Featured Application
Heterogeneous electro-Fenton-like processes based on catalyst and electrocatalyst using transition metals for 2-phenylphenol degradation.
Abstract
The hunt for efficient and environmentally friendly degradation processes has positioned the heterogeneous advanced oxidation processes as an alternative more interesting and economical rather than homogenous processes. Hence, the current study lies in investigating the efficiency of different heterogeneous catalysts using transition metals in order to prevent the generation of iron sludge and to extend the catalogue of possible catalysts to be used in advanced oxidation processes. In this study, nickel and zinc were tested and the ability for radical-generation degradation capacity of both ions as homogeneous was evaluated in the electro-Fenton-like degradation of 2-phenylphenol. In both cases, the degradation profiles followed a first-order kinetic model with the highest degradation rate for nickel (1 mM) with 2-phenylphenol removal level of 90.12% and a total organic reduction near 70% in 2 h. To synthesise the heterogeneous nickel catalyst, this transition metal was fixed on perlite by hydrothermal treatment and in a biochar or carbon nanofibers by adsorption. From the removal results using the three synthesized catalysts, it is concluded that the best catalysts were obtained by inclusion of nickel on biochar or nanofibers achieving in both with removal around 80% before 1 h. Thus, to synthetize a nickel electrocatalyst, nickel doped nanofibers were included on carbon felt. To do this, the amount of carbon black, nickel nanofibers and polytetrafluoroethylene to add on the carbon felt was optimized by Taguchi design. The obtained results revealed that under the optimised conditions, a near-complete removal was achieved after 2 h with high stability of the nickel electrocatalyst that open the applicability of this heterogeneous system to operate in flow systems.
1. Introduction
Nowadays, the need for various organic compounds to facilitate people’s daily lives, such as pesticides, pharmaceuticals, cosmetics, etc., is a reality. Among these organic compounds, pesticides stand out because their use is essential to ensure the production and quality of foodstuffs demanded by the population [1,2,3].
In this study, the fungicide 2-phenylphenol, also called ortho-phenylphenol, has been chosen, which belongs to the hydroxybiphenyl chemical family [4]. The use of the fungicide 2-phenylphenol is mainly as disinfection, but it is also bactericide and virucide. As an example, in hospitals, shops and industries, it is generally used to disinfect surfaces and equipment, and in agriculture, it could be used to disinfect fruits, especially citrus fruits and pears, vegetables and eggs [5,6,7].
However, having all these advantages, the main problem is its appearance and persistence on the surface, such as food paper packages and canned soft drinks and aquatic environmental compartments [5]. In fact, since the early 2000s, it has been tracked down at low levels, in several environments: marine sediments and urban wastewaters [8], in sewage sludge (63–172 μg/kg), surface water (47 ng/l) and river sediments (2–69 μg/kg) from diverse streams and rivers in the state Baden-Württemberg (Germany) [9] or Bouregreg River (Morocco) at a concentration range of 54–257 ng/L [10].
According to the revision made, in 2003, by the World Health Organization (WHO), the permissible level in drinking-water is 0.1 mg/L, at which toxic effects can be expected, was calculated from the acceptable daily intake (ADI) for humans of 0.4 mg/kg of body weight and this data has not been updated [11]. Recently, interesting research evaluated the effects of pesticides on humans, specifically on the younger population [12]. In this study, 703 infants were selected, who are under 1-year-old, and young children, being between 1 and 3 years old. It was noted that in more than 5% of them, pesticides could be detected, and the most common pesticide detected was 2-phenylphenol (18%).
Several studies have reported its acute and chronic toxicity to creatures [5,11,13,14]. Indeed, the acute toxicity (LD50/LC50) in rats and mice have been determined in a range of concentration of 2880–3600 and 3152–3499 mg/kg body weight, respectively [13]. In addition, other studies determined that, when rats were fed with 2-phenylphenol level of 0.5–4% in their diet, they suffer urinary bladder carcinoma [5].
These reported drawbacks encourage progress in emerging technologies to reduce this kind of pollutants from aquatic environmental compartments. These approaches include advanced oxidation processes stand out since they are green processes and very effective in the reduction of organic pollutants [15,16,17,18]. In addition, these types of processes can be performed in homogeneous and heterogeneous systems [19,20].
Within each of these classes, multiple types can be also found, making advanced oxidation processes very versatile. Among them, electrochemical processes based on the use of electrical energy for the generation of radicals, such as hydroxyl, sulphate, etc., which have a high oxidising capacity and make it possible to break the bonds that form the molecules of these organic compounds [15,21]. Amongst, the electro-Fenton process represents an encouraging technology able to generate in situ hydrogen peroxide via cathodic reduction of oxygen (Equation (1)) and the generation of hydroxyl radical by Fenton reaction between the ferrous ion and hydrogen peroxide (Equation (2)) and the regeneration of ferric ion to ferrous ion on cathode (Equation (3)) to maintain the catalytic cycle of Fenton’s reaction [15,21].
O2 (g) + 2H+ + 2e− → H2O2
Fe2+ + H2O2 → •OH + OH− + Fe3+
Fe3+ + e− → Fe2+
More recent approaches for the use of different transition metals as Co, Mn, Cu, … in several advanced oxidation processes for the removal of organic pollutants have been reported [22,23,24,25]. A clear example of the efficiency of these uses can be found in spent Lithium-ion batteries that contain Li, Ni, Mn, Al, and Co, that after a simple washing−recycling process was employed as an easily recoverable catalyst for peroxymonosulfate (PMS) activation [26,27] with levels removal of more than 96% in more than 5 cycles of use [27].
These ions could be used instead of iron for the hydrogen peroxide decomposition and the reaction that takes place is similar to the classical Fenton process (Equation (4)) [28]. In addition, in the electrochemical process, the Me(n+1)+ could be regenerated to Men+ on the cathode, avoiding the generation of sludge and increasing the efficiency of this process [28,29].
Men+ + H2O2 + H+ → Me(n+1)+ + •OH + H2O
In recent years, the evaluation of various heterogeneous catalysts with application to electro-Fenton technology have been increasing number of studies. This increase is because it allows solving several drawbacks of the conventional process. In which is remarkable the requirements for catalyst amount optimization as well as the expansion of the working pH range or the operation in a flow system [30,31]. In this sense, iron and/or transition metals have been used to modify or functionalised cathode materials in the heterogeneous electro-Fenton process. This electrocatalytic material presents a dual benefit because the modified material could be used as a cathode and as a catalyst during the electrochemical treatment process [32,33,34].
