Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review
Abstract
1. Introduction
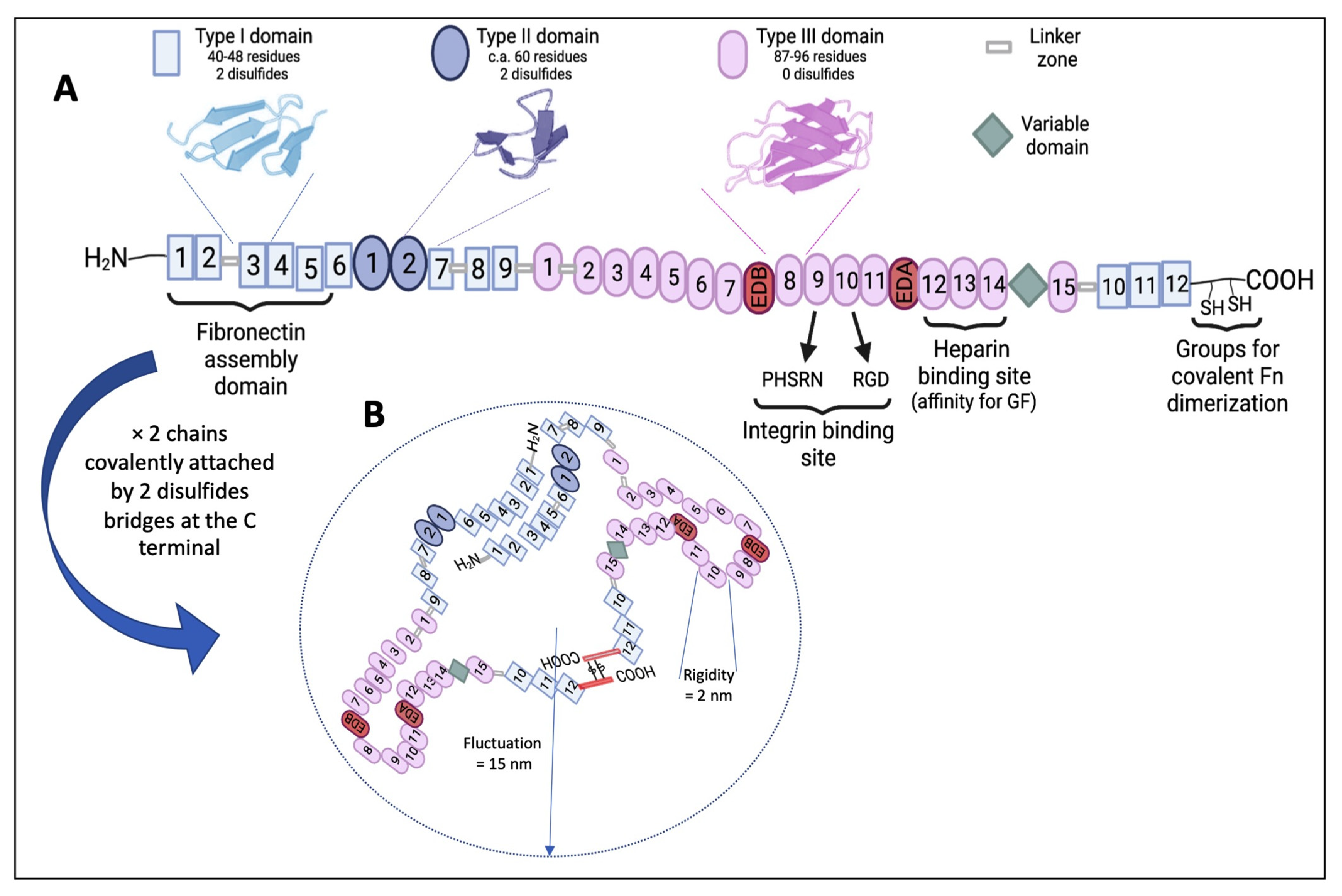
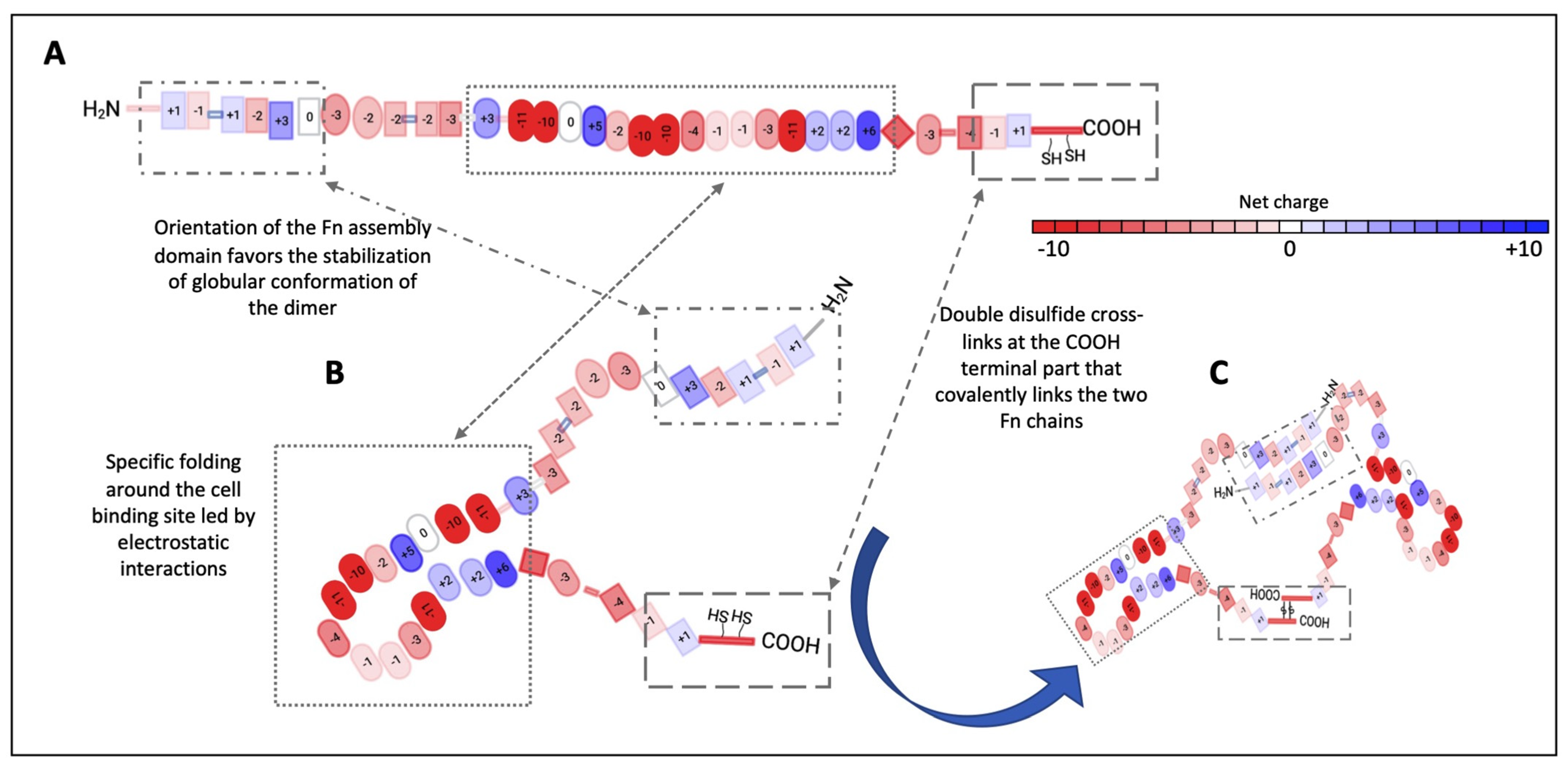
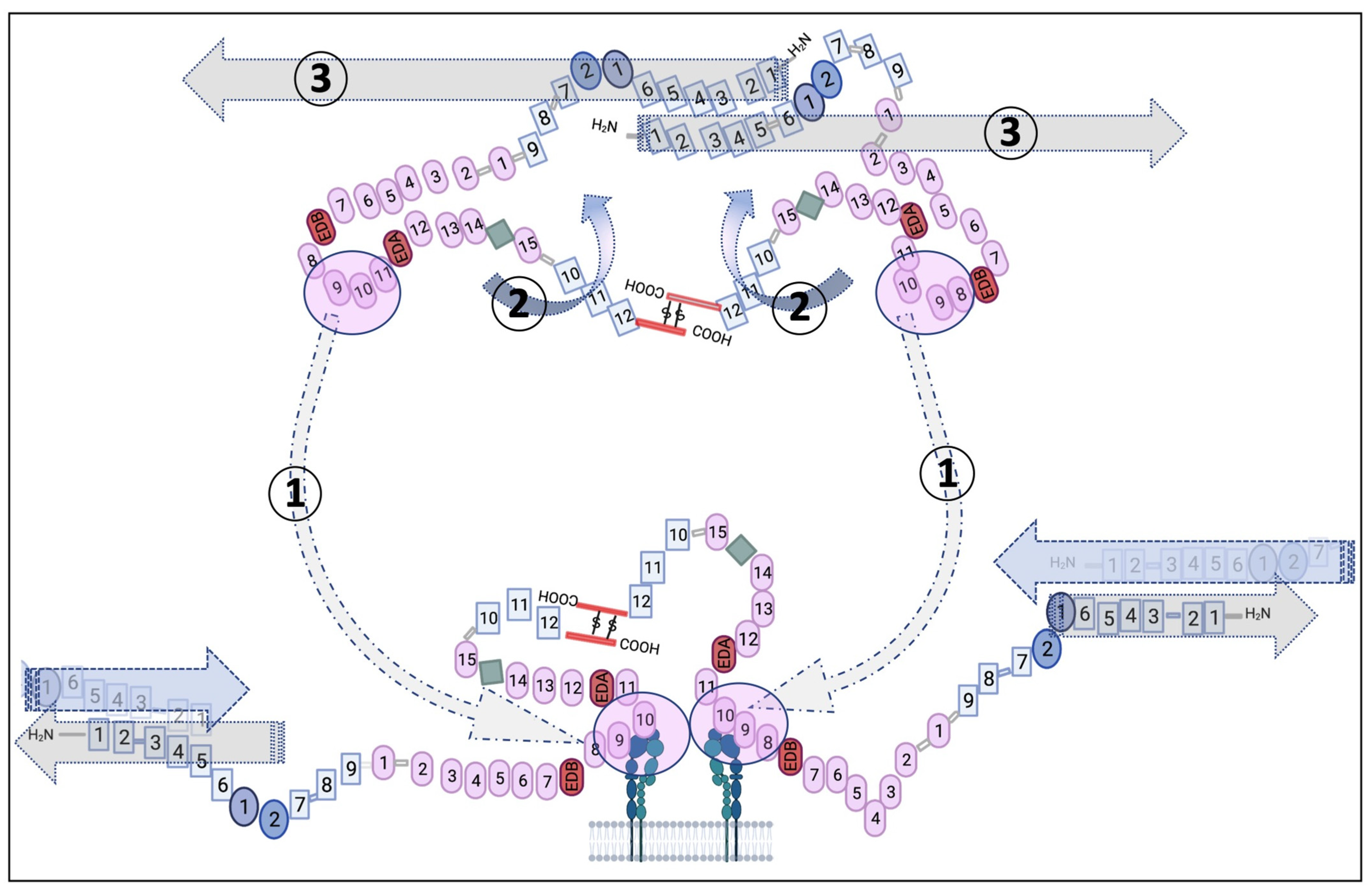
2. Fn as a Pertinent Key Actor in the ECM
2.1. Fn’s General Properties
2.2. Fn’s Role and the Structure–Function Relationship within the ECM and Cells
3. Fn Application and Involvement in Biomaterials and Medical Device Engineering
- Simple molecular two-dimensional (2D) coatings at interfaces via covalent binding or adsorption and the use of aptamers to favor Fn adsorption in monolayers;
- Complex coated interfaces, where Fn is combined with other molecules in order to form bioengineered multilayered interfaces generating thin films and interfaces in 2.5D;
- Fn distribution in a three-dimensional (3D) volume entrapped in hydrogels via physical dispersion and covalent cross-linking.
3.1. Fn 2D Molecular Coatings
3.1.1. Fn Covalent Binding
3.1.2. Fn Physical Adsorption
3.2. Fn in Complex Coated Interfaces
3.3. Fn in Volume
3.3.1. Fn Physical Dispersion
3.3.2. Fn Covalent Cross-Linking
3.4. Potential for the Use of Fn in Medical Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, H.E.; Leach, J.K. Designing Bioactive Delivery Systems for Tissue Regeneration. Ann. Biomed. Eng. 2011, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Dvir, T.; Kohane, D.S. Delivering Bioactive Molecules as Instructive Cues to Engineered Tissues. Expert. Opin. Drug Deliv. 2012, 9, 473–492. [Google Scholar] [CrossRef] [PubMed]
- DeForest, C.A.; Anseth, K.S. Advances in Bioactive Hydrogels to Probe and Direct Cell Fate. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the Road to Smart Biomaterials for Bone Research: Definitions, Concepts, Advances, and Outlook. Bone Res. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Ruoslahti, E. Fibronectin and its receptors. Annu. Rev. Biochem. 1988, 57, 375–413. [Google Scholar] [CrossRef]
- Mosher, D.F. Physiology of Fibronectin. Annu. Rev. Med. 1984, 35, 561–575. [Google Scholar] [CrossRef]
- Potts, J.R.; Campbell, I.D. Fibronectin Structure and Assembly. Curr. Opin. Cell Biol. 1994, 6, 648–655. [Google Scholar] [CrossRef]
- Tsyguelnaia, I.; Doolittle, R.F. Presence of a Fibronectin Type III Domain in a Plant Protein. J. Mol. Evol. 1998, 46, 612–614. [Google Scholar] [CrossRef]
- Yamada, K.M. Fibronectin Domains and Receptors. In Fibronection; Elsevier: Amsterdam, The Netherlands, 1989; pp. 47–121. ISBN 978-0-12-508470-3. [Google Scholar]
- Kornblihtt, A.R.; Pesce, C.G.; Alonso, C.R.; Cramer, P.; Srebrow, A.; Werbajh, S.; Muro, A.F. The Fibronectin Gene as a Model for Splicing and Transcription Studies. FASEB J. 1996, 10, 248–257. [Google Scholar] [CrossRef]
- Dickinson, C.D.; Veerapandian, B.; Dai, X.-P.; Hamlin, R.C.; Xuong, N.; Ruoslahti, E.; Ely, K.R. Crystal Structure of the Tenth Type III Cell Adhesion Module of Human Fibronectin. J. Mol. Biol. 1994, 236, 1079–1092. [Google Scholar] [CrossRef]
- Williams, M.J.; Phan, I.; Harvey, T.S.; Rostagno, A.; Gold, L.I.; Campbell, I.D. Solution Structure of a Pair of Fibronectin Type 1 Modules with Fibrin Binding Activity. J. Mol. Biol. 1994, 235, 1302–1311. [Google Scholar] [CrossRef]
- Sticht, H.; Pickford, A.R.; Potts, J.R.; Campbell, I.D. Solution Structure of the Glycosylated Second Type 2 Module of Fibronectin. J. Mol. Biol. 1998, 276, 177–187. [Google Scholar] [CrossRef][Green Version]
- Alexander, S.S.; Colonna, G.; Yamada, K.M.; Pastan, I.; Edelhoch, H. Molecular Properties of a Major Cell Surface Protein from Chick Embryo Fibroblasts. J. Biol. Chem. 1978, 253, 5820–5824. [Google Scholar] [CrossRef]
- Alexander, S.S.; Colonna, G.; Edelhoch, H. The Structure and Stability of Human Plasma Cold-Insoluble Globulin. J. Biol. Chem. 1979, 254, 1501–1505. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Chen, A.B.; Huseby, R.M. The Cold-Insoluble Globulin of Human Plasma: Studies of Its Essential Structural Features. Biochim. Biophys. Acta (BBA)-Protein Struct. 1975, 386, 509–524. [Google Scholar] [CrossRef]
- Koteliansky, V.E.; Glukhova, M.A.; Benjamin, M.V.; Smirnov, V.N.; Filimonov, V.V.; Zalite, O.M.; Venyaminov, S.Y. A Study of the Structure of Fibronectin. Eur. J. Biochem. 1981, 119, 619–624. [Google Scholar] [CrossRef]
- Pelta, J.; Berry, H.; Fadda, G.C.; Pauthe, E.; Lairez, D. Statistical Conformation of Human Plasma Fibronectin. Biochemistry 2000, 39, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Baujard-Lamotte, L.; Noinville, S.; Goubard, F.; Marque, P.; Pauthe, E. Kinetics of Conformational Changes of Fibronectin Adsorbed onto Model Surfaces. Colloids Surf. B Biointerfaces 2008, 63, 129–137. [Google Scholar] [CrossRef]
- Kornblihtt, A.R.; Umezawa, K.; Vibe-Pedersen, K.; Baralle, F.E. Primary Structure of Human Fibronectin: Differential Splicing May Generate at Least 10 Polypeptides from a Single Gene. EMBO J 1985, 4, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; García, A.J.; Salmeron-Sanchez, M. Receptor Control in Mesenchymal Stem Cell Engineering. Nat. Rev. Mater. 2018, 3, 1–14. [Google Scholar] [CrossRef]
- Martino, M.M.; Hubbell, J.A. The 12th–14th Type III Repeats of Fibronectin Function as a Highly Promiscuous Growth Factor-Binding Domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Available online: https://www.liebertpub.com/doi/abs/10.1089/ten.2005.11.1 (accessed on 4 October 2021).
