Abstract
Galactolipids are a class of lipids present, inter alia, in the plastid membranes of plant cells. Apart from their biological significance, they are recognized as an important group of bioactive agents, especially in the treatment of osteoarthritis. The aim of this research was to evaluate the usefulness of the green chemistry approach in the extraction of these compounds. Waste products of food processing were selected as a raw material to improve the sustainability of the process even further, and their galactolipid content was investigated using an LC-MS analysis. The rosehip pomace, which was recognized as the most promising amongst materials used in this study, was subjected to supercritical fluid extraction (SFE) and ultrasound-assisted extraction (UAE). It transpired that SFE using pure CO2 was not an effective method for the extraction of galactolipids, although the use of ethanol as a cosolvent favored the separation. The results of UAE were also very promising—the improvement of the extraction yield up to 74% was observed. The green chemistry approaches used for galactolipid isolation were compared with a conventional processing method and proved to be an interesting alternative.
1. Introduction
Galactolipids are a group of lipids that occur mainly in the thylakoids of higher plants and algae [1]. It is the main class of lipids found in plastid membranes, and their presence is crucial for the synthesis of the photosynthetic apparatus. Two main groups of galactolipids are distinguished: monogalactosyldiacylglycerols (MGDGs), which account for 50% of all lipids in thylakoids, and digalactosyldiacylglycerols (DGDGs), which in turn account for 30% [2]; their structures are presented in Figure 1. Their synthesis takes place in the plastid envelope membranes by galactosyltransferase enzymes [3] An impact of a balanced diet rich in vegetables and fruits on consumers’ health due to the presence of bioactive compounds has been known for decades. These compounds include galactolipids, which are considered to be an important class of nutraceuticals. So far, several positive effects have been attributed to galactolipids, e.g., MGDGs isolated from fresh spinach leaves inhibit the activation of the Epstein–Barr virus (EBV) [4]. Another study by Kuriyama et al. showed that the glycolipid fraction of spinach, containing, among other things, MGDGs and DGDGs, shows potential anti-cancerogenic features [5]. Moreover, the presence of galactolipids along with other compounds (such as carotenoids or terpenoids) present in rosehip (Rosa canina L.) counteract osteoarthritis [6]. Its anti-inflammatory as well as anti-rheumatic properties were attributed to a galactolipid named GOPO. So far, many studies have been carried out that clearly show that in patients suffering from chronic inflammation of the bones and joints, there was a reduction in pain and an improvement in joint mobility after the administration of powdered rosehips [7]. More importantly, no side effects of the administered substances have been observed so far [8]. Products containing GOPO are commercially available on the pharmaceutical market; producers claim that thanks to the presence of this galactolipid, they can delay arthritic changes and slow down the degradation of cartilage tissue. It must, however, be noted, that the EFSA has so far not made any health claims connected with galactolipids. The details of the methods used by the manufacturers of galactolipid-based diet supplements are confidential; however, based on chemical properties of galactolipids, it can be assumed that extraction with organic solvents is the method of choice.
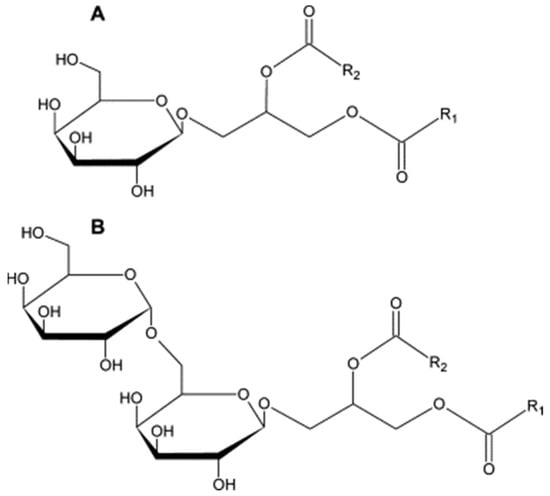
Figure 1.
Structure of MGDGs (A) and DGDGs (B) [9]. Reproduced from [9] with permission. Copyright 2012 Elsevier.
In recent decades, there has been an increasing awareness of the environmental impact of production and processing, which has led to a global tendency to ensure the sustainability of such operations. The term “green chemistry” was coined to describe an alternative approach to the operations that were developed to be more environmentally friendly, often at the price of their profitability. The main principles of green extraction include the use of alternative solvents, innovative technologies, and the selection of renewable resources [10]. Some of the techniques have already found a place as the methods of choice in industrial applications, while others are still in the first testing stage. A broader description of the various methods of green extraction can be found in excellent reviews on the subject [11,12].
Ultrasound-assisted extraction (UAE) refers to the modification of a typical solid–liquid extraction by applying high-intensity ultrasonic waves to the sample. Typical implementations of the process include the use of baths, with generators built in the wall of the vessel, and horns, which are immersed in the sample. Compared to other novel processing techniques, UAE is very inexpensive. The main driving force of the process is acoustic cavitation—the creation, expansion, and implosive collapse of microbubbles in ultrasound-irradiated liquids. The use of ultrasound also causes agitation, vibrations, shockwaves, microjets, and radical formation. The UAE benefits from enhanced mass transfer and an increase of the surface area due to the disintegration of the solid matrix [13].
Supercritical fluid extraction (SFE) takes advantage of the properties of substances in a supercritical state and uses them instead of typical liquid solvents. Many compounds can be used in supercritical processes, although the solvent of choice in the vast majority of applications is carbon dioxide, which is characterized by mild critical-point conditions, chemical inertia, low price, and negligible toxicity. Apart from that, the low viscosity of supercritical fluids improves mass transfer during extraction, while the transition to gas after the pressure decrease results in obtaining solvent-free extracts [14]. The advantages of SFE led to its industrial implementation in the production of, among other things, decaffeinated coffee, hop extracts, alcohol-free wine and beer, and spice extracts [15].
The aim of the work was to evaluate the possibility of using green extraction technology for the extraction of galactolipids from plant material. SFE and UAE were the two techniques selected for the experiments. The techniques were selected according to their status as the method that is already implemented in industrial practice. The methods differ in principle of the operation: SFE uses an alternative solvent, while UAE improves currently existing techniques by an intensification of the mass transfer. Additionally, waste products from fruit and vegetable processing were selected as a material to further improve the sustainability of the process. This paper is the first report on the extraction of galactolipids using green chemistry methods, and the authors believe that it can provide valuable information for prospective industrial applications.
2. Materials and Methods
2.1. Materials
Certified standards of MGDGs and DGDGs were acquired from Avanti Polar Lipids (Alabaster, AL, USA). Chromatographic solvents of appropriate purity were acquired from Merck (Darmstadt, Germany), while other chemicals came from either Merck or Avantor (Radnor, PA, USA).
As significant amounts of galactolipids are present exclusively in plant material, the selection of matrices to be analyzed was limited to the waste products of fruit and vegetable processing. The selected material included pomace from apple (Malus domestica Borkh.), carrot (Daucus carota Hoffm.), and rosehip (Rosa canina L.), as well as wastes from the processing of broccoli (Brassica oleracea var. italica), fava beans (Vicia faba L.), and potato (Solanum tuberosum L.). The material was produced on a laboratory scale using typical processing methods used in the industry. Samples were kept at a temperature of −18 °C prior to analysis and extraction.
2.2. Supercritical Fluid Extraction (SFE)
The SFE process was conducted on a SFE Spe-ed 4 apparatus (Applied Separations, Allentown, PA, USA) using a 24 mL extraction vessel. The portions of material were thermostated for 15 min prior to extraction; in the experiments using cosolvent, the cosolvent was added along with the sample at this point. Each extraction run was conducted with a constant flow rate of approx. 100 g h−1 for 1 h; our previous publications using similar parameters proved that such a duration is sufficient for extraction [16,17,18,19].
The solvating strength of supercritical fluids is strongly connected with their parameters of state; therefore various temperatures and pressures were evaluated. Their lower limits were set by critical parameters of carbon dioxide (31.0 °C, 7.38 MPa), while the upper limit was set by the thermal stability of the galactolipids (75 °C) and technical limitations of the equipment (40 MPa). A 515 HPLC Pump (Waters, Milford, MA, USA) was used to volumetrically deliver the co-solvent to the vessel. Ethanol and carbon dioxide were mixed in a small chamber prior to entering the vessel. The experiments included extraction runs at combinations of three levels of the temperature (35, 55, and 75 °C) and the levels of the pressure (10, 20, and 40 MPa). Next, the three levels of cosolvent flow (0.0, 0.2 and 0.4 mL min−1) were evaluated in combinations with the temperatures.