To this day, studies about 2-phenylphenol degradation by advanced oxidation processes are limited in the literature, in which no one has evaluated the efficiency of electro-Fenton processes. Several of them were focused on the elimination of several compounds of the phenylphenol family by ultraviolet light (UV) and peroxide as an oxidising agent, achieving a level of 89%, while in the other isomers the levels are in a range of 71–87% [35]. TiO2 and B-doped TiO2 catalysts were synthesised and several pesticides (2-methyl-4-chlorophenoxyacetic acid, diuron, ter-buthylazine and o-phenylphenol) were eliminated by ozonation and photocatalytic processes alone or in combination under simulated solar irradiation. Having been determined combination of processes led to faster mineralization of the pollutants mixture keeping around 75% after to perform three successive cycles [36]. Recently, selective degradation of o-phenylphenol was obtained by applying N-doped TiO2 photocatalysts under simulated solar light irradiation showing a degradation efficiency of around 46% [24].
In this context, this work aimed to assess the degradation of 2-phenylphenol by electro-Fenton-like process, using as a catalyst transition metal ion (nickel and zinc). Based on the preliminary results, the synthesis of new catalysts and electrocatalyst (used as the cathode) is carried out to open the possibility of using this degradation process in a heterogeneous mode. According to our knowledge, this research is one of the first endeavours where nickel and zinc are used as heterogeneous catalysts and electrocatalysts in Fenton processes. In this situation, the present report intends to establish the use of these transition metals as an alternative to the conventional advanced oxidation processes for 2-phenylphenol removal, using different heterogeneous designs (catalysts and electrocatalyst).
2. Materials and Methods
2.1. Pollutants
Synthetic polluted effluents were obtained using ultrapure-water at desired concentrations of 2-phenylphenol (Sigma-Aldrich, Barcelona, Spain). Nickel (II) chloride hexahydrate, zinc (II) sulfate heptahydrate, anhydrous sodium sulphate, expanded perlite beads (grade 5), carbon black, polytetrafluoroethylene (PTFE) and carbon nanofiber were purchased from Sigma-Aldrich. Carbon-felt RVG 2000 and boron-doped diamond (BDD) were supplied by Carbon-Lorraine and Condias, respectively.
2.2. Electrochemical Processes
Experiments were performed in a 250 mL electrochemical undivided cylindrical cell (Figure 1) with a solution volume of 150 mL of 2-phenylphenol solution 25 mg/L with 10 mM Na2SO4 as a supporting electrolyte. The initial solution pH was 3 by acidification with 0.1 M H2SO4. BDD (3.5 × 2.0 × 0.2 cm) as anode BDD and different cathodes (described in the following section) were connected to a direct current power supply (HP model 3662) at different current intensities. To in situ electrogeneration of hydrogen peroxide, continuous compressed air was bubbled (0.5 L/min) on the cathode surface and the solution was stirred to prevent concentration gradients into the bulk solution. Electric parameters were recorded with a digital multimeter (BRYMEN BM-257). Anodic oxidation was performed using electrodes BDD and carbon felt without airflow to limit the hydrogen peroxide generation. In the homogeneous electro-Fenton-like process nickel and zinc was added in a range of 1–5 mM. In a heterogeneous process, several catalysts were synthesised as is described in the next section.
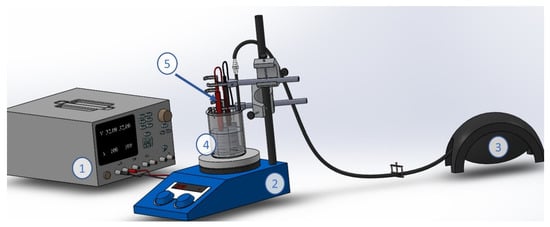
Figure 1.
Experimental device for anodic oxidation and electro-Fenton-like process. (1) current power supply, (2) magnetic plate, (3) air pump, (4) cylindrical electrochemical cell and (5) electrodes.
2.3. Heterogeneous Catalyst
Several transition metal catalysts were synthesised using different materials and techniques. Thus, nickel perlite was obtained by inclusion of 20 g of perlite in a 200 mL of nickel solution in a Teflon autoclave reactor, which was kept in a muffle (Thermolyne F3040CM-33 Fisher Scientific, Madrid, Spain) at 200 °C during 1 h for its hydrothermal treatment and finally was cooled at room temperature, filtered, cleaned with ultrapure-water and dried at 105 °C for 12 h. Nickel nanofibers were manufactured by immersion of carbon nanofibers in a nickel solution at 70 °C and NaOH (5 M) was added dropwise to generate the nanoparticles. Rice bran biochar was produced by thermal decomposition of grinded and sieved rice bran material (size lower than 0.250 mm) at 320 °C for 30 min under an inert nitrogen atmosphere by heating 10 °C/min [37]. Then, it was added in nickel solution for 12 h. In both cases, after immersion in the solution, the material was cleaned with ultrapure-water and dried at 60 °C for 24 h.
2.4. Cathodes
Different cathodes were used: (i) an unmodified carbon felt cute in rectangular size 5 × 2.5 cm and (ii) functionalised cathodes. In the last, previously the carbon felt was cleaned with acetone and, thereafter, with ultrapure-water using an ultrasonic bath for 1 h (Fisherbrand FB11203, Fisher Scientific, Madrid, Spain) and dried at 60 °C for 24 h. After that, nickel nanofibers were included by immersion of carbon felt in a dispersed solution that contained carbon black, carbon nanofibers and PTFE in a solution 1:2.5 ethanol:ultrapure-water. In this study, the proportion of each component was optimized by the Taguchi design experiment (see Section “Taguchi Design Experiment”). Thereafter, following the procedure described in the literature [38,39], the material was sonicated for 30 min and dried at 80 °C for 12 h, and as a final point annealed at 360 °C for 30 min.
Taguchi Design Experiment
This method is based on the orthogonal array experimental design proposed by Taguchi and allows the study of the effect of different parameters employing a minimum number of runs. It is a simple and effective way for the identification and optimisation of the parameter conditions on the selected response thought the use of ANOVA as analysis tool.
In the present study, the Taguchi experiment was used for the selection of significant components and their optimisation in the electrocatalyst composition (A: carbon black, B: nickel loaded nanofibers and C: PFTE content) applied for the degradation of the selected pollutant. The parameters affecting the development of the process and their values have been identified in the Table 1.

Table 1.
Screening experiment parameters and level.
The performance of the method outputs is optimized in relation to the response’s signal/noise ratio (S/N) which decreases the variability of the process. In this method, S/N represents the measurement of the deviation of the response from the desired value. A lower variability in the process is ensured through maximizing the S/N ratio, and in this study, the larger-the-better category (Equation (5)) was selected.
where yi is the response and n is the number of experiments.
S/N = −10 · log (1/n ∑1/y2i)
2.5. Sample Preparation and Analytical Methods
Periodically, samples were taken from the electrochemical cell and centrifuged at 10,000 rpm for 10 min. The liquid was used to determine the pH, 2-phenylphenol, hydrogen peroxide, total organic carbon (TOC) and transition metals concentration.
2.5.1. 2-Phenylphenol Concentration
2-phenylphenol degradation was determined by HPLC and spectrophotometrically. For HPLC determination, 2-phenylphenol concentration decrease was measured with an Agilent 1100 HPLC equipped with Diode Array Detector and using a Zorbax Eclipse XD8-C8 reverse-phase column (150 × 4.6 mm i.d. 5 µm). Mobile phase composed of water:acetonitrile (40:60) was pumped in isocratic mode at 1 mL/min for 20 min. Separation was carried out at room temperature and the column effluent was monitored spectrophotometrically at 252 nm wavelength. Prior to chromatographic analysis, all samples were filtered through 0.45 µm PVDF filters. Spectrophotometric measurements were performed with a GENESYS 150 UV-Vis Spectrophotometer from 240 to 350 nm, and the pollutant removal was associated with the area under curve decrease and expressed in terms of percentage in relation to the initial sample.
2.5.2. Hydrogen Peroxide Concentration
It was determined according to the Titanium oxalate method developed by Sellers [40]. In this method, 2 mL of filtrated sample were mixed with 0.25 mL of sulphuric acid solution (1:17), 0.2 mL of potassium titanium oxide oxalate dihydrate (50 g/L) and 0.05 mL of distilled water for a final volume of 2.5 mL. After 5 min of reaction, the generation of yellow-orange titanium (IV) peroxide complex was spectrophotometrically measured at the maximum absorbance at 400 nm in a GENESYS 150 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Madrid, Spain). Reagents were provided by Sigma-Aldrich (Barcelona, Spain).
Current efficiency for hydrogen peroxide production (CE, %) and electric energy consumption (EEC, kWh/kg) was determined following Equations (6) and (7) [41]:
where n (=2) is the number of electrons transferred for oxygen reduction to hydrogen peroxide, F: Faraday constant, I: current intensity (A), t: time (s), CH2O2: hydrogen peroxide concentration (mg/L) and V: volume of the solution and U: voltage (V).
EEC = 1000·U·I·t/CH2O2·V
2.5.3. Metal Leaching Concentration
The metal concentration was monitored by Atomic Absorption Spectroscopy (Agilent 240FS) (C.A.C.T.I., University of Vigo).
2.5.4. TOC Concentration
Mineralization degree was determined via combustion by multi N/C 3100 Autoanalyzer (Analytik Jena) coupled with a nondispersive infrared (NDIR) detector (C.A.C.T.I. University of Vigo).
2.5.5. Material Characterization
For the characterization of the different synthesised materials, scanning electron microscopy (SEM) images were used to know the morphological characterization of catalyst and electrocatalyst. SEM images and Energy Dispersive Microanalysis (EDS) were performed on a JEOL JSM-6700F equipped with an EDS Oxford Inca Energy 300 SEM using an accelerating voltage of 20 kV (Electron Microscopy Service, C.A.C.T.I., University of Vigo, Spain).
The hydrophilicity/hydrophobicity of the prepared electrodes was determined based on the contact angle. For this purpose, a Mobile Drop Analyzer (Kruss) was used and the contact angle between a water drop (volume 0.005 mL) and the electrode material was measured.
3. Results
3.1. Screening of Transition Metals in Homogeneous Electro-Fenton-like Process
Initially, nickel and zinc ions were evaluated in the electro-Fenton-like process as catalysts for generation of hydroxyl radicals from hydrogen peroxide, which is produced in an electrochemical cell by two-electron reduction of oxygen on a carbonous cathode surface using electrical energy to overcome the unfavourable reactions [42]. In this study, a carbon felt cathode and BDD anode were used, and the hydrogen peroxide production was evaluated at different current intensities. This parameter affects the efficiency of the electro-Fenton process due to the oxygen reduction and generation of hydrogen peroxide on the cathode are directly related to the applied current. The continuous production of hydrogen peroxide at two current intensities (100–300 mA) showed that the efficiencies for the production were increased with the reduction of the current intensity. In both cases, the generation reached an apparent steady-state level after 60 min, which could be explained by the in situ decomposition of hydrogen peroxide [43]. In addition, the energy consumption of 100 mA (161 kWh/kg) was lower than that of 300 mA (998.79 kWh/kg) after 60 min. On the other hand, the current efficiency proved a better efficiency in the hydrogen peroxide production at a lower intensity (9.36 and 1.93 at 60 min for 100 and 300 mA, respectively).
Based on these results a current intensity of 100 mA was selected to evaluate the ability of the different transition metals (nickel and zinc) in the degradation of 2-phenylphenol. In the electro-Fenton the optimal concentration of used iron catalyst is lower than the conventional Fenton process, thus in the Fenton process is required catalyst concentration in a range of g/L, while in the electro-Fenton process is mg/L [44]. Moreover, the catalyst concentration is one of the key parameters to control the production of hydroxyl radical and the efficiency of the electro-Fenton process [31]. For this reason, as an attempt to optimize the electro-Fenton process, three concentrations (1, 3 and 5 mM) of each transition metal ions were tested.
Figure 2a shows the degradation profiles of 2-phenylphenol in the three electro-Fenton tests carried out in comparison to the anodic oxidation test, used as control experiment. BDD electrodes have great characteristics that distinguish them from other materials, such as high oxygen evolution potentials, inert surface with low adsorption and very low double layer capacitance and background current. Thanks to its properties, BDD anodes attain higher oxidation rates and mineralization efficiencies, producing •OH from water electrolysis (Equation (8)) that are strongly adsorbed on its surface and unselectively attack the 2-phenylphenol.
BDD + H2O → BDD(•OH) + H+ + e−
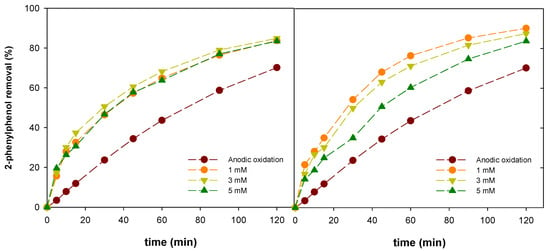
Figure 2.
Profiles of 2-phenylphenol removal in anodic oxidation and electro-Fenton-like using zinc (right) and nickel (left) at 1 mM (●), 3 mM (▼) and 5 mM (▲).
As is shown in Figure 2, anodic oxidation achieved a 2-phenylphenol degradation level of around 70.15%, meanwhile, in the electro-Fenton treatment tests, the degradation increased to a range of 80–90%, which represents an increase in efficiency of more than 10%. Concerning the concentration of Ni used, the percentage of degradation is around 5% between each of them, increasing the degradation level from 5 to 1 mM of Ni. These presented experimental data seem to reveal that 2-phenylphenol removal is described by a kinetic model of first-order (Equation (9)):
where C (mg/L): measured 2-phenylphenol concentration along the reaction time, t (min) and k: kinetic constant (min−1).
Table 2 summarizes the kinetic values and TOC reduction after 120 min of all tests performed. It should also be noticed that the test using nickel as catalyst, which gives the highest reaction rate, was 1 mM with a value 2.5 times higher than the constant of anodic oxidation. This electro-Fenton-like process shows a similar degradation rate in comparison with other previous studies using other more expensive degradation processes such as ozonation or UVC irradiation combined with ozonation processes [45]. Analogous to the electro-Fenton process with iron, the decrease in the efficiency with the increase of the catalyst could be due to the scavenging effect of an excess of nickel that will produce an excess of Ni3+, which react with hydrogen peroxide to produce hydroperoxyl radical of less oxidation capability than hydroxyl radical. Another reaction that can take place when an excess of Ni2+ is present is its reaction with hydroxyl radicals, decreasing the concentration of both and reducing the degradation reaction rate [21].

Table 2.
Parameter of first order reaction kinetic model (Equation (9)) fitting to the experimental data and TOC reduction after 2 h of treatment.
Similarly, the electro-Fenton-like test using zinc as homogeneous catalyst showed also better results than anodic oxidation (Figure 3). Concerning the zinc concentration, in this case, the differences among them are minimal, achieving the highest reaction rate at a zinc concentration of 3 mM (Table 2). However, the maximum removal was around 84.88% with a corresponding 64.36% TOC reduction after 120 min, values that were lower than the obtained using nickel as a catalyst with 90.12 and 69.08% of pollutant removal and TOC reduction, respectively. Thus, the best performance was attained when the electro-Fenton process was performed with nickel and the next assays were carried out using this transition metal ion.
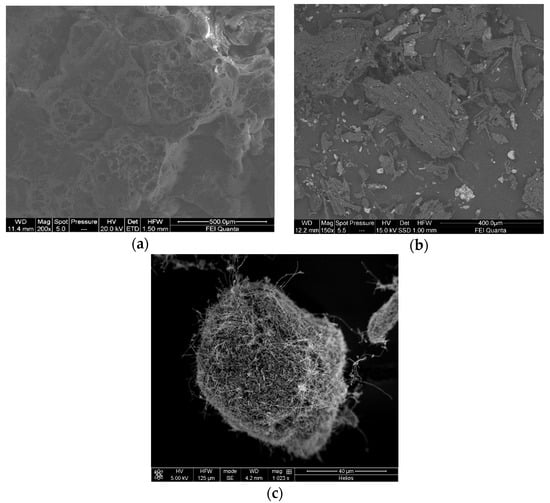
Figure 3.
SEM images of (a) perlite, (b) biochar and (c) carbon nanofibers.
3.2. Heterogeneous Nickel Catalyst
Once established nickel as the best electro-Fenton-like catalyst, several heterogeneous catalysts were synthesised in order to allow the operation in a flow system process.
As was described in material and methods, the nickel was included in different materials such as perlite, biochar or nanofibers by hydrothermal and adsorption treatments (Figure 3). Perlite was selected because it has been proved to be a good material for the fixation of metals such as iron by hydrothermal treatment [46]. In addition, this material has a floating characteristic that allows the catalyst to be on the liquid surface so that it can be recovered very easily [47]. Both of these characteristics are considered suitable for use in the design of a heterogeneous system electro-Fenton treatment. Biochar and nanofiber are materials with higher surface area and porosity [33,48], that allow the retention of metal ions by adsorption that can be used as a catalyst. The morphology and structural characteristics were determined by SEM, which confirmed the high porosity of these materials that favours the contact of the catalyst with the hydrogen peroxide increasing the removal rate [37]. Attending at Figure 3, it can be observed that the perlite presents a high amount of macropores for the fixation of the metal as suggested previously; however, biochar and carbon nanofibers have a superior surface area (45 and 54 m2/g) with small-sized pores.
Figure 4 shows the 2-phenylphenol removal profiles using the different catalysts tested in this study. It is clear that with catalyst supported in nanofiber and biochar the removal levels achieved after 5 min are quite similar (70–75%) which could be explained by the adsorption of the pollutant in the carbonaceous materials (biochar and nanofiber).
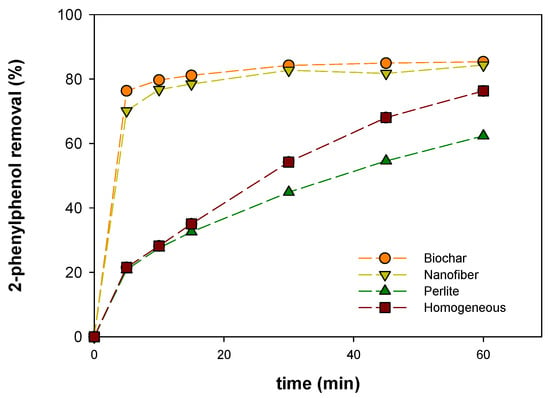
Figure 4.
Profiles of 2-phenylphenol removal (%) in different heterogeneous electro-Fenton-like and homogeneous electro-Fenton processes.
To elucidate this hypothesis, the adsorption of 2-phenylphenol in these materials was evaluated and compared with the electro-Fenton process. It was detected a high adsorption rate on carbonaceous materials, with levels around 60% after 5 min, achieving the maximum removal (76%) after 60 min. These values are lower than obtained when the electro-Fenton process was evaluated. Desorption process was accomplished in order to elucidate if the 2-phenylphenol was adsorbed upon the carbonous materials after the adsorption and electro-Fenton process. Desorption of 2-phenylphenol from nickel catalysts with acetonitrile did not show a residual concentration, however from nanofiber and biochar used in the adsorption test was possible the total recovery of the adsorbed pollutant in the acetonitrile solution.
These results confirm that the electro-Fenton treatment with this kind of catalyst is an efficient process for the disposal of pollutants that takes place in two steps: adsorption and oxidation. Even when adsorption has an important role, the adsorbed 2-phenylphenol was finally oxidized, resulting in a high removal from the bulk solution. This behaviour is in accordance with other previous studies in which the combination of adsorption and oxidation process had been detected. Thus, the efficiency of a hybrid process such as adsorption-Fenton oxidation using granular activated carbon showed, that this two-step process is an excellent combination for the effective removal of bisphenol A [49]. This synergistic interaction of adsorption and photocatalysis process has been considered in several studies as one of the most efficient techniques for environmental purification of wastewater [50,51]. Recently, doped porous carbon prepared by in-situ doping after carbonization of nitrogen-containing Metal-organic framework materials showed high efficiency for removal of tetracycline hydrochloride as a catalyst of PMS advanced oxidation process. It was demonstrated that the high degradation level achieved is closely related to the adsorption and catalytic performance of the catalyst [52]. Thus, the capability of carbonaceous catalysts to adsorb the target pollutant makes them excellent candidates for the two-step process of treating organic pollutants. In the literature, there are several studies in which electro-Fenton-process was used to regenerate spent adsorbent-like granular activates carbon [53,54], biochar [37] or aerogels used as adsorbents of pharmaceuticals pollutants [55,56].
In addition, no nickel leaching was detected which confirms that the use of these carbonaceous materials as a catalyst is a good option to design a system to operate in a flow system avoiding the presence of nickel in the output stream.
3.3. Heterogeneous Nickel Electrocatalyst
Nowadays, a challenge of the modern electro-Fenton systems is to improve the characteristics of the cathodes to enhance hydrogen peroxide production. In this sense, several authors have focused their studies on the evaluation of several cathode modification strategies that can improve its properties and increase electric conductivity [57,58]. Among the wide range of approaches reported for the enhancement of the production, cathodes synthesised by merging carbonaceous materials and Teflon (polytetrafluoroethylene or PTFE) have achieved interesting results [59,60].
In addition, to improve the electro-Fenton process, the inclusion of metals in the cathode can act as a heterogeneous catalyst with the capacity to react with electrogenerated hydrogen peroxide [31,61,62]. In this context, the inclusion of nanofibers doped with nickel has been tested to perform the heterogeneous electro-Fenton process. Due to a large number of experiments need to evaluate the effect of cathode composition, in this study, a Taguchi design was used to reduce the number of trials required to obtain a valid conclusion [63]. Taguchi uses orthogonal matrices, which organise the variables that affect the process and their levels, in the most likely to affect the process. In addition, it offers the opportunity to reduce the number of experiments by testing pairs of combinations, saving time and resources. It allows the calculation of a signal-to-noise ratio (S/N) based on experimental data, which defines the best experimental conditions, considering product quality or process performance within the range of process performance within the experimental range tested.
In this study, the cathodes were prepared by immersion of carbon felt in a dispersion solution of ethanol:ultrapure water that contained carbon black, nickel nanofibers and PTFE (Figure 5). In this study, the Taguchi method was used to investigate the effect of three operating parameters to obtain an optimal process within the evaluated range. The selected variables were A: carbon black, B: nickel nanofibers and C: PTFE and the used concentrations were summarised in Table 2.
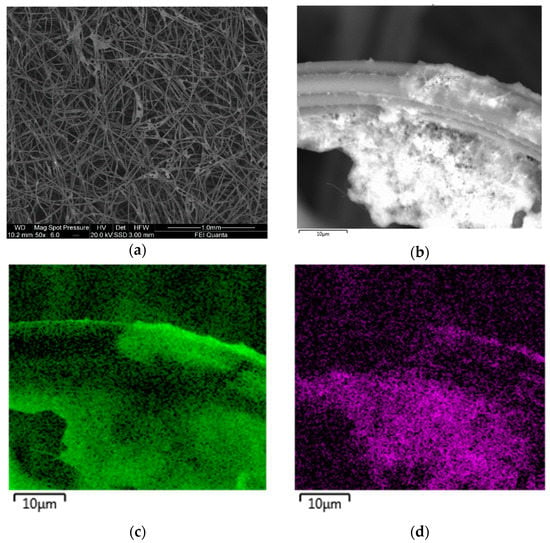
Figure 5.
Detailed SEM image of an electrocatalyst containing carbon black, nanofibers, PTFE and nickel (a,b) and EDS analysis: fluorine (c) and nickel (d).
The current intensity was also tested since depending on the composition, the response of the prepared electrodes could be different about carbon felt cathode. Thus, the Taguchi method to ascertain the significance of each of the factors chosen in the hydrogen peroxide production was evaluated operating at 100 and 300 mA.
The effect of the input variables on the hydrogen peroxide has been determined by the S/N ratio with the larger-the-better criteria and the attained response values were therefore converted into S/N. As an example, Figure 6 shows the main effect plots for the response at intensity current of 100 mA. Thus, the strongest influence is the nickel nanofibers, followed by carbon black and PTFE. Moreover, the main effect plots determine the optimum factor levels. As can be seen, A2B2C1 are the optimal condition operating at both current intensities. These improvements of hydrogen peroxide could be attributed to the change in the surface cathode with, for example, the formation of microporous by deposition of carbon black and nanofibers.
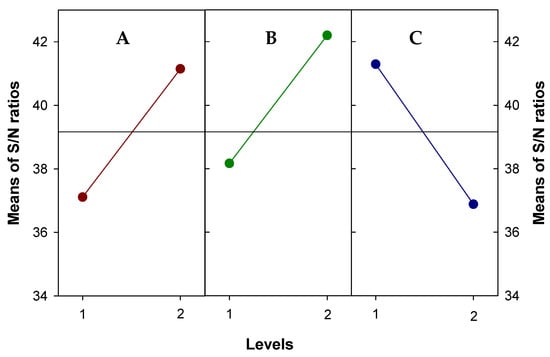
Figure 6.
Main effects plot for S/N ratios with the larger-the better criteria of screening experiment yield. Responses of hydrogen peroxide produced at 100 mA. (A: carbon black, B: nickel loaded nanofibers and C: PFTE content).
In Figure 7, the profiles of produced hydrogen peroxide and 2-phenylphenol removal operating with the optimized cathode at 100 and 300 mA are shown.
