TQ-6, a Novel Ruthenium Derivative Compound, Possesses Potent Free Radical Scavenging Activity in Macrophages and Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TQ-6
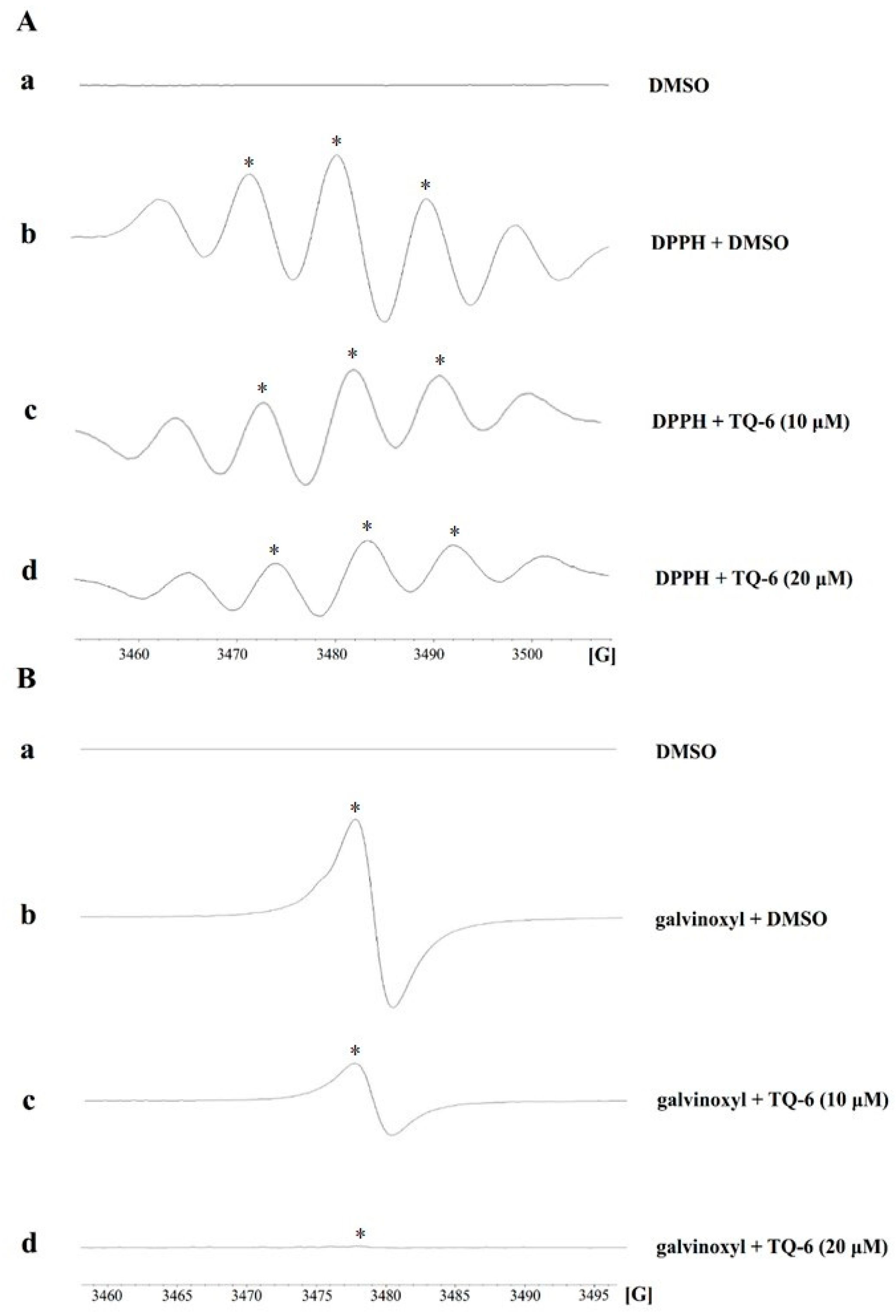
2.3. DPPH and Galvinoxyl Radical Scavenging Assay
2.4. Superoxide Scavenging Assay
2.5. Fenton Reaction Analysis by ESR Spectrometry
2.6. Cell Cultivation
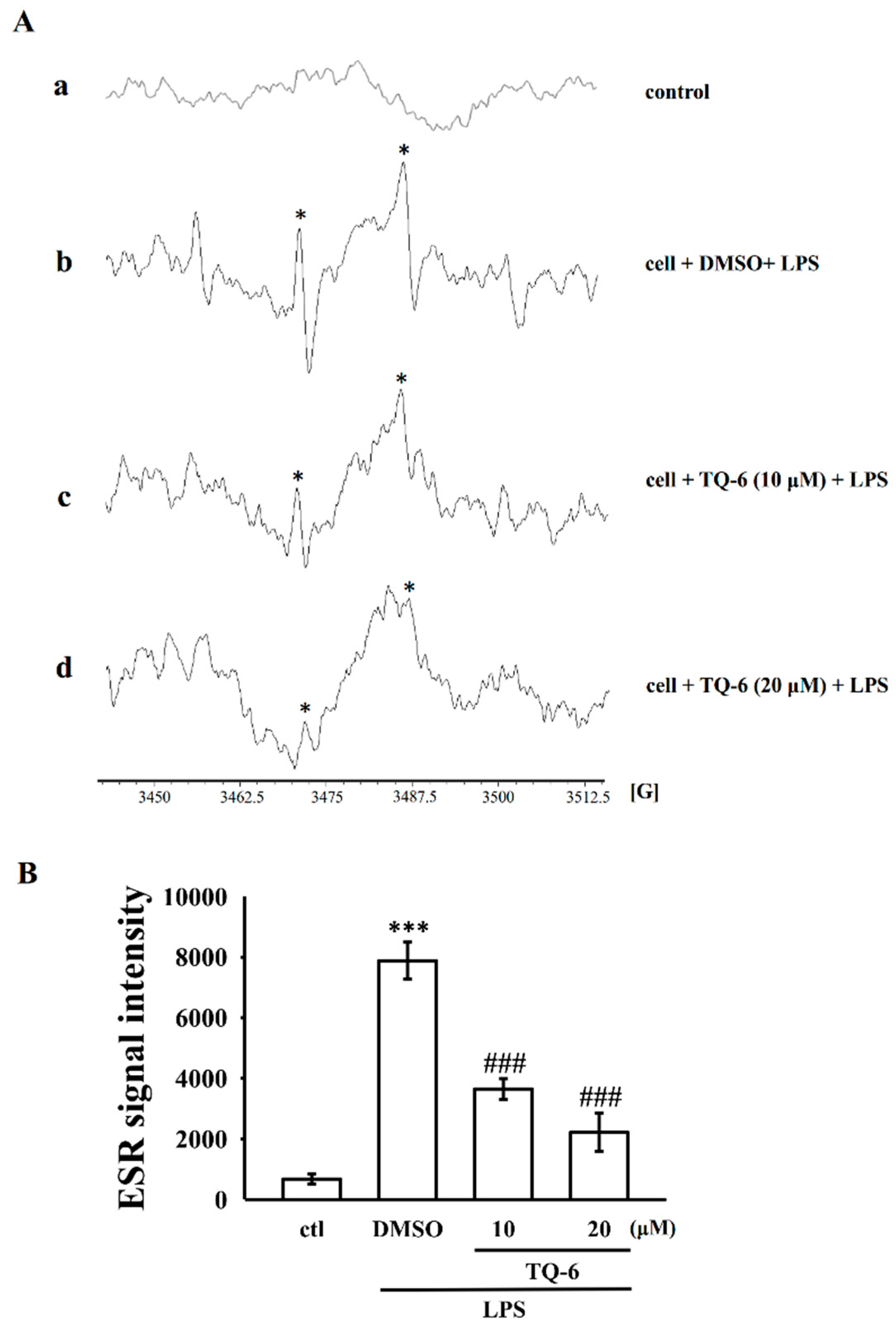
2.7. Measurement of OH• Formation
2.8. Quantitative Real-Time PCR (RT-qPCR)
2.9. Immunoblotting
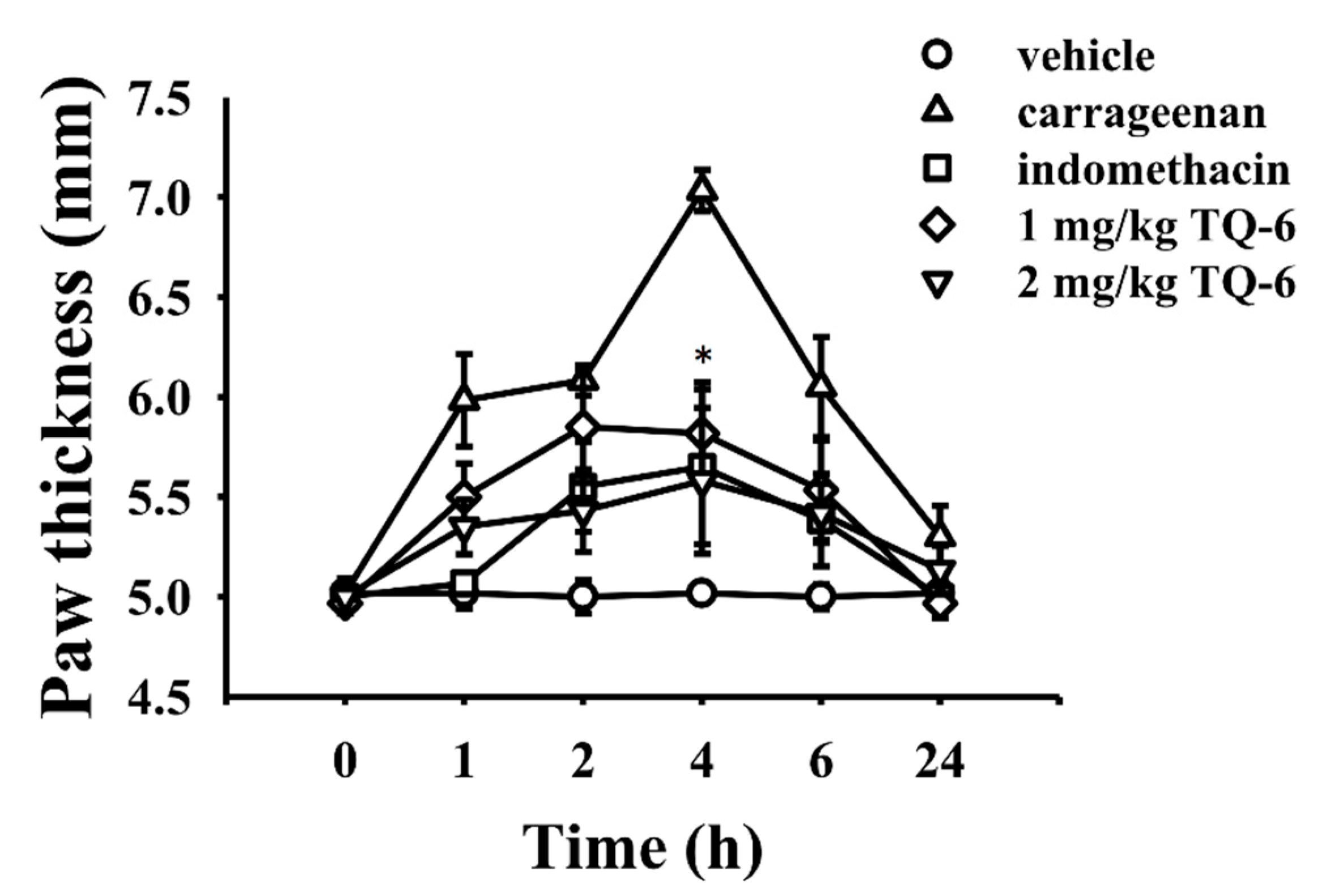
2.10. Carrageenan-Induced Rat Paw Edema Measurement
2.11. Statistical Analysis
3. Results
3.1. TQ-6 Reduces DPPH and Galvinoxyl Free Radical Formation
3.2. Effect of TQ-6 on Superoxide Anion Formation-Derived from X/XO and OH• Formation
3.3. TQ-6 Inhibits OH• and ROS Formation
3.4. TQ-6 Enhances Nrf2/HO-1 mRNA and Protein Expression
3.5. Carrageenan-Induced Inflammatory Response in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Crunkhorn, S. Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat. Rev. Drug Discov. 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Tamion, F.; Richard, V.; Renet, S.; Thuillez, C. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, antioxidant, and antibacterial studies of some metal(ii) complexes of tetradentate Schiff base ligand: (4E)-4-[(2-{(E)-[1-(2,4-Dihydroxyphenyl) ethylidene]amino}ethyl)imino] pentan-2-one. Bioinorg. Chem. Appl. 2015, 2015, 890734. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Pothiraj, K.; Baskaran, T. DNA-binding, oxidative DNA cleavage and coordination mode of later 3d transition metal complexes of a Schiff base derived from isatin as antimicrobial agents. J. Coord. Chem. 2011, 64, 3900–3917. [Google Scholar] [CrossRef]
- Pascu, G.I.; Hotze, A.C.G.; Sanchez-Cano, C.; Kariuki, B.M.; Hannon, M.J. Dinuclear Ruthenium(II) triple-stranded helicates: Luminescent supramolecular cylinders that bind and coil DNA and exhibit activity against cancer cell lines. Angew. Chem. Int. Ed. 2007, 46, 4374–4378. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, in vitro antioxidant and anticancer studies of ruthenium(III) complexes of symmetric and asymmetric tetradentate Schiff bases. J. Coord. Chem. 2015, 68, 2552–2564. [Google Scholar] [CrossRef]
- Hsia, C.H.; Velusamy, M.; Jayakumar, T.; Chen, Y.J.; Hsia, C.W.; Tsai, J.H.; Teng, R.D.; Sheu, J.R. Mechanisms of TQ-6, a Novel Ruthenium-Derivative Compound, against Lipopolysaccharide-Induced In Vitro Macrophage Activation and Liver Injury in Experimental Mice: The Crucial Role of p38 MAPK and NF-κB Signaling. Cells 2018, 7, 217. [Google Scholar] [CrossRef]
- Hsia, C.H.; Velusamy, M.; Sheu, J.R.; Khamrang, T.; Jayakumar, T.; Lu, W.J.; Lin, K.H.; Chang, C.C. A novel ruthenium (II)-derived organometallic compound, TQ-6, potently inhibits platelet aggregation: Ex vivo and in vivo studies. Sci. Rep. 2017, 7, 9556. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.S.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R.; Chen, Z.C.; Hsu, M.J.; Wang, S.H.; Jung, K.W.; Wu, W.F.; Pan, S.H.; Teng, R.D.; Yang, C.H.; Hsieh, C.Y. CME-1, a novel polysaccharide, suppresses iNOS expression in lipopolysaccharide-stimulated macrophages through ceramide-initiated protein phosphatase 2A activation. J. Cell. Mol. Med. 2018, 22, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Roy, P.; Singh, V.; Kumar, A.; Singh, R.; Sharma, P. Anti-inflammatory activity of lactobacillus on carrageenan-induced paw edema in male wistar rats. Int. J. Inflam. 2012, 2012, 752015. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001, 335, 157–166. [Google Scholar]
- Brüne, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264.7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/ HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, L.; Zhou, P.; Dong, Z.; Feng, S.; Liu, K.; Gong, Z. CHP1002, a novel andrographolide derivative, inhibits pro-inflammatory inducible nitric oxide synthase and cyclooxygenase-2 expressions in RAW264.7 macrophages via up-regulation of heme oxygenase-1 expression. Int. Immunopharmacol. 2013, 15, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4, 7-Dimethoxy-5-methyl-1, 3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Shurui, C.; Zipeng, Z.; Hongliang, D.; Hongyu, W.; Yuanlong, L.; Kang, Z.; Zhaoliang, S.; Yue, G.; Chang, L.; et al. Aspirin suppresses neuronal apoptosis, reduces tissue inflammation, and restrains astrocyte activation by activating the Nrf2/HO-1 signaling pathway. Neuroreport 2018, 29, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Natanson, C.; Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Peart, K.W.; Banks, S.M.; MacVittie, T.J.; Walker, R.I.; Parrillo, J.E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J. Clin. Investig. 1989, 83, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Lin, M.H.; Chou, Y.S.; Tsai, Y.J.; Chou, D.S. Antioxidant properties of 5,7-dihydroxycoumarin derivatives in in vitro cell-free and cell-containing systems. J. Exp. Clin. Med. 2011, 3, 126–131. [Google Scholar] [CrossRef]
- Leung, C.H.; Lin, S.; Zhong, H.J.; Ma, D.L. Metal complexes as potential modulators of inflammatory and autoimmune responses. Chem. Sci. 2015, 6, 871–884. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Pink, M.; Fan, H.; Caulton, K.G. Redox chemistry of the triplet complex (PNP)Co(I). J. Am. Chem. Soc. 2008, 130, 4262–4276. [Google Scholar] [CrossRef]
- Li, W.; Yang, S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar]
- You, Z.L.; Shi, D.H.; Xu, C.; Zhang, Q.; Zhu, H.L. Schiff base transition metal complexes as novel inhibitors of xanthine oxidase. Eur. J. Med. Chem. 2008, 43, 862–871. [Google Scholar] [CrossRef]
