Amoxicillin in Water: Insights into Relative Reactivity, Byproduct Formation, and Toxicological Interactions during Chlorination
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug and Reagents
2.2. Chlorination Reaction
2.2.1. Apparatus and Equipment
2.2.2. Chlorination Experiments
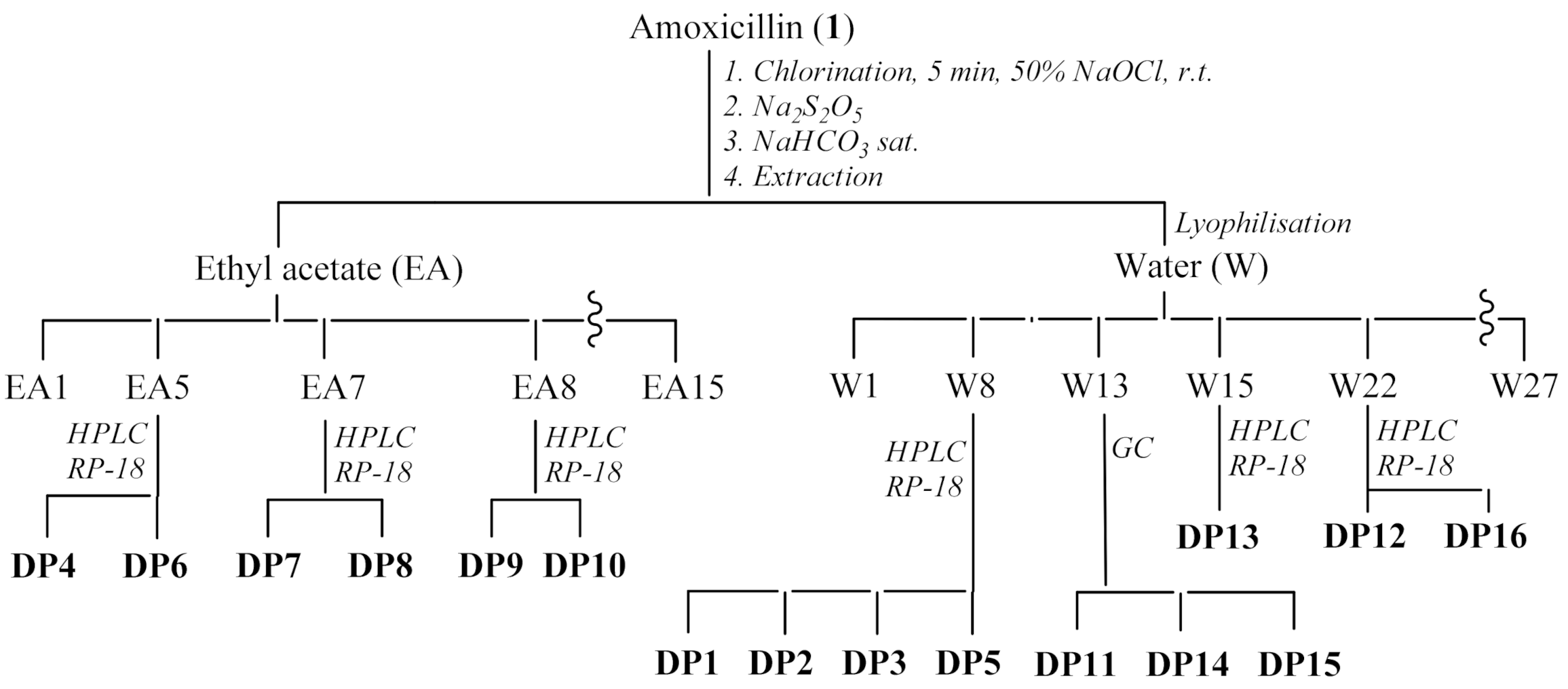
2.2.3. Chlorination Procedure and Product Isolation
2.3. Spectral Data
2.4. Measurement of Antibiotic Activity
3. Results and Discussion
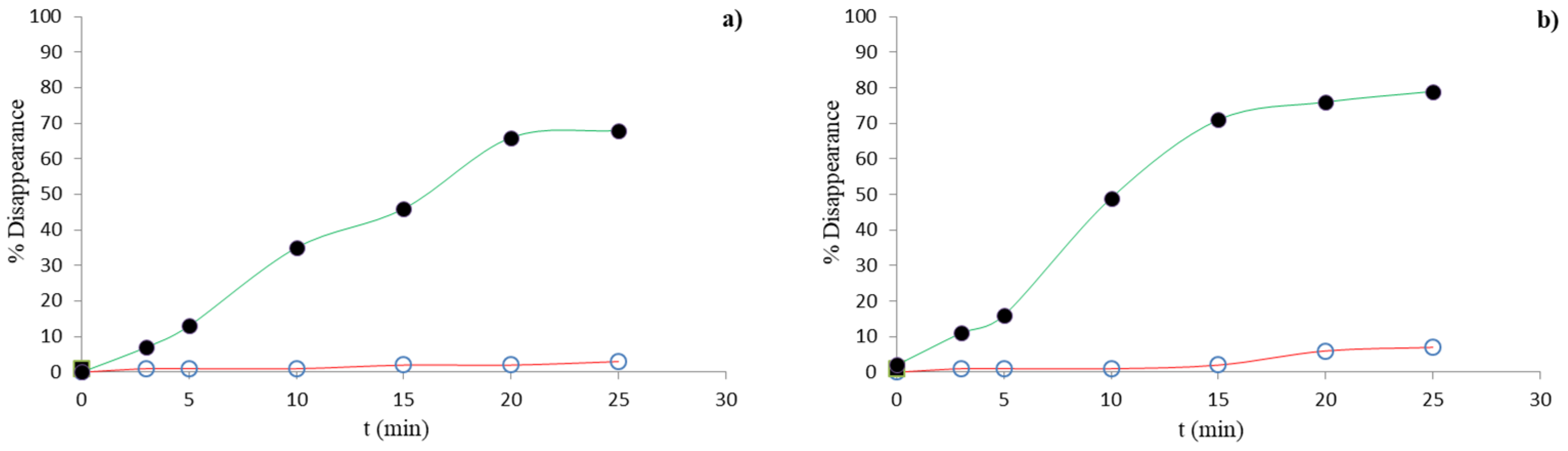
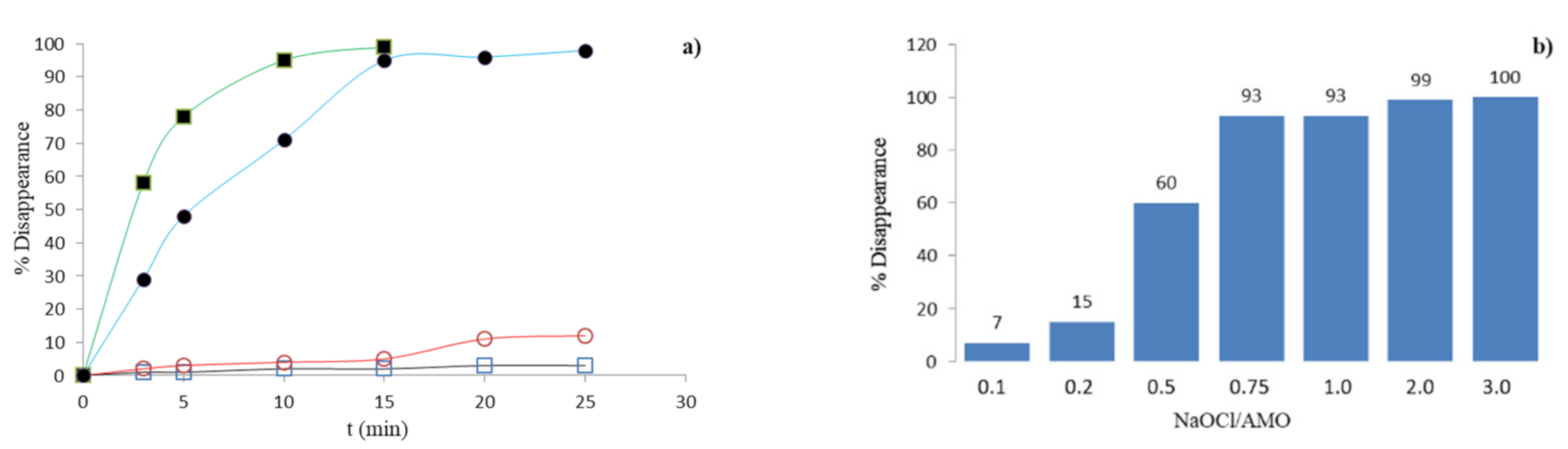
3.1. Chlorination Experiments
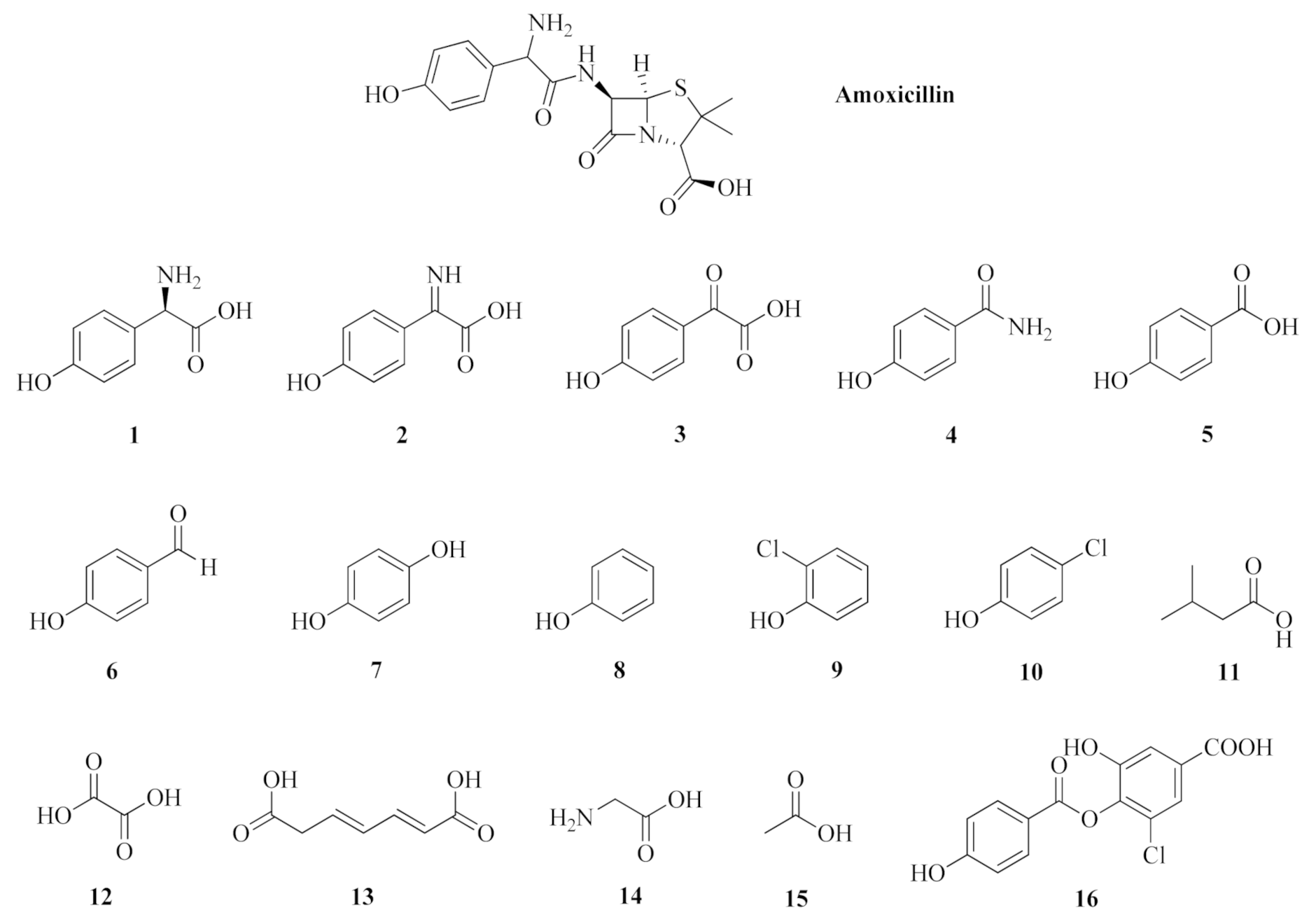
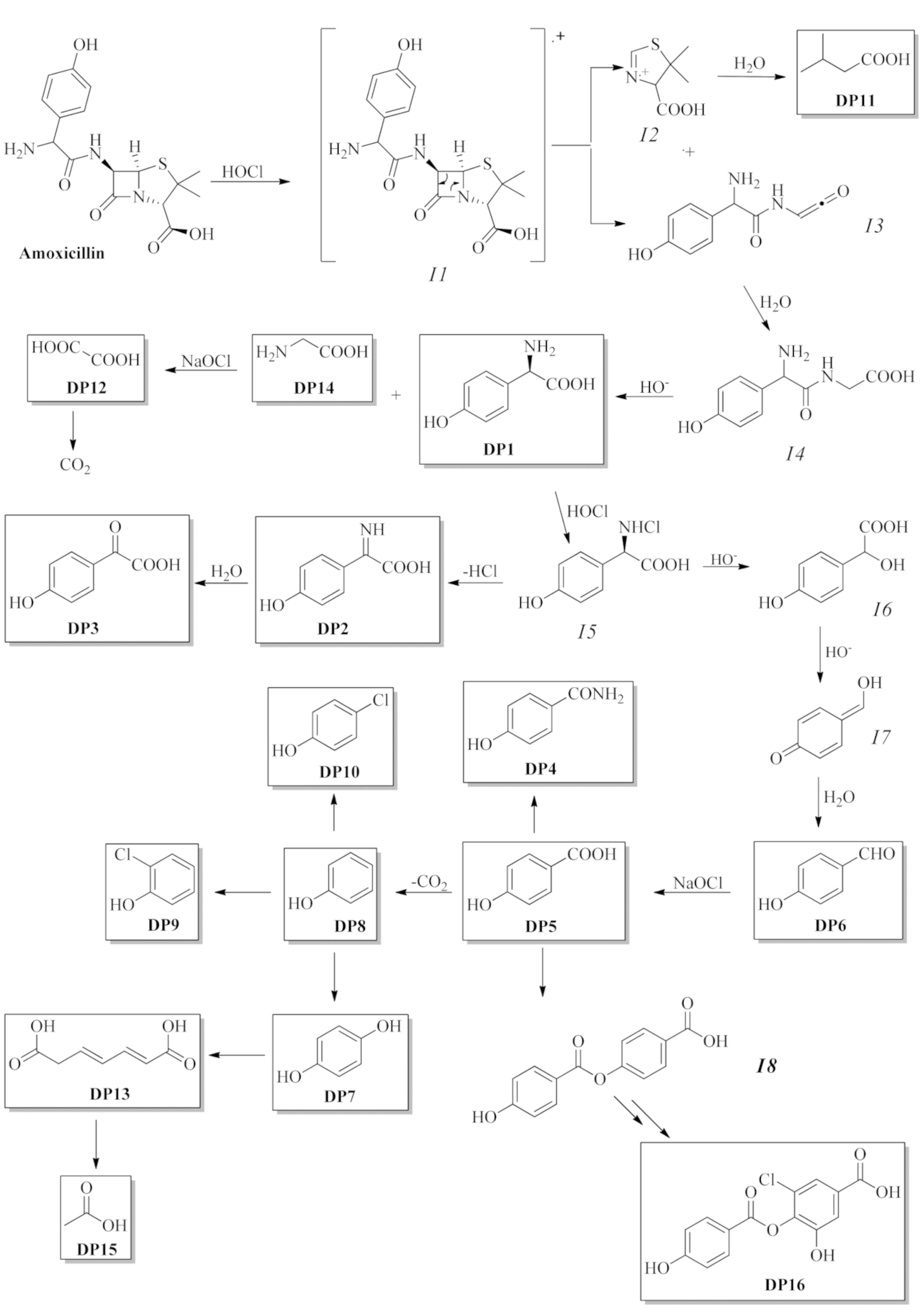
3.2. Structure Elucidation of Degradation Byproducts DP1—DP16
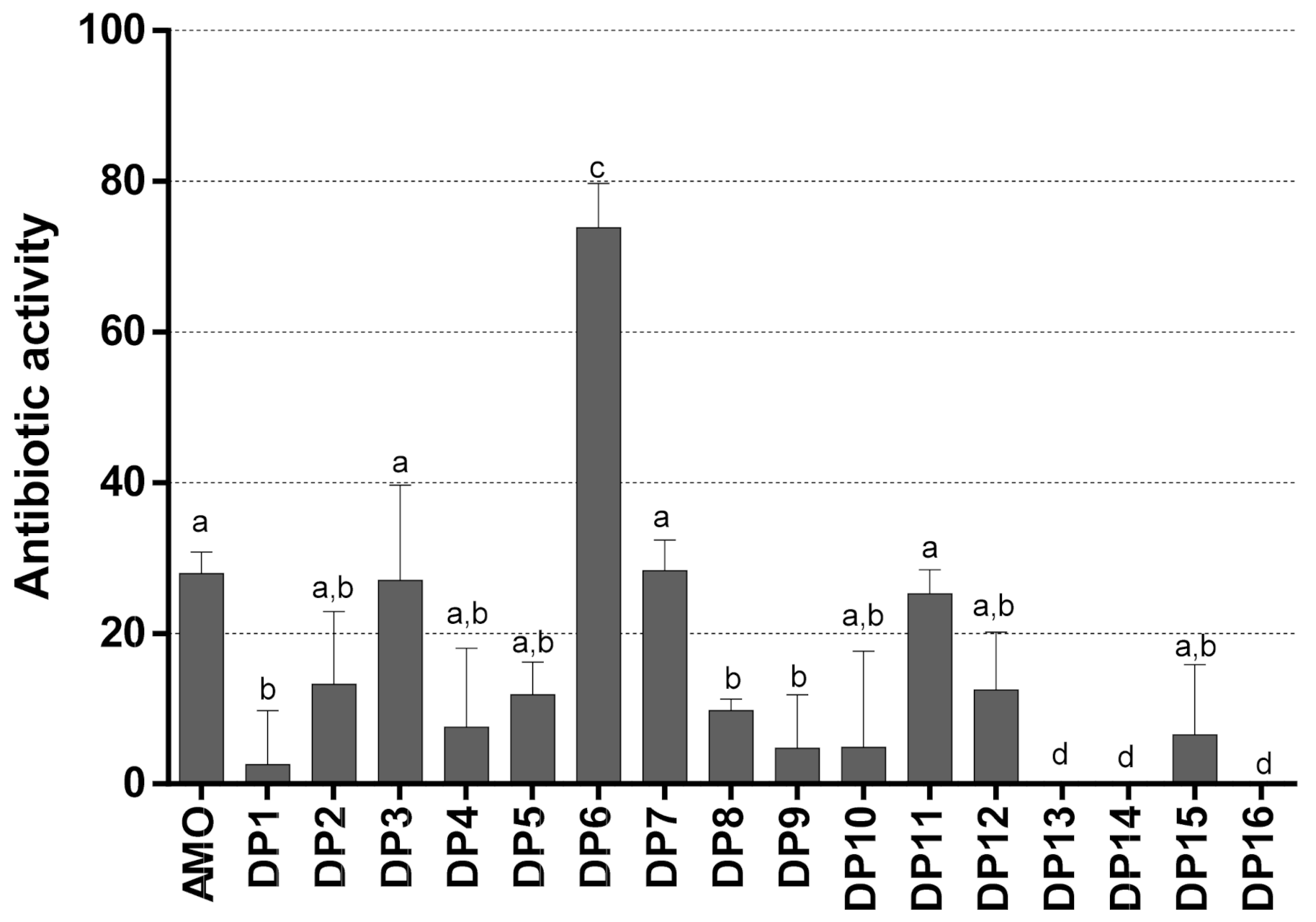
3.3. Antibiotic Activity Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Gabarrón, S.; Gernjak, W.; Valero, F.; Barceló, A.; Petrovic, M.; Rodríguez-Roda, I. Evaluation of emerging contaminants in a drinking water treatment plant using electrodialysis reversal technology. J. Hazard. Mater. 2016, 309, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef]
- Boix, C.; Ibáñez, M.; Sancho, J.V.; Parsons, J.R.; de Voogt, P.; Hernández, F. Biotransformation of pharmaceuticals in surface water and during waste water treatment: Identification and occurrence of transformation products. J. Hazard. Mater. 2016, 302, 175–187. [Google Scholar] [CrossRef]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef]
- Christensen, F.M. Pharmaceuticals in the environment: A human risk? Regul. Toxicol. Pharmacol. 1998, 28, 212–221. [Google Scholar] [CrossRef]
- Stuer-Lauridsen, F.; Birkved, M.; Hansen, L.P.; Lutzhoft, H.C.H.; Halling-Sorensen, B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 2000, 40, 783–793. [Google Scholar] [CrossRef]
- Merlin, C.; Bonot, S.; Courtois, S.; Block, J.-C. Persistence and dissemination of the multiple-antibiotic-resistance plasmid pB10 in the microbial communities of wastewater sludge microcosms. Water Res. 2011, 45, 2897–2905. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist. Updates 2012, 15, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; Tawe, G.S.; Bum, E.N.; Chabrol, T.; Beney, C.; Sinniger, V.; Haudecoeur, R.; Marcourt, L.; Challal, S.; Ferreira–Queiroz, E.; et al. Occurrence of the synthetic analgesic tramadol in an African medicinal plant. Angew. Chem. Int. Ed. Engl. 2013, 52, 11780–11784. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Perrodin, Y.; Pivot, C.; Trepo, D.; Perraud, M.; Droguet, J.; Tissot-Guerraz, F.; Locher, F. Identification and prioritization of bioaccumulable pharmaceutical substances discharged in hospital effluents. J. Environ. Manag. 2012, 103, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.A.; Bellingham, M.; Sinclair, K.D.; Evans, N.P.; Pocar, P.; Fischer, B.; Schaedlich, K.; Schmidt, J.-S.; Amezaga, M.R.; Bhattacharya, S.; et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 2012, 355, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hess-Wilson, J.K.; Knudsen, K.E. Endocrine disrupting compounds and prostate cancer. Cancer Lett. 2006, 241, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Austin, B. Antibiotic pollution from fish farms: Effects on aquatic microflora. Microbiol. Sci. 1985, 2, 113–117. [Google Scholar]
- Miranda, C.D.; Castillo, G. Resistance to antibiotic and heavy metals of motile aeromonads from Chilean freshwater. Sci. Total Environ. 1998, 224, 167–176. [Google Scholar] [CrossRef]
- Lalumera, G.M.; Calamari, D.; Galli, P.; Castiglioni, S.; Crosa, S.; Fanelli, R. Preliminary investigation on the environmental occurrence and effects of antibiotics used in aquaculture in Italy. Chemosphere 2004, 54, 661–668. [Google Scholar] [CrossRef]
- Jones, O.A.; Voulvoulis, N.; Lester, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Garcia-Reiriz, A.; Damiani, P.C.; Olivieri, A.C. Different strategies for the direct determination of amoxicillin in human urine by second-order multivariate analysis of kinetic-spectrophotometric data. Talanta 2007, 71, 806–815. [Google Scholar] [CrossRef]
- Calderon-Preciado, D.; Matamoros, V.; Bayona, J.M. Occurrence and potential crop uptake of emerging contaminants and related compounds in an agricultural irrigation network. Sci. Total Environ. 2011, 412–413, 14–19. [Google Scholar] [CrossRef] [PubMed]
- CSTEE (Scientific Committee on Toxicity, Ecotoxicity and the Environment). Opinion on draft CPMP discussion paper on environmental risk assessment of medicinal products of human use [Non-genetically modified organism (non-GMO) containing]. In Proceedings of the 24th CSTEE Plenary Meeting, Brussels, Belgium, 12 June 2001.