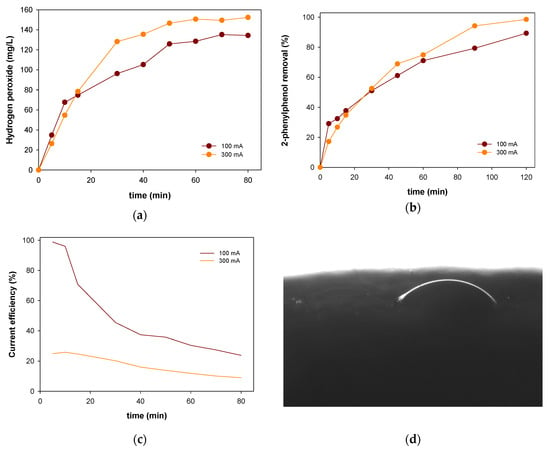
Figure 7.
Profiles of hydrogen peroxide (a) and 2-phenylphenol removal (b) using the optimized electrocatalyst in electro-Fenton-like process at 100 mA (marron) and 300 mA (orange) and current efficiency evolution along time (c). Water contact angle determined by drop assay (d).
This cathode provides an increase until more than 4 times de produced hydrogen peroxide after 60 min. This behaviour can be partially explained by the measurement of the hydrophilic characteristics related to the electrode using water-drop contact angle experiments (Figure 6d). The water-drop contact angle of the electrode was 36.6°, which decreased from the unmodified CF (133.25°). This small contact angle implies a good hydrophilic characteristic of the optimized electrode toward the solution and changing the surface of the material. Figure 6a,b illustrate the changes in the electrode surface. Higher hydrophilicity enhances the diffusion of the dissolved oxygen through the electrode suggesting an increase in the generation of hydrogen peroxide as it was detected [33]. In addition, high degradation levels were obtained with near-complete degradation after 2 h. Although the experiment carried out at 300 mA with the optimized electrocatalyst achieves higher levels, taking into account its energy consumption (4655.92 kWh/kg of 2-phenylphenol removal) the operation at high current intensity could be considered inefficient, since operating at 100 mA the consumption drops to 622.75 kWh/kg. Those results are also confirmed by the determination of the current efficiencies (Figure 7c) showing that the operation at a higher intensity decreases the efficiency of the electrodes.
Thus, operating at the minimum current intensity the energetic consumption decreased 7.5 times with a minimal impact on process effectiveness that was around a 10% decrease from optimal efficiency. Similar results have been determined in previous studies using different electrocatalysts, such as carbon felt cathodes modified by the inclusion of nano-Fe3O4. Operating over a wide range of conditions, it was concluded that operating at the lowest applied current resulted in a tenfold decrease in energy consumption within a decrease in optimum efficiency of less than 10% [64].
4. Conclusions
In the present study, the preparation of new catalysts and electrocatalysts using a transition metal as nickel supported in different clay and carbonaceous materials were successfully performed. Their evaluation on the degradation of a pollutant such as a fungicide 2-phenylphenol proved their efficiency and feasibility for their use in a heterogeneous Fenton based process. However, it is concluded the superiority of heterogeneous nickel electrocatalyst synthesised by the inclusion of nanofibers doped with nickel into carbon felt. The preparation of this electrocatalyst adding carbon black, nickel doped nanofibers and PTFE to the carbon felt increased significantly the potential to produce hydrogen peroxide (more than 4 times the unmodified carbon felt). In addition, the presence of the nickel catalyst on the cathode favoured the expected generation of hydroxyl radicals. The optimisation of the ratios among carbon black, nickel doped nanofibers and PTFE allowed concluding that the best production of hydrogen peroxide and the degradation yields were attained when the amount of carbon black and nanofibers were increased while the added PTFE decreased in the range evaluated. Moreover, an increase in the current efficiency and reduction in energy consumption was also achieved when a current intensity was 100 mA. Thus, the heterogeneous nickel electrocatalyst shows an excellent behaviour in the electro-Fenton process, with high hydrogen peroxide production, pollutant removal and stability. Summing up, the use of this new electrocatalyst can be an alternative to the current cathodes that increase the catalogue of catalysts offering advantages such as low leaching, high stability and pollutants removal. These open the possibility to develop more effective electrocatalysts extending the type of catalysts in the electro-Fenton process that helps to increase the viability of electro-advanced oxidation processes showing high potential to be used in the removal of pollutants. In a future more investigations are requested in order to evaluate its behaviour in the operation at high scale.
Author Contributions
Conceptualization, E.R. and M.Á.S.; methodology, E.R. and M.Á.S.; formal analysis, A.F.-S. and E.R.; investigation, R.M.-T. and A.F.-S.; resources, M.P. and M.Á.S.; data curation, A.F.-S. and E.R.; writing—original draft preparation, A.F.-S.; writing—review and editing, E.R. and M.Á.S.; visualization, A.F.-S., M.P. and M.Á.S.; supervision, M.P. and M.Á.S.; project administration, M.P. and M.Á.S.; funding acquisition, M.Á.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the R&D Project CTM 2017-87326-R funded by 10.13039/501100011033/FEDER “Una manera de hacer Europa”, Project PID2020-113667GB-I00 funded by MCIN/AEI/10.13039/501100011033 and PDC2021-121394-I00 funded by MCIN/AEI/10.13039/501100011033 and by the European Union Next GenerationEU/PRTR, and Xunta de Galicia and European Regional Development Fund (ED431C 2021-43). Antía Fdez-Sanromán acknowledges the University of Vigo for training fellowships.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- El Balkhi, S.; Dulaurent, S.; Saint-Marcoux, F. Analytical Strategies to Measure Herbicide Active Ingredients and their Metabolites; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128236741. [Google Scholar]
- Visconti, V.; Coton, E.; Rigalma, K.; Dantigny, P. Effects of disinfectants on inactivation of mold spores relevant to the food industry: A review. Fungal Biol. Rev. 2021, 38, 44–66. [Google Scholar] [CrossRef]
- Bocos, E.; Fernandez-Costas, C.; Pazos, M.; Sanroman, M.A. Removal of PAHs and pesticides from polluted soils by enhanced electrokinetic-Fenton treatment. Chemosphere 2015, 125, 168–174. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Reregistration Eligibility Decision for 2-Phenylphenol and Salts (Orthophenylphenol or OPP). 2006. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1009GW3.txt (accessed on 3 December 2021).