- Bylund, J.; Brown, K.L.; Movitz, C.; Dahlgren, C.; Karlsson, A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: How, where, and what for? Free Radic. Biol. Med. 2010, 49, 1834–1845. [Google Scholar] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zeng, C.H.; Huang, H.L.; He, L.X.; Wu, F.H. Synthesis, DNA-binding, photocleavage, cytotoxicity and antioxidant activity of ruthenium (II) polypyridyl complexes. Eur. J. Med. Chem. 2010, 45, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Chung, H.T.; Pae, H.O. Differential effects of resveratrol and its natural analogs, piceatannol and 3, 5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. Biofactors 2014, 40, 138–145. [Google Scholar] [CrossRef]
- Choo, Y.Y.; Lee, S.; Nguyen, P.H.; Lee, W.; Woo, W.H.; Min, B.S.; Lee, J.H. Caffeoylglycolic acid methyl ester, a major constituent of sorghum, exhibits anti-inflammatory activity via the Nrf2/heme oxygenase-1 pathway. RSC Adv. 2015, 5, 17786–17796. [Google Scholar] [CrossRef]
- Li, B.Z.; Guo, B.; Zhang, H.Y.; Liu, J.; Tao, S.S.; Pan, H.F.; Ye, D.Q. Therapeutic potential of HO-1 in autoimmune diseases. Inflammation 2014, 37, 1779–1788. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Xaus, J.; Comalada, M.; Valledor, A.F.; Lloberas, J.; López-Soriano, F.; Argilés, J.M.; Bogdan, C.; Celada, A. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-α. Blood 2000, 95, 3823–3831. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.A.; Shelke, R.; Nawale, R.B. Zinc-aceclofenac complex: Synthesis, hydrolysis study and antiinflammatory studies. Antiinflamm. Antiallergy Agents Med. Chem. 2014, 13, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rademaker-Lakhai, J.M.; van den Bongard, D.; Pluim, D.; Beijnen, J.H.; Schellens, J.H.M. A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin. Cancer Res. 2004, 10, 3717–3727. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer Sequence | Accession No. |