- Schreiber, F.; Szewzyk, U. Environmentally relevant concentrations of pharmaceuticals influence the initial adhesion of bacteria. Aquatic Toxicol. 2008, 87, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; De Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the environment: Occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Dogruel, S.; Baykal, E.; Gerone, G. Combined chemical and biological oxidation of penicillin formulation effluent. J. Environ. Manag. 2004, 73, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three β-lactam antibiotics with ClO2. Water Res. 2008, 42, 1935–1942. [Google Scholar] [CrossRef]
- Kurt, A.; Mert, B.K.; Özengin, N.; Sivrioğlu, Ö.; Yonar, T. Treatment of antibiotics in wastewater using advanced oxidation processes (AOPs). In Physico-Chemical Wastewater Treatment And Resource Recovery; IntechOpen: London, UK, 2017; Volume 175. [Google Scholar] [CrossRef]
- Moles, S.; Mosteo, R.; Gómez, J.; Szpunar, J.; Gozzo, S.; Castillo, J.R.; Ormad, M.P. Towards the removal of antibiotics detected in wastewaters in the POCTEFA territory: Occurrence and TiO2 photocatalytic pilot-scale plant performance. Water 2020, 12, 1453. [Google Scholar] [CrossRef]
- Abbassi, B.E.; Saleem, M.A.; Zytner, R.G.; Gharabaghi, B.; Rudra, R. Antibiotics in wastewater: Their degradation and effect on wastewater treatment efficiency. J. Food Agric. Environ. 2016, 14, 95–99. [Google Scholar]
- Calisto, V.; Domingues, M.R.M.; Erny, G.L.; Esteves, V.I. Direct photodegradation of carbamazepine followed by micellar electrokinetic chromatography and mass spectrometry. Water Res. 2011, 45, 1095–1104. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Kolar, B. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Luongo, G.; Previtera, L.; Ladhari, A.; Di Fabio, G.; Zarrelli, A. Peracetic acid vs. sodium hypochlorite: Degradation and transformation of drugs in wastewater. Molecules 2020, 25, 2294. [Google Scholar] [CrossRef] [PubMed]
- Luongo, G.; Guida, M.; Siciliano, A.; Libralato, G.; Saviano, L.; Amoresano, A.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Oxidation of diclofenac in water by sodium hypochlorite: Identification of new degradation by-products and their ecotoxicological evaluation. J. Pharm. Biomed. Anal. 2021, 194, 113762. [Google Scholar] [CrossRef] [PubMed]
- Chusaksri, S.; Sutthivaiyakit, S.; Sedlak, D.L.; Sutthivaiyakit, P. Reactions of phenylurea compounds with aqueous chlorine: Implications for herbicide transformation during drinking water degradation. J. Hazard. Mater. 2012, 209, 484–491. [Google Scholar] [CrossRef]
- Sandín-España, P.; Magrans, J.O.; García-Baudín, J.M. Study of clethodim degradation and by-product formation in chlorinated water by HPLC. Chromatographia 2005, 62, 133–137. [Google Scholar] [CrossRef]
- ISO 6341:2012. Water Quality-Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organisation for Standardisation, Geneva, Switzerland. 2012. Available online: https://www.iso.org/standard/54614.html (accessed on 23 December 2020).
- Bedner, M.; MacCrehan, W.A. Transformation of acetaminophen bychlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.W.; Mckim, J.M.; Martin, R.A., Jr. HPLC Determination of oxalic acid in cocoa. J. Liq. Chromatogr. 1986, 9, 2781–2789. [Google Scholar] [CrossRef]
- Liu, C.; Molinski, T.F. Preparation of α-amino acids by oxidative oxazoline–oxazinone rearrangement–hydrogenation (OOOH). Scope and limitations. Chem. Asian J. 2011, 6, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.R.; Chakraborty, D. Fe-III-catalyzed synthesis of primary amides from aldehydes. Eur. J. Org. Chem. 2011, 201, 2226–2229. [Google Scholar] [CrossRef]
- Fujita, M.; Nagai, M.; Inoue, T. Carbon-13 nuclear magnetic resonance spectral study. Effect of O-methylation of ortho-substituted phenols on the aryl carbon shielding and its application to interpretation of the spectra of some flavonoids. Chem. Pharm. Bull. 1982, 30, 1151–1156. [Google Scholar] [CrossRef][Green Version]
- Bovonsombat, P.; Ali, R.; Khan, C.; Leykajarakul, J.; Pla-on, K.; Aphimanchindakul, S.; Natchapon, P.; Nisit, T.; Anchalee, A.; Punpongjareorn, N. Facile p-toluenesulfonic acid-promoted para-selective monobromination and chlorination of phenol and analogues. Tetrahedron 2010, 66, 6928–6935. [Google Scholar] [CrossRef]
- Trincado, M.; Grützmacher, H.; Vizza, F.; Bianchini, C. Domino rhodium/palladium-catalyzed dehydrogenation reactions of alcohols to acids by hydrogen transfer to inactivated alkenes. Chem. Eur. J. 2010, 16, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Siciliano, A.; Galdiero, E.; Guida, M.; Luongo, G.; Liguori, R.; Di Fabio, G.; Previtera, L.; Zarrelli, A. Degradation by-products and ecotoxic risk associated with hypochlorite treatment of tramadol. Molecules 2019, 24, 693. [Google Scholar] [CrossRef] [PubMed]
- Zarrelli, A.; Della Greca, M.; Parolisi, A.; Iesce, M.R.; Cermola, F.; Isidori, M.; Lavorgna, M.; Passananti, M.; Previtera, L. Chemical fate and genotoxic risk associated with hypochlorite treatment of nicotine. Sci. Total Environ. 2012, 426, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zarrelli, A.; Della Greca, M.; Iesce, M.R.; Lavorgna, M.; Temussi, F.; Schiavone, L.; Criscuolo, E.; Parrella, A.; Previtera, L.; Isidori, M. Ecotoxicological evaluation of caffeine and its derivatives from a simulated chlorination step. Sci. Total Environ. 2014, 470, 453–458. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Amoxicillin in Water: Insights into Relative Reactivity, Byproduct Formation, and Toxicological Interactions during Chlorination. Appl. Sci. 2021, 11, 1076. https://doi.org/10.3390/app11031076
Siciliano A, Guida M, Libralato G, Saviano L, Luongo G, Previtera L, Di Fabio G, Zarrelli A. Amoxicillin in Water: Insights into Relative Reactivity, Byproduct Formation, and Toxicological Interactions during Chlorination. Applied Sciences. 2021; 11(3):1076. https://doi.org/10.3390/app11031076
Chicago/Turabian StyleSiciliano, Antonietta, Marco Guida, Giovanni Libralato, Lorenzo Saviano, Giovanni Luongo, Lucio Previtera, Giovanni Di Fabio, and Armando Zarrelli. 2021. "Amoxicillin in Water: Insights into Relative Reactivity, Byproduct Formation, and Toxicological Interactions during Chlorination" Applied Sciences 11, no. 3: 1076. https://doi.org/10.3390/app11031076
APA StyleSiciliano, A., Guida, M., Libralato, G., Saviano, L., Luongo, G., Previtera, L., Di Fabio, G., & Zarrelli, A. (2021). Amoxicillin in Water: Insights into Relative Reactivity, Byproduct Formation, and Toxicological Interactions during Chlorination. Applied Sciences, 11(3), 1076. https://doi.org/10.3390/app11031076