- Davis, D.H.; Giannoulis, C.S.; Johnson, R.W.; Desai, T.A. Immobilization of RGD to <1 1 1> Silicon Surfaces for Enhanced Cell Adhesion and Proliferation. Biomaterials 2002, 23, 4019–4027. [Google Scholar] [CrossRef]
- Jin Yoon, J.; Ho Song, S.; Sung Lee, D.; Park, T.G. Immobilization of Cell Adhesive RGD Peptide onto the Surface of Highly Porous Biodegradable Polymer Scaffolds Fabricated by a Gas Foaming/Salt Leaching Method. Biomaterials 2004, 25, 5613–5620. [Google Scholar] [CrossRef] [PubMed]
- García, A.J.; Schwarzbauer, J.E.; Boettiger, D. Distinct Activation States of A5β1 Integrin Show Differential Binding to RGD and Synergy Domains of Fibronectin. Biochemistry 2002, 41, 9063–9069. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ikacia, Y. Corneal Cell Adhesion and Proliferation on Hydrogel Sheets Bound with Cell-Adhesive Proteins. Curr. Eye Res. 1991, 10, 899–908. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ikada, Y.; Moritera, T.; Ogura, Y.; Honda, Y. Collagen-Immobilized Hydrogel as Material for Lamellar Keratoplasty. J. Appl. Biomater. 1991, 2, 261–267. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ikada, Y. Covalent Immobilization of Proteins on to the Surface of Poly(Vinyl Alcohol) Hydrogel. Biomaterials 1991, 12, 747–751. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of Fibronectin to Poly(Vinyl Alcohol) Hydrogels Promotes NIH3T3 Cell Adhesion, Proliferation, and Migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Zajaczkowski, M.B.; Cukierman, E.; Galbraith, C.G.; Yamada, K.M. Cell–Matrix Adhesions on Poly(Vinyl Alcohol) Hydrogels. Tissue Eng. 2003, 9, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Twigg, P.; Egan, A.; Moody, A.; Smith, A.; Eagland, D.; Crowther, N.; Britland, S. Poly(Vinyl Alcohol) Hydrogel as a Biocompatible Viscoelastic Mimetic for Articular Cartilage. Biotechnol. Prog. 2006, 22, 1400–1406. [Google Scholar] [CrossRef]
- Millon, L.E.; Padavan, D.T.; Hamilton, A.M.; Boughner, D.R.; Wan, W. Exploring Cell Compatibility of a Fibronectin-Functionalized Physically Crosslinked Poly(Vinyl Alcohol) Hydrogel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1–10. [Google Scholar] [CrossRef]
- Hynd, M.R.; Frampton, J.P.; Dowell-Mesfin, N.; Turner, J.N.; Shain, W. Directed Cell Growth on Protein-Functionalized Hydrogel Surfaces. J. Neurosci. Methods 2007, 162, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Weiss, J.B.; Lee, J.; Kilian, K.A. Matrix Composition and Mechanics Direct Proangiogenic Signaling from Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 2737–2745. [Google Scholar] [CrossRef]
- Lee, Y.B.; Lee, J.; Byun, H.; Ahmad, T.; Akashi, M.; Matsusaki, M.; Shin, H. One-Step Delivery of a Functional Multi-Layered Cell Sheet Using a Thermally Expandable Hydrogel with Controlled Presentation of Cell Adhesive Proteins. Biofabrication 2018, 10, 025001. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Riedel, J.; Walles, H.; Scherner, M.; Awad, G.; Varghese, S.; Schürlein, S.; Garke, B.; Veluswamy, P.; Wippermann, J.; et al. Comparative Evaluation on Impacts of Fibronectin, Heparin–Chitosan, and Albumin Coating of Bacterial Nanocellulose Small-Diameter Vascular Grafts on Endothelialization In Vitro. Nanomaterials 2021, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Custódio, C.A.; Alves, C.M.; Reis, R.L.; Mano, J.F. Immobilization of Fibronectin in Chitosan Substrates Improves Cell Adhesion and Proliferation. J. Tissue Eng. Regen. Med. 2010, 4, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, T.; An, S.S.A. Improved Fibronectin-Immobilized Fibrinogen Microthreads for the Attachment and Proliferation of Fibroblasts. Int. J. Nanomed. 2013, 8, 1037–1049. [Google Scholar] [CrossRef][Green Version]
- Warner, J.; Soman, P.; Zhu, W.; Tom, M.; Chen, S. Design and 3D Printing of Hydrogel Scaffolds with Fractal Geometries. ACS Biomater. Sci. Eng. 2016, 2, 1763–1770. [Google Scholar] [CrossRef]
- Yamaki, K.; Harada, I.; Goto, M.; Cho, C.-S.; Akaike, T. Regulation of Cellular Morphology Using Temperature-Responsive Hydrogel for Integrin-Mediated Mechanical Force Stimulation. Biomaterials 2009, 30, 1421–1427. [Google Scholar] [CrossRef]
- Schulte, A.; Alhusaini, Q.F.M.; Schönherr, H. Anodic Aluminum Oxide Nanopore Template-Assisted Fabrication of Nanostructured Poly(Vinyl Alcohol) Hydrogels for Cell Studies. ACS Appl. Bio. Mater. 2020, 3, 2419–2427. [Google Scholar] [CrossRef]
- Shinohara, S.; Kihara, T.; Sakai, S.; Matsusaki, M.; Akashi, M.; Taya, M.; Miyake, J. Fabrication of in Vitro Three-Dimensional Multilayered Blood Vessel Model Using Human Endothelial and Smooth Muscle Cells and High-Strength PEG Hydrogel. J. Biosci. Bioeng. 2013, 116, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ferracci, G.; Wang, Y.; Fan, T.F.; Cho, N.-J.; Chow, P.K.H. Porcine Hepatocytes Culture on Biofunctionalized 3D Inverted Colloidal Crystal Scaffolds as an in Vitro Model for Predicting Drug Hepatotoxicity. RSC Adv. 2019, 9, 17995–18007. [Google Scholar] [CrossRef]
- Sharma, R.I.; Snedeker, J.G. Biochemical and Biomechanical Gradients for Directed Bone Marrow Stromal Cell Differentiation toward Tendon and Bone. Biomaterials 2010, 31, 7695–7704. [Google Scholar] [CrossRef] [PubMed]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular Matrix Type Modulates Mechanotransduction of Stem Cells. Acta Biomater. 2019, 96, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhao, R.; Feng, F.; Yang, L. The Effect of Matrix Stiffness on Human Hepatocyte Migration and Function—An In Vitro Research. Polymers 2020, 12, 1903. [Google Scholar] [CrossRef]
- Vallières, K.; Petitclerc, E.; Laroche, G. Covalent Grafting of Fibronectin onto Plasma-Treated PTFE: Influence of the Conjugation Strategy on Fibronectin Biological Activity. Macromol. Biosci. 2007, 7, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Vallières, K.; Chevallier, P.; Sarra-Bournet, C.; Turgeon, S.; Laroche, G. AFM Imaging of Immobilized Fibronectin: Does the Surface Conjugation Scheme Affect the Protein Orientation/Conformation? Langmuir 2007, 23, 9745–9751. [Google Scholar] [CrossRef]
- Ghadhab, S.; Bilem, I.; Guay-Bégin, A.-A.; Chevallier, P.; Auger, F.A.; Ruel, J.; Pauthe, E.; Laroche, G. Fibronectin Grafting to Enhance Skin Sealing around Transcutaneous Titanium Implant. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef]
- Vanslambrouck, S.; Chevallier, P.; Guay-Bégin, A.-A.; Laroche, G. Effect of Linking Arm Hydrophilic/Hydrophobic Nature, Length and End-Group on the Conformation and the RGD Accessibility of Surface-Immobilized Fibronectin. Mater. Sci. Eng. C 2020, 107, 110335. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, C.; Jiang, X.S.; Teoh, S.H.; Leong, K.W. Fibronectin Immobilized by Covalent Conjugation or Physical Adsorption Shows Different Bioactivity on Aminated-PET. Mater. Sci. Eng. C 2007, 27, 213–219. [Google Scholar] [CrossRef]
- Allen, L.T.; Tosetto, M.; Miller, I.S.; O’Connor, D.P.; Penney, S.C.; Lynch, I.; Keenan, A.K.; Pennington, S.R.; Dawson, K.A.; Gallagher, W.M. Surface-Induced Changes in Protein Adsorption and Implications for Cellular Phenotypic Responses to Surface Interaction. Biomaterials 2006, 27, 3096–3108. [Google Scholar] [CrossRef]
- Barrias, C.C.; Martins, M.C.L.; Almeida-Porada, G.; Barbosa, M.A.; Granja, P.L. The Correlation between the Adsorption of Adhesive Proteins and Cell Behaviour on Hydroxyl-Methyl Mixed Self-Assembled Monolayers. Biomaterials 2009, 30, 307–316. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial Organization of Osteoblast Fibronectin Matrix on Titanium Surfaces: Effects of Roughness, Chemical Heterogeneity and Surface Energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.C.; Zink, M.; Weidt, A.; Mayr, S.G.; Markaki, A.E. Effect of Microgrooved Surface Topography on Osteoblast Maturation and Protein Adsorption. J. Biomed. Mater. Res. A 2015, 103, 2689–2700. [Google Scholar] [CrossRef] [PubMed]
- González-García, C.; Sousa, S.R.; Moratal, D.; Rico, P.; Salmerón-Sánchez, M. Effect of Nanoscale Topography on Fibronectin Adsorption, Focal Adhesion Size and Matrix Organisation. Colloids Surf. B Biointerfaces 2010, 77, 181–190. [Google Scholar] [CrossRef]
- Grinnell, F.; Feld, M.K. Fibronectin Adsorption on Hydrophilic and Hydrophobic Surfaces Detected by Antibody Binding and Analyzed during Cell Adhesion in Serum-Containing Medium. J. Biol. Chem. 1982, 257, 4888–4893. [Google Scholar] [CrossRef]
- Toworfe, G.K.; Composto, R.J.; Adams, C.S.; Shapiro, I.M.; Ducheyne, P. Fibronectin Adsorption on Surface-Activated Poly(Dimethylsiloxane) and Its Effect on Cellular Function. J. Biomed. Mater. Res. A 2004, 71, 449–461. [Google Scholar] [CrossRef]
- Bergkvist, M.; Carlsson, J.; Oscarsson, S. Surface-Dependent Conformations of Human Plasma Fibronectin Adsorbed to Silica, Mica, and Hydrophobic Surfaces, Studied with Use of Atomic Force Microscopy. J. Biomed. Mater. Res. Part A 2003, 64A, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface Chemistry Modulates Fibronectin Conformation and Directs Integrin Binding and Specificity to Control Cell Adhesion. J. Biomed. Mater. Res. Part A 2003, 66A, 247–259. [Google Scholar] [CrossRef]
- Lin, M.; Wang, H.; Ruan, C.; Xing, J.; Wang, J.; Li, Y.; Wang, Y.; Luo, Y. Adsorption Force of Fibronectin on Various Surface Chemistries and Its Vital Role in Osteoblast Adhesion. Biomacromolecules 2015, 16, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Rico, P.; Hernández, J.C.R.; Moratal, D.; Altankov, G.; Pradas, M.M.; Salmerón-Sánchez, M. Substrate-Induced Assembly of Fibronectin into Networks: Influence of Surface Chemistry and Effect on Osteoblast Adhesion. Tissue Eng. Part A 2009, 15, 3271–3281. [Google Scholar] [CrossRef]
- Salmerón-Sánchez, M.; Rico, P.; Moratal, D.; Lee, T.T.; Schwarzbauer, J.E.; García, A.J. Role of Material-Driven Fibronectin Fibrillogenesis in Cell Differentiation. Biomaterials 2011, 32, 2099–2105. [Google Scholar] [CrossRef]
- Llopis-Hernández, V.; Cantini, M.; González-García, C.; Cheng, Z.A.; Yang, J.; Tsimbouri, P.M.; García, A.J.; Dalby, M.J.; Salmerón-Sánchez, M. Material-Driven Fibronectin Assembly for High-Efficiency Presentation of Growth Factors. Sci. Adv. 2016, 2, e1600188. [Google Scholar] [CrossRef] [PubMed]
- Moulisová, V.; Gonzalez-García, C.; Cantini, M.; Rodrigo-Navarro, A.; Weaver, J.; Costell, M.; Sabater I Serra, R.; Dalby, M.J.; García, A.J.; Salmerón-Sánchez, M. Engineered Microenvironments for Synergistic VEGF—Integrin Signalling during Vascularization. Biomaterials 2017, 126, 61–74. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Shen, M.; Garcia, I.; Maier, R.V.; Horbett, T.A. Effects of Adsorbed Proteins and Surface Chemistry on Foreign Body Giant Cell Formation, Tumor Necrosis Factor Alpha Release and Procoagulant Activity of Monocytes. J. Biomed. Mater. Res. Part A 2004, 70A, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Gossart, A.; Gand, A.; Ollivier, V.; Boissière, M.; Santerre, J.P.; Letourneur, D.; Pauthe, E. Coating of Cobalt Chrome Substrates with Thin Films of Polar/Hydrophobic/Ionic Polyurethanes: Characterization and Interaction with Human Immunoglobulin G and Fibronectin. Colloids Surf. B Biointerfaces 2019, 179, 114–120. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Galli, C.; Bianchera, A.; Lagonegro, P.; Elviri, L.; Smerieri, A.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G.M. Anti-Fibronectin Aptamers Improve the Colonization of Chitosan Films Modified with D-(+) Raffinose by Murine Osteoblastic Cells. J. Mater. Sci. Mater. Med. 2017, 28, 136. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Bianchi, M.G.; Bergonzi, C.; Bianchera, A.; Bettini, R.; Elviri, L.; Macaluso, G.M. Functional Fibronectin Adsorption on Aptamer-Doped Chitosan Modulates Cell Morphology by Integrin-Mediated Pathway. Materials 2019, 12, 812. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Machado, V.; Chevallier, P.; Mantovani, D.; Pauthe, E. On the Potential for Fibronectin/Phosphorylcholine Coatings on PTFE Substrates to Jointly Modulate Endothelial Cell Adhesion and Hemocompatibility Properties. Biomatter 2015, 5, e979679. [Google Scholar] [CrossRef]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The Effect of Coimmobilizing Heparin and Fibronectin on Titanium on Hemocompatibility and Endothelialization. Biomaterials 2011, 32, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.R.; Lamghari, M.; Sampaio, P.; Moradas-Ferreira, P.; Barbosa, M.A. Osteoblast Adhesion and Morphology on TiO2 Depends on the Competitive Preadsorption of Albumin and Fibronectin. J. Biomed. Mater. Res. Part A 2008, 84A, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, M.; Albutt, D.; Alexander, M.R.; Russell, N.A. The Role of Albumin and Fibronectin in the Adhesion of Fibroblasts to Plasma Polymer Surfaces. Plasma Process. Polym. 2012, 9, 149–156. [Google Scholar] [CrossRef]
- Hindié, M.; Camand, E.; Agniel, R.; Carreiras, F.; Pauthe, E.; Van Tassel, P. Effects of Human Fibronectin and Human Serum Albumin Sequential Adsorption on Preosteoblastic Cell Adhesion. Biointerphases 2014, 9, 029008. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2012, 24, 854–869. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: III. Consecutively Alternating Adsorption of Anionic and Cationic Polyelectrolytes on Charged Surfaces. Thin Solid Film. 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Wu, C.; Aslan, S.; Gand, A.; Wolenski, J.S.; Pauthe, E.; Van Tassel, P.R. Porous Nanofilm Biomaterials Via Templated Layer-by-Layer Assembly. Adv. Funct. Mater. 2013, 23, 66–74. [Google Scholar] [CrossRef]
- Gand, A.; Hindié, M.; Chacon, D.; Van Tassel, P.R.; Pauthe, E. Nanotemplated Polyelectrolyte Films as Porous Biomolecular Delivery Systems. Application to the Growth Factor BMP-2. Biomatter 2014, 4, e28823. [Google Scholar] [CrossRef]
- Guan, B.; Wang, H.; Xu, R.; Zheng, G.; Yang, J.; Liu, Z.; Cao, M.; Wu, M.; Song, J.; Li, N.; et al. Establishing Antibacterial Multilayer Films on the Surface of Direct Metal Laser Sintered Titanium Primed with Phase-Transited Lysozyme. Sci. Rep. 2016, 6, 36408. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef]
- Hartmann, H.; Krastev, R. Biofunctionalization of Surfaces Using Polyelectrolyte Multilayers. BioNanoMaterials 2017, 18. [Google Scholar] [CrossRef]
- Ouni, O.A.; Subbiahdoss, G.; Scheberl, A.; Reimhult, E. DNA Polyelectrolyte Multilayer Coatings Are Antifouling and Promote Mammalian Cell Adhesion. Materials 2021, 14, 4596. [Google Scholar] [CrossRef]
- Wittmer, C.R.; Phelps, J.A.; Saltzman, W.M.; Van Tassel, P.R. Fibronectin Terminated Multilayer Films: Protein Adsorption and Cell Attachment Studies. Biomaterials 2007, 28, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Ngankam, A.P.; Mao, G.; Van Tassel, P.R. Fibronectin Adsorption onto Polyelectrolyte Multilayer Films. Langmuir 2004, 20, 3362–3370. [Google Scholar] [CrossRef]
- Kreke, M.R.; Badami, A.S.; Brady, J.B.; Akers, R.M.; Goldstein, A.S. Modulation of Protein Adsorption and Cell Adhesion by Poly(Allylamine Hydrochloride) Heparin Films. Biomaterials 2005, 26, 2975–2981. [Google Scholar] [CrossRef]
- Li, M.; Mills, D.K.; Cui, T.; McShane, M.J. Cellular Response to Gelatin- and Fibronectin-Coated Multilayer Polyelectrolyte Nanofilms. IEEE Trans. NanoBioscience 2005, 4, 170–179. [Google Scholar] [CrossRef]
- Reyes, D.R.; Hong, J.S.; Elliott, J.T.; Gaitan, M. Hybrid Cell Adhesive Material for Instant Dielectrophoretic Cell Trapping and Long-Term Cell Function Assessment. Available online: https://pubs.acs.org/doi/pdf/10.1021/la200762j (accessed on 20 October 2021).
- Bhadriraju, K.; Hong, J.S.; Lund, S.P.; Reyes, D.R. Fibronectin in Layer-by-Layer Assembled Films Switches Tumor Cells between 2D and 3D Morphology. Available online: https://pubs.acs.org/doi/pdf/10.1021/acsbiomaterials.7b00608 (accessed on 18 October 2021).
- Gand, A.; Tabuteau, M.; Chat, C.; Ladam, G.; Atmani, H.; Van Tassel, P.R.; Pauthe, E. Fibronectin-Based Multilayer Thin Films. Colloids Surf B Biointerfaces 2017, 156, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mauquoy, S.; Dupont-Gillain, C. Combination of Collagen and Fibronectin to Design Biomimetic Interfaces: Do These Proteins Form Layer-by-Layer Assemblies? Colloids Surf. B Biointerfaces 2016, 147, 54–64. [Google Scholar] [CrossRef]
- Kadowaki, K.; Matsusaki, M.; Akashi, M. Control of Cell Surface and Functions by Layer-by-Layer Nanofilms. Langmuir 2010, 26, 5670–5678. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Matsusaki, M.; Akashi, M. Effectiveness of Nanometer-Sized Extracellular Matrix Layer-by-Layer Assembled Films for a Cell Membrane Coating Protecting Cells from Physical Stress. Available online: https://pubs.acs.org/doi/pdf/10.1021/la303459v (accessed on 19 October 2021).
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as Artificial Matrices for Cell Seeding in Microfluidic Devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Ahearne, M. Introduction to Cell–Hydrogel Mechanosensing. Interface Focus 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, S.; Vega, S.L.; Song, K.H.; Félix, A.S.; Dalby, M.J.; Burdick, J.A.; Salmeron-Sanchez, M. Engineered Full-Length Fibronectin–Hyaluronic Acid Hydrogels for Stem Cell Engineering. Adv. Healthc. Mater. 2020, 9, 2000989. [Google Scholar] [CrossRef]
- Jun, I.; Ahmad, T.; Bak, S.; Lee, J.-Y.; Kim, E.M.; Lee, J.; Lee, Y.B.; Jeong, H.; Jeon, H.; Shin, H. Spatially Assembled Bilayer Cell Sheets of Stem Cells and Endothelial Cells Using Thermosensitive Hydrogels for Therapeutic Angiogenesis. Adv. Healthc. Mater. 2017, 6, 1601340. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Drinnan, C.T.; Petersen, R.R.; Shear, J.B.; Suggs, L.J.; Schmidt, C.E. Fibronectin–Hyaluronic Acid Composite Hydrogels for Three-Dimensional Endothelial Cell Culture. Acta Biomater. 2011, 7, 2401–2409. [Google Scholar] [CrossRef]
- Gilmozzi, V.; Gentile, G.; Riekschnitz, D.A.; Von Troyer, M.; Lavdas, A.A.; Kerschbamer, E.; Weichenberger, C.X.; Rosato-Siri, M.D.; Casarosa, S.; Conti, L.; et al. Generation of HiPSC-Derived Functional Dopaminergic Neurons in Alginate-Based 3D Culture. Front. Cell Dev. Biol. 2021, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Karoubi, G.; Ormiston, M.L.; Stewart, D.J.; Courtman, D.W. Single-Cell Hydrogel Encapsulation for Enhanced Survival of Human Marrow Stromal Cells. Biomaterials 2009, 30, 5445–5455. [Google Scholar] [CrossRef] [PubMed]
- Mosahebi, A.; Wiberg, M.; Terenghi, G. Addition of Fibronectin to Alginate Matrix Improves Peripheral Nerve Regeneration in Tissue-Engineered Conduits. Tissue Eng. 2003, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O.; Novikov, L.N. Alginate Hydrogel and Matrigel as Potential Cell Carriers for Neurotransplantation. J. Biomed. Mater. Res. Part A 2006, 77A, 242–252. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, R.; Chen, T.; Zhang, K.; Gui, J. 3D Bioprinting Modified Autologous Matrix-Induced Chondrogenesis(AMIC) Technique for Repair of Cartilage Defects. Mater. Des. 2021, 203, 109621. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Hernández, J.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Schwann Cells and Mesenchymal Stem Cells in Laminin- or Fibronectin-Aligned Matrices and Regeneration across a Critical Size Defect of 15 Mm in the Rat Sciatic Nerve. J. Neurosurg. Spine 2017, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Norris, E.G.; Dalecki, D.; Hocking, D.C. Acoustic Fabrication of Collagen–Fibronectin Composite Gels Accelerates Microtissue Formation. Appl. Sci. 2020, 10, 2907. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Anselmi, C.; Hebling, J.; Costa, C.A. de S. Fibronectin-Loaded Collagen/Gelatin Hydrogel Is a Potent Signaling Biomaterial for Dental Pulp Regeneration. J. Endod. 2021, 47, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Nii, M.; Lai, J.H.; Keeney, M.; Han, L.-H.; Behn, A.; Imanbayev, G.; Yang, F. The Effects of Interactive Mechanical and Biochemical Niche Signaling on Osteogenic Differentiation of Adipose-Derived Stem Cells Using Combinatorial Hydrogels. Acta Biomater. 2013, 9, 5475–5483. [Google Scholar] [CrossRef] [PubMed]
- Goldshmid, R.; Seliktar, D. Hydrogel Modulus Affects Proliferation Rate and Pluripotency of Human Mesenchymal Stem Cells Grown in Three-Dimensional Culture. ACS Biomater. Sci. Eng. 2017, 3, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Ingenito, E.P.; Sen, E.; Tsai, L.W.; Murthy, S.; Hoffman, A. Design and Testing of Biological Scaffolds for Delivering Reparative Cells to Target Sites in the Lung. J. Tissue Eng. Regen. Med. 2010, 4, 259–272. [Google Scholar] [CrossRef]
- Ao, Q.; Wang, S.; He, Q.; Ten, H.; Oyama, K.; Ito, A.; He, J.; Javed, R.; Wang, A.; Matsuno, A. Fibrin Glue/Fibronectin/Heparin-Based Delivery System of BMP2 Induces Osteogenesis in MC3T3-E1 Cells and Bone Formation in Rat Calvarial Critical-Sized Defects. ACS Appl. Mater. Interfaces 2020, 12, 13400–13410. [Google Scholar] [CrossRef]
- Nie, T.; Akins, R.E.; Kiick, K.L. Production of Heparin-Containing Hydrogels for Modulating Cell Responses. Acta Biomater. 2009, 5, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Lee, Y.B.; Choi, Y.S.; Engler, A.J.; Park, H.; Shin, H. Transfer Stamping of Human Mesenchymal Stem Cell Patches Using Thermally Expandable Hydrogels with Tunable Cell-Adhesive Properties. Biomaterials 2015, 54, 44–54. [Google Scholar] [CrossRef]
- Walia, R.; Akhavan, B.; Kosobrodova, E.; Kondyurin, A.; Oveissi, F.; Naficy, S.; Yeo, G.C.; Hawker, M.; Kaplan, D.L.; Dehghani, F.; et al. Hydrogel−Solid Hybrid Materials for Biomedical Applications Enabled by Surface-Embedded Radicals. Adv. Funct. Mater. 2020, 30, 2004599. [Google Scholar] [CrossRef]
- Okada, M.; Blombäck, B.; Chang, M.D.; Horowitz, B. Fibronectin and Fibrin Gel Structure. J. Biol. Chem. 1985, 260, 1811–1820. [Google Scholar] [CrossRef]
- Robinson, K.G.; Nie, T.; Baldwin, A.D.; Yang, E.C.; Kiick, K.L.; Akins, R.E. Differential Effects of Substrate Modulus on Human Vascular Endothelial, Smooth Muscle, and Fibroblastic Cells. J. Biomed. Mater. Res. Part A 2012, 100A, 1356–1367. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ho, K.-N.; Feng, S.-W.; Huang, H.-M.; Chang, C.-H.; Lin, C.-T.; Teng, N.-C.; Pan, Y.H.; Chang, W.-J. Fibronectin-Grafted Titanium Dental Implants: An In Vivo Study. BioMed Res. Int. 2016, 2016, e2414809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, R.; González-García, C.; Torstrick, B.; Guldberg, R.E.; Salmerón-Sánchez, M.; García, A.J. Simple Coating with Fibronectin Fragment Enhances Stainless Steel Screw Osseointegration in Healthy and Osteoporotic Rats. Biomaterials 2015, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive Coatings for Orthopaedic Implants—Recent Trends in Development of Implant Coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Assmann, A.; Struß, M.; Schiffer, F.; Heidelberg, F.; Munakata, H.; Timchenko, E.V.; Timchenko, P.E.; Kaufmann, T.; Huynh, K.; Sugimura, Y.; et al. Improvement of the in Vivo Cellular Repopulation of Decellularized Cardiovascular Tissues by a Detergent-Free, Non-Proteolytic, Actin-Disassembling Regimen. J. Tissue Eng. Regen. Med. 2017, 11, 3530–3543. [Google Scholar] [CrossRef]
| Surface Activation/Fn Grafting | Substrate | Biological Activity | References |
|---|---|---|---|
| Modification with isocyanates | Polyvinyl alcohol (PVA) | Increasing cell adhesion and proliferation in rabbit corneal epithelial cells | [27,28,29] |
| Carbonyl diimidazole activation | PVA | Enhancing cell adhesion and proliferation in murine (NIH3T3) and human fibroblasts, murine chondrocytes, and porcine radial arterial and endothelial cells | [30,31,32,33] |
| Avidin–biotin | Polyethylene glycol diacrylate (PEGDA)/polyacrylamide (PA) | Improving cell adhesion and supporting long-term survival of rat astroglioma cells | [34] |
| Hydrazine hydrate activation | PA | Leading to higher cell adhesion of human marrow stromal cells (hMSCs); enhancing the secretion of proangiogenic factors | [35] |
| Polydopamine coating | Poloxamine | Enhancing cell adhesion and the proliferation of human dermal fibroblasts | [36] |
| Modification with cyanate ester | Nanocellulose | Increasing cell adhesion and proliferation in static and dynamic culture conditions of human saphenous vein endothelial and endothelial progenitor cells | [37] |
| Carbodiimide cross-linker | Chitosan | Increasing cell adhesion and proliferation in osteoblasts, murine myoblasts (C2C12), hMSCs, NIH3T3, and pancreatic tumor cells | [38] |
| Fibrinogen | [39] | ||
| PEGDA/PA | [40] | ||
| Poly(N-isopropylacrylamide) | [41] | ||
| PVA | [42] | ||
| Polymer modification PEG–NHS | PEG | Improving cell adhesion and proliferation in human aortic smooth muscle cells and human umbilical vein endothelial cells (HUVECs) | [43] |
| PEGDA | Increasing cell proliferation and metabolic activity in porcine hepatocyte cells; enhancing albumin secretion | [44] | |
| Modification with Sulfo-SANPAH | PA | Enhancing osteoblast differentiation in bone marrow stromal cells; influencing translocation of yes-associated protein (YAS) in hMSCs. | [45] |
| [46] | |||
| PVA | Enhancing optimal migratory behavior in human hepatocytes | [47] | |
| GA cross-linking | Plasma-treated silica and polytetrafluoroethylene (PTFE) | Increasing CBD accessibility and bovine aortic endothelial cell adhesion | [48,49] |
| Modification with phosphonate | Titanium | Increasing dermal fibroblast adhesion, spreading, and proliferation; enhancing the strength of adhesion to bioengineered dermal tissue | [50] |
| Type of Binding | Mechanism of Fn Addition | Polymer | Biological Activity | References |
|---|---|---|---|---|
| Physical entrapment | Dispersed into a hydrogel | Alginate | Improving cell proliferation and metabolic activity in rat Schwann cells and olfactory ensheathing cells; enhancing nerve reparation in vivo | [104,105] |
| Increasing cell viability for the long-term culture of human induced pluripotent stem cells (hiPSCs) and cell differentiation | [102] | |||
| Alginate/gelatin/hyaluronic acid | Enhancing the proliferation, migration, and chondrogenic differentiation of rat chondrogenic progenitor cells | [106] | ||
| Collagen | Improving nerve regeneration in adult rats | [107] | ||
| Co-localizing and reorganizing Fn in fibrils after the seeding of fibronectin-null mouse embryonic fibroblasts | [108] | |||
| Collagen/gelatin | Improving the adhesion, spreading, and viability and migration of human apical papilla cells; gene expression of α5 integrin, αV integrin, and type I and type III collagens | [109] | ||
| Agarose | Enhancing cell survival, adhesion, and the metabolic activity of hMSCs in complex with fibrinogen | [103] | ||
| PEGDA/collagen | Enhancing the differentiation of human adipose-derived stem cells (hADSCs) and cell proliferation and pluripotency of hMSCs | [110] | ||
| PEGDA/fibrinogen | [111] | |||
| Covalent cross-linking | Cross-linking with XIII factor | Fibrin | Increasing cell adhesion and spreading in sheep lung mesenchymal cells and tissue regeneration in vivo | [112] |
| Sustaining the release of bone morphogenic protein (BMP2) and the expression of osteogenic markers in preosteoblast cells; improving tissue regeneration in vivo | [113] | |||
| Photo cross-linking | Hyaluronic acid | Increasing cell viability and tubular organization of HUVECs | [101] | |
| Improving the cell adhesion and proliferation of MSCs; expressing YAS protein in encapsulated cells | [99] | |||
| Maleimide reaction | PEG | Increasing cell adhesion and proliferation in human cardiovascular cell types | [114] | |
| Enzymatic cross-linking | Poloxamine | Enhancing the cell adhesion and proliferation of hMSCs and HUVECs | [100,115] | |
| Cross-linking by radicals | Plasma immersion PA | Improving the cell adhesion and proliferation of human dermal fibroblast | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomino-Durand, C.; Pauthe, E.; Gand, A. Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review. Appl. Sci. 2021, 11, 12111. https://doi.org/10.3390/app112412111
Palomino-Durand C, Pauthe E, Gand A. Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review. Applied Sciences. 2021; 11(24):12111. https://doi.org/10.3390/app112412111
Chicago/Turabian StylePalomino-Durand, Carla, Emmanuel Pauthe, and Adeline Gand. 2021. "Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review" Applied Sciences 11, no. 24: 12111. https://doi.org/10.3390/app112412111
APA StylePalomino-Durand, C., Pauthe, E., & Gand, A. (2021). Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review. Applied Sciences, 11(24), 12111. https://doi.org/10.3390/app112412111