|---|---|---|
| Nrf2 | Forward 5′-AGC AGG ACA TGG AGC AAG TT-3′ Reverse 5′-TTC TTT TTC CAG CGA GGA GA-3 | NM_010902.4 |
| HO-1 | Forward 5′-GCA CTA TGT AAA GCG TCT CC-3′ Reverse 5′-GAC TCT GGT CTT TGT GTT CC-3’ | NM_010442.2 |
| GAPDH | Forward 5′-GAA CAT CAT CCC TGC ATC CA-3′ Reverse 5′-GCC AGT GAG CTT CCC GTT CA-3′ | NM_001289726.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, K.-W.; Chang, C.-C.; Jayakumar, T.; Velusamy, M.; Hsia, C.-W.; Trang, N.T.T.; Chou, D.-S.; Hsieh, C.-Y.; Hsia, C.-H. TQ-6, a Novel Ruthenium Derivative Compound, Possesses Potent Free Radical Scavenging Activity in Macrophages and Rats. Appl. Sci. 2021, 11, 1008. https://doi.org/10.3390/app11031008
Hung K-W, Chang C-C, Jayakumar T, Velusamy M, Hsia C-W, Trang NTT, Chou D-S, Hsieh C-Y, Hsia C-H. TQ-6, a Novel Ruthenium Derivative Compound, Possesses Potent Free Radical Scavenging Activity in Macrophages and Rats. Applied Sciences. 2021; 11(3):1008. https://doi.org/10.3390/app11031008
Chicago/Turabian StyleHung, Kao-Wei, Chao-Chien Chang, Thanasekaran Jayakumar, Marappan Velusamy, Chih-Wei Hsia, Nguyen Thi Thu Trang, Duen-Suey Chou, Cheng-Ying Hsieh, and Chih-Hsuan Hsia. 2021. "TQ-6, a Novel Ruthenium Derivative Compound, Possesses Potent Free Radical Scavenging Activity in Macrophages and Rats" Applied Sciences 11, no. 3: 1008. https://doi.org/10.3390/app11031008
APA StyleHung, K.-W., Chang, C.-C., Jayakumar, T., Velusamy, M., Hsia, C.-W., Trang, N. T. T., Chou, D.-S., Hsieh, C.-Y., & Hsia, C.-H. (2021). TQ-6, a Novel Ruthenium Derivative Compound, Possesses Potent Free Radical Scavenging Activity in Macrophages and Rats. Applied Sciences, 11(3), 1008. https://doi.org/10.3390/app11031008