- Hou, J.; Li, X.; Li, J.; Sun, J.; Zheng, S. Enhanced adsorption of o-phenylphenol on zeolites: A combing pore filling and hydrophobic effects. Microporous Mesoporous Mater. 2020, 294, 109860. [Google Scholar] [CrossRef]
- Hou, J.; Yang, S.; Wan, H.; Fu, H.; Qu, X.; Xu, Z.; Zheng, S. Highly effective catalytic peroxymonosulfate activation on N-doped mesoporous carbon for o-phenylphenol degradation. Chemosphere 2018, 197, 485–493. [Google Scholar] [CrossRef]
- Wang, H.; Deng, W.; Shen, M.; Yan, G.; Zhao, W.; Yang, Y. A laccase Gl-LAC-4 purified from white-rot fungus Ganoderma lucidum had a strong ability to degrade and detoxify the alkylphenol pollutants 4-n-octylphenol and 2-phenylphenol. J. Hazard. Mater. 2021, 408, 124775. [Google Scholar] [CrossRef] [PubMed]
- Agüera, A.; Fernández-Alba, A.R.; Piedra, L.; Mézcua, M.; Gómez, M.J. Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry. Anal. Chim. Acta 2003, 480, 193–205. [Google Scholar] [CrossRef]
- Bolz, U.; Hagenmaier, H.; Ko, W. Phenolic xenoestrogens in surface water, sediments, and sewage sludge from Baden-Württemberg, south-west Germany. Environ. Pollut. 2001, 115, 291–301. [Google Scholar] [CrossRef]
- Chafi, S.; Azzouz, A.; Ballesteros, E. Occurrence and distribution of endocrine disrupting chemicals and pharmaceuticals in the river Bouregreg (Rabat, Morocco). Chemosphere 2022, 287, 132202. [Google Scholar] [CrossRef]
- World Health Organization. 2-Phenylphenol in Drinking-water. In Background Document for Development of WHO Guidelines for Drinking-Water Quality; (WHO/SDE/WSH/03.04/69); World Health Organization: Geneva, Switzerland, 2011; Volume 216, pp. 303–304. [Google Scholar]
- Nougadère, A.; Sirot, V.; Cravedi, J.; Vasseur, P.; Feidt, C.; Fussell, R.J.; Hu, R.; Leblanc, J.; Jean, J.; Rivière, G.; et al. Dietary exposure to pesticide residues and associated health risks in infants and young children—Results of the French infant total diet study. Environ. Int. 2020, 137, 105529. [Google Scholar] [CrossRef] [PubMed]
- Erdemli köse, S.B.; Kocasari, F. Toxicity of Ortho-Phenylphenol (OPP) and Sodyum Ortho-Phenylphenate (SOPP). Mehmet Akif Ersoy Üniversitesi Sağlık Bilimleri Enstitüsü Dergisi 2020, 8, 18–29. [Google Scholar] [CrossRef]
- Additives, F. Novel sequential separation and determination of a quaternary mixture of fungicides by using an automatic fluorimetric optosensor. Food Addit. Contam. Part A 2019, 36, 278–288. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N. Synthesis of Spinel Ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for Persulfate Activation to Remove Aqueous Organics: Effects of M-Site Metal and Synthetic Method. Front. Chem. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef] [PubMed]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Antiñolo, L.; Mart, J.; Muñio, M.; Manuel, J.; Capilla, P. Effectiveness of Advanced Oxidation Processes in Wastewater Treatment: State of the Art. Water 2021, 13, 2094. [Google Scholar] [CrossRef]
- Rosales, E.; Pazos, M.; Sanromán, M.A. Advances in the Electro-Fenton Process for Remediation of Recalcitrant Organic Compounds. Chem. Eng. Technol. 2012, 35, 609–617. [Google Scholar] [CrossRef]
- Meijide, J.; Rosales, E.; Pazos, M.; Sanromán, M.A. p-Nitrophenol degradation by electro-Fenton process: Pathway, kinetic model and optimization using central composite design. Chemosphere 2017, 185, 726–736. [Google Scholar] [CrossRef]
- Dave, S.; Das, J. 2—Technological Model on Advanced Stages of Oxidation of Wastewater Effluent from Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128210116. [Google Scholar]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J. Recent advances in waste water treatment through transition metal sulfides-base d advance d oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di, A.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly imprinted N-doped TiO 2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater. Sci. Semicond. Process. 2020, 112, 105019. [Google Scholar] [CrossRef]
- Bouzayani, B.; Rosales, E.; Pazos, M.; Elaoud, S.C.; Sanromán, M.A. Homogeneous and heterogeneous peroxymonosulfate activation by transition metals for the degradation of industrial leather dye. J. Clean Prod. 2019, 228, 222–230. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dai, L.; Guo, H.; Shi, P.; Min, Y.; Xu, Q. Recycling the Cathode Scrap of Spent Lithium-Ion Batteries as an Easily Recoverable Peroxymonosulfate Catalyst with Enhanced Catalytic Performance. ACS Sustain. Chem. Eng. 2020, 8, 11337–11347. [Google Scholar] [CrossRef]
- Dang, S.; Zhou, P.; Shi, P.; Min, Y.; Xu, Q. In Situ Aluminothermic Reduction Induced by Mechanochemical Activation Enhances the Ability of the Spent LiCoO 2 Cathode to Activate Peroxymonosulfate. ACS Sustain. Chem. Eng. 2021, 9, 15375–15385. [Google Scholar] [CrossRef]
- Fernández de Dios, M.Á.; Rosales, E.; Fernández-Fernández, M.; Pazos, M.; Sanromán, M.Á. Degradation of organic pollutants by heterogeneous electro-Fenton process using Mn-alginate composite. J. Chem. Technol. Biotechnol. 2015, 90, 1439–1447. [Google Scholar] [CrossRef]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton technology for wastewater treatment: A bibliometric analysis of current research trends, future perspectives and energy consumption analysis. J. Water Process. Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Angeles, M. Chemosphere Current advances and trends in electro-Fenton process using heterogeneous catalysts e A review Ver o Sanrom a. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; Van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Acevedo-García, V.; Pazos, M.; Sanromán, M.Á.; Rosales, E. Electrochimica Acta Iron-doped cathodes for electro-Fenton implementation: Application for pymetrozine degradation. Electrochim. Acta 2020, 338, 1–11. [Google Scholar] [CrossRef]
- Popescu, M.; Sandu, C.; Rosales, E.; Pazos, M.; Lazar, G.; Sanromán, M.Á. Evaluation of different cathodes and reaction parameters on the enhancement of the electro-Fenton process. J. Electroanal. Chem. 2018, 808, 455–463. [Google Scholar] [CrossRef]
- Olak-kucharczyk, M.; Ledakowicz, S. Advanced oxidation of preservative agents in H2O2/UVC system—Kinetics study, transformation products and toxicity assessment. J. Hazard. Mater. 2017, 333, 348–357. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Li Puma, G. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2015, 178, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M.A. Synthesis and use of efficient adsorbents under the principles of circular economy: Waste valorisation and electroadvanced oxidation process regeneration. Sep. Purif. Technol. 2020, 242, 116796. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Rezaee, A.; Khataee, A.R.; Godini, H. Electrochemical generation of hydrogen peroxide using carbon black-, carbon nanotube-, and carbon black/carbon nanotube-coated gas-diffusion cathodes: Effect of operational parameters and decolorization study. Res. Chem. Intermed. 2013, 39, 4277–4286. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Rajic, L.; Xue, Y.; Chen, S.; Ding, Y.; Kou, K.; Wang, Y.; Gao, J.; Qin, Y.; et al. “Floating” cathode for efficient H2O2 electrogeneration applied to degradation of ibuprofen as a model pollutant. Electrochem. Commun. 2018, 96, 37–41. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.; Ren, G.; Li, Y.; Li, Y.; Du, X. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion. Nat. Commun. 2020, 11, 1731. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, J.S.J.; Chung, Y.; Ahn, W.; Hisatomi, T.; Domen, K.; Kung, M.C.; Kung, H.H. Applied Catalysis A, General Minimizing energy demand and environmental impact for sustainable NH3 and H2O2 production—A perspective on contributions from thermal, electro-, and photo-catalysis. Appl. Catal. A Gen. 2020, 594, 117419. [Google Scholar] [CrossRef]
- Lim, J.; Hoffmann, M.R. Substrate oxidation enhances the electrochemical production of hydrogen peroxide. Chem. Eng. J. 2019, 374, 958–964. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Velmathi, S.; Sanjini, N.S. Magnetite as a heterogeneous electro Fenton catalyst for the removal of Rhodamine B from aqueous solution. RSC Adv. 2014, 4, 5698–5708. [Google Scholar] [CrossRef]
- Olak-Kucharczyk, M.; Ledakowicz, S. Decomposition of Phenylphenol Isomers by UVC-Enhanced Ozonation Process. Ozone Sci Eng. 2017, 39, 333–342. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Pazos, M.; Sanromán, M.A. Prompt removal of antibiotic by adsorption/electro-Fenton degradation using an iron-doped perlite as heterogeneous catalyst. Process. Saf. Environ. Prot. 2020, 144, 100–110. [Google Scholar] [CrossRef]
- Díez, A.M.; Pazos, M.; Sanromán, M.A. Bifunctional floating catalyst for enhancing the synergistic effect of LED-photolysis and electro-Fenton process. Sep. Purif. Technol 2020, 230, 115880. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Applied sciences Unravelling the Environmental Application of Biochar as Low-Cost Biosorbent: A Review. Appl. Sci. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Kim, J.R.; Huling, S.G.; Kan, E. Effects of temperature on adsorption and oxidative degradation of bisphenol A in an acid-treated iron-amended granular activated carbon. Chem. Eng. J. 2015, 262, 1260–1267. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Kumar, A.; Naushad, M.; Vo, D.V.N.; Al-Misned, F.A.; El-Serehy, H.A.; Stadler, F.J. Ag0-Ag2O embedded nanocomposite hydrogel for adsorption-coupled-photocatalytic removal of triclosan. Mater. Lett. 2020, 276, 128169. [Google Scholar] [CrossRef]
- Sheng, Y.; Wei, Z.; Miao, H.; Yao, W.; Li, H.; Zhu, Y. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem. Eng. J. 2019, 370, 287–294. [Google Scholar] [CrossRef]
- Xie, J.; Chen, L.; Luo, X.; Huang, L.; Li, S.; Gong, X. Degradation of tetracycline hydrochloride through efficient peroxymonosulfate activation by B, N co-doped porous carbon materials derived from metal-organic frameworks: Nonradical pathway mechanism. Sep. Purif. Technol. 2022, 281, 119887. [Google Scholar] [CrossRef]
- Bañuelos, J.A.; Rodríguez, F.J.; Manríquez Rocha, J.; Bustos, E.; Rodríguez, A.; Cruz, J.C.; Arriaga, L.G.; Godínez, L.A. Novel electro-fenton approach for regeneration of activated carbon. Environ. Sci. Technol. 2013, 47, 7927–7933. [Google Scholar] [CrossRef]
- Bury, N.A.; Mumford, K.A.; Stevens, G.W. The electro-Fenton regeneration of Granular Activated Carbons: Degradation of organic contaminants and the relationship to the carbon surface. J. Hazard. Mater. 2021, 416, 125792. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.; Pazos, M.; Rosales, E.; Sanromán, M.A. Electro-reversible adsorption as a versatile tool for the removal of diclofenac from wastewater. Chemosphere 2021, 280, 130778. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Sanromán, M.A.; Pazos, M. Environmental application of monolithic carbonaceous aerogels for the removal of emerging pollutants. Chemosphere 2020, 248, 125995. [Google Scholar] [CrossRef]
- Jung, E.; Shin, H.; Lee, B.H.; Efremov, V.; Lee, S.; Lee, H.S.; Kim, J.; Hooch Antink, W.; Park, S.; Lee, K.S.; et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 2020, 19, 436–442. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Zheng, Y.; Xia, B.; Zhou, X.; Xu, H.; Qiao, S.Z. Tailoring Selectivity of Electrochemical Hydrogen Peroxide Generation by Tunable Pyrrolic-Nitrogen-Carbon. Adv. Energy Mater. 2020, 10, 2000789. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, M.; Yu, X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration. Electrochim. Acta 2015, 163, 182–189. [Google Scholar] [CrossRef]
- Pérez, J.F.; Sáez, C.; Llanos, J.; Cañizares, P.; López, C.; Rodrigo, M.A. Improving the Efficiency of Carbon Cloth for the Electrogeneration of H2O2: Role of Polytetrafluoroethylene and Carbon Black Loading. Ind. Eng. Chem. Res. 2017, 56, 12588–12595. [Google Scholar] [CrossRef]
- Liu, K.; Yu, J.C.C.; Dong, H.; Wu, J.C.S.; Hoffmann, M.R. Degradation and Mineralization of Carbamazepine Using an Electro-Fenton Reaction Catalyzed by Magnetite Nanoparticles Fixed on an Electrocatalytic Carbon Fiber Textile Cathode. Environ. Sci. Technol. 2018, 52, 12667–12674. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, F.; Qiu, S.; Ma, F.; Zheng, Y.; Gao, L. A self-sufficient electro-Fenton system with enhanced oxygen transfer for decontamination of pharmaceutical wastewater. Chem. Eng. J. 2022, 429, 132176. [Google Scholar] [CrossRef]
- Li, L.; Yang, T.; Li, Z. Parameter optimization and yield prediction of cathode coating separation process for direct recycling of end-of-life lithium-ion batteries. RSC Adv. 2021, 11, 24132–24136. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).