2.3. Ultrasound-Assisted Extraction (UAE)
The UAE process was conducted using an ultrasound bath MKD-6 (MKD Ultrasonic, Warsaw, Poland) with a sonication power of 300 W and an ambient temperature. The plant material was placed inside a Falcon vessel (50 mL) with solvent, sonicated for a fixed amount of time and shaken for 1 min at 300 min−1 (SK-O330 Pro, DragonLab Instruments, Beijing, China). The control samples were prepared using the same matrix–solvent ratio and kept for a fixed amount of time. and shaken for 1 min.
The first stage of the experiment was to compare the efficiency of twelve solvents for the extraction of galactolipids—the yield of both sonicated and control samples was compared. The results along with properties of the solvents were used to select the most appropriate solvent for further experiments. The second part was an analysis of an impact of process parameters (sonication time, mass–solvent ratio) on the efficiency of the process.
2.4. Analysis of Galactolipid Content
The method presented by Zábranská and co-workers was used for the qualitative and quantitative analysis of galactolipids [9]. The ground plant sample (6 g) was placed in a flask, 15 mL of isopropanol was added, and the mixture was kept at a temperature of 75 °C for 15 min. Subsequently, 7.5 mL of chloroform and 3 mL of water were added to the flask and then shaken for 1 h at 300 min−1. The extract was collected and a sample was reextracted with 10 mL of chloroform–methanol mixture (2:1) three times. The extracts were collected and washed with 20 mL of 1M potassium chloride. The organic phase was dehydrated with 5 g of anhydrous magnesium sulfate, filtered through cotton wool, and concentrated on a rotary evaporator (R-300 Rotavapor, Büchi, Flawil, Switzerland) to approximately 20% of the initial volume. The extracts obtained using SFE and UAE methods were only subjected to the last part of the aforementioned protocol (from dehydration with MgSO4).
Semi-preparative thin-layer chromatography was used to isolate the main groups of galactolipids. The extract was transferred to a 10 × 20 cm TLC silica gel 60G (0.2 mm) plate (Merck, Darmstadt, Germany) and eluted with a mixture of chloroform–methanol–water (80:18:2). The zones corresponding to MGDGs and DGDGs typically had retention factors of 0.7 and 0.4, respectively. Nevertheless, standards were also placed on the plate and visualized under UV light after spraying with a primuline solution (0.05% in methanol-water (8:2) mixture). The zones corresponding to galactolipid groups were scraped, extracted with a mixture of chloroform-methanol (2:1), and filtered through cotton glass.
Liquid chromatography analyses were performed on an H-class liquid chromatograph coupled to a mass spectrometer with a time-of-flight analyzer (UPLC-TOF-HRMS; Waters, Milford, MA, USA). The samples (5 µL) were separated using a UPLC C18 Cortecs (2.1 × 100 mm, 1.6 μm) column (Waters). Despite using the same equipment, separate gradients were employed for the galactolipid groups—the details are presented below in Table 1. The mass spectrometer operated in the positive electrospray ionization mode. The ion source temperature was 150 °C, while the desolvation temperature was 350 °C. The nebulizing gas (N2) flow rate was 750 L min−1, the cone gas flow rate was 40 L min−1, and the capillary bias was 3.2 kV.

Table 1.
Gradients used for the LC-MS analysis of the galactolipids.
The method implemented allowed the separation of galactolipids and their partial identification. As reported by the authors of the method, galactolipids in plant samples are present as a mixture of compounds with various fatty acids attached to the glycerol scaffold (Figure 1) [9]. The implementation of MS detection made it possible to distinguish between the compounds based on their m/z ratio, as the elongation of the carbon chain or saturation of the double bond can be easily identified. GOPO, the compound that, according to the literature, has the highest pharmacological potential is MGