
| 3–10 g/L | ↑ | |||||||||
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↑ | ||||||||
| Ethyl isobutanoate | Sweet, ethereal, fruity, alcoholic, fusel, rummy | 200 μg/L | 1.66 | Model wine solution | 12 | Catechin | 2 g/L | NA | ↓ | [66] |
| Gallic acid | 2 g/L | NA | NA | |||||||
| Isobutyl acetate | Sweet, fruity, ethereal, banana, tropical | 0–0.675 mg/L | 1.78 | White wine | 12 | TPC = 230 | ↓ | [62] | ||
| Young-red wine | TPC = 1820 | ↑ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| Butyl acetate | Ethereal, solvent, fruity, banana | 0–0.713 mg/L | 1.78 | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↑ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| Ethyl butanoate | Fruity, fruit juice, pineapple, cognac | 200 μg/L | 1.80 | Model wine solution | 12 | Catechin | 2 g/L | NA | ↓ | [66] |
| Gallic acid | 2 g/L | NA | NA | |||||||
| - | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | |||||
| 0–1.456 mg/L | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | |||||
| Young-red wine | TPC = 1820 | ↑ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| Ethyl 2-methyl butanoate | Sharp, sweet, green apple, fruity | - | 2.16 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ (ns) | [67] | ||
| 0–0.803 mg/L | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | |||||
| Young-red wine | TPC = 1820 | ↑ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| Isoamyl acetate | Sweet, fruity, banana, solvent | 4 mg/L | 2.25 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↑ | [63] | |
| 200 μg/L | Model wine solution | 12 | Catechin | 2 g/L | NA | NA | [66] | |||
| Gallic acid | 2 g/L | NA | NA | |||||||
| - | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | |||||
| 0–1.619 mg/L | White wine | 12 | TPC = 230 | ↓ | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| Ethyl hexanoate | Sweet, fruity, pineapple, waxy, green banana | - | 2.85 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | ||
| 0–2.356 mg/L | White wine | 12 | TPC = 230 | ↓ | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ (ns) | ||||||||
| Ethyl cinnamate | Sweet, balsamic, fruity, spicy, powdery, berry plum | 0–0.825 mg/L | 2.99 | White wine | 12 | TPC = 230 | ↓ | [62] | ||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| Ethyl octanoate | Fruity, winey, waxy, sweet, apricot, banana, brandy, pear | 1 mg/L | 3.84 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↓ | ||||||||
| 600 μg/L | Model wine solution | 12 | Catechin | 2 g/L | ↓ | ↓ | [66] | |||
| Gallic acid | 2 g/L | NA | NA | |||||||
| - | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | |||||
| 0–2.124 mg/L | White wine | 12 | TPC = 230 | ↓ | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| Ethyl decanoate | Sweet, waxy, fruity, apple, grape, oily, brandy | 1.5 mg/L | 4.86 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↓ | ||||||||
| 0–0.931 mg/L | White wine | 12 | TPC = 230 | ↓ | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| Ethyl dodecanoate | Sweet, waxy, floral, soapy, clean | 2 mg/L | 5.71 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Seed tannins extract | 0.5–5 g/L | ↑ | ||||||||
| 5–10 g/L | ↓ | |||||||||
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↓ | ||||||||
| ALCOHOLS | ||||||||||
| Isobutanol | Ethereal, winey | 80 mg/L | 0.76 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↓ | ||||||||
| Benzyl alcohol | Floral, rose, phenolic, balsamic | 0–1.563 mg/L | 1.10 | White wine | 12 | TPC = 230 | ↑ | [62] | ||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| 3-methyl-1-butanol | Fusel, alcoholic, whiskey, fruity, banana | 50 mg/L | 1.16 | Model wine solution | 10 | Grape tannins | 0.5–1.5 g/L | ↓ | [69] | |
| 2-methyl-1-butanol | Roasted, winey, onion, fruity, fusel, alcoholic, whiskey | 150 mg/L | 1.29 | Model wine solution | 10 | Skin tannins extract | 0.5–10 g/L | ↑ | [63] | |
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | slight ↑ | ||||||||
| β-phenylethanol | Floral, rose, dried rose | 50 mg/L | 1.36 | Model wine solution | 10 | Skin tannins extract | 0.5–1 g/L | ↓ | [63] | |
| 1–10 g/L | ↑ | |||||||||
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↑ | ||||||||
| trans-3-hexen-1-ol | Green, cortex, privet, leafy, floral, petal, oily, earthy | 0–0.875 mg/L | 1.61 | White wine | 12 | TPC = 230 | ↓ | [62] | ||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ (ns) | ||||||||
| Hexanol | Ethereal, fusel, oily, fruity, alcoholic, sweet, green | 6 mg/L | 2.03 | Model wine solution | 10 | Seed tannins extract | 0.5–10 g/L | ↓ | [63] | |
| Model wine solution | 10 | Grape tannins | 0.5–1.5 g/L | ↓ | [69] | |||||
| 0–2.200 mg/L | White wine | 12 | TPC = 230 | ↓ (ns) | [62] | |||||
| Young-red wine | TPC = 1820 | ↑ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| ACIDS | ||||||||||
| Butyric acid | Sharp, acetic, cheesy, buttery, fruity | - | 0.79 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | ||
| Hexanoic acid | Sour, fatty, sweaty, cheesy | - | 1.92 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | ||
| Octanoic acid | Fatty, waxy, rancid, oily, vegetable, cheesy | 200 mg/L | 3.05 | Model wine solution | 10 | Skin tannins extract | 0.5–1 g/L | ↓ | [63] | |
| 1–10 g/L | ↑ | |||||||||
| Seed tannins extract | 0.5–5 g/L | ↑ | ||||||||
| 5–10 g/L | ↓ | |||||||||
| Seed/Skin tannins mixture (4:1 w/w) | 1–10 g/L | ↑ | ||||||||
| - | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ↓ | [67] | |||||
| 0–4.656 mg/L | White wine | 12 | TPC = 230 | ↑ | [62] | |||||
| Young-red wine | TPC = 1820 | ↑ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ (ns) | ||||||||
| VOLATILE PHENOLS | ||||||||||
| Guaiacol | Phenolic, smoky, spicy, vanilla, woody | 4 mg/L | 1.32 | Model wine solution | 10 | Grape tannins | 0.5–1.5 g/L | ↑ | [69] | |
| Eugenol | Sweet, spicy, clove, woody | 0.5 mg/L | 2.27 | Model wine solution | 10 | Grape tannins | 0.5–1.5 g/L | ↑ | [69] | |
| 0–0.400 mg/L | White wine | 12 | TPC = 230 | ↓ | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| 4-ethylguaiacol | Spicy, smoky, bacon, phenolic, clove | 135 μg/L | 2.43 | Model wine solution | Not specified | Grape polyphenolic extract | 0–3 g/L | ↓ | ↓ | [73] |
| 4-ethylphenol | Phenolic, castoreum, smoky, guaiacol | 440 μg/L | 2.58 | Model wine solution | Not specified | Grape polyphenolic extract | 0–3 g/L | ↓ | ↓ | [73] |
| 4-vinylphenol | Chemical, phenolic, medicinal, sweet | 0–0.432 mg/L | 2.61 | White wine | 12 | TPC = 230 | ↓ | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| KETONES | ||||||||||
| 1-octen-3-one | Herbal, mushroom, earthy, musty, dirty | 1 mg/L | 2.18 | Model wine solution | 10 | Grape tannins | 0.5–1.5 g/L | ↑ | [69] | |
| - | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ND | [67] | |||||
| α-ionone | Sweet, woody, floral, violet, orris, tropical, fruity | 0–0.228 mg/L | 3.99 | White wine | 12 | TPC = 230 | ↑ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| β-damascenone | Natural, sweet, fruity, rose, plum, grape, raspberry, sugar | - | 4.04 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ND | [67] | ||
| 0–0.425 mg/L | White wine | 12 | TPC = 230 | ↑ (ns) | [62] | |||||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| OXYGEN HETEROCYCLES (FURANS/LACTONES) | ||||||||||
| Sotolon | Sweet, caramellic, maple, sugar, burnt sugar, coffee | - | −0.29 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ND | [67] | ||
| Furaneol | Sweet, cotton candy, caramellic, strawberry, sugar, brown sugar | - | −0.08 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ND | [67] | ||
| Ethyl furaneol | Sweet, caramellic, candy, butterscotch | - | 0.43 | Red wine non-volatile extract + white wine VOCs extract | 12 | TPI = 60.1 | ND | [67] | ||
| 5-methyl furfural | Spicy, caramellic, maple | 0–1.475 mg/L | 0.67 | White wine | 12 | TPC = 230 | ↑ | [62] | ||
| Young-red wine | TPC = 1820 | ↑ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↑ | ||||||||
| γ-nonalactone | Coconut, creamy, waxy, sweet, buttery, oily | 0–0.413 mg/L | 1.94 | White wine | 12 | TPC = 230 | ↓ | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| trans-whiskey lactone | Spicy, coconut, clove, celery, incense | 0–0.868 mg/L | White wine | TPC = 230 | ↑ | [62] | ||||
| Young-red wine | TPC = 1820 | ↓ (ns) | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
| cis-whiskey lactone | Sweet, spicy, coconut, vanilla | 0–0.682 mg/L | 2.63 | White wine | 12 | TPC = 230 | ↑ (ns) | [62] | ||
| Young-red wine | TPC = 1820 | ↓ | ||||||||
| Oak barrel aged-red wine | TPC = 2142 | ↓ | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. https://doi.org/10.3390/app11031157
Pittari E, Moio L, Piombino P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Applied Sciences. 2021; 11(3):1157. https://doi.org/10.3390/app11031157
Chicago/Turabian StylePittari, Elisabetta, Luigi Moio, and Paola Piombino. 2021. "Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights" Applied Sciences 11, no. 3: 1157. https://doi.org/10.3390/app11031157
APA StylePittari, E., Moio, L., & Piombino, P. (2021). Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Applied Sciences, 11(3), 1157. https://doi.org/10.3390/app11031157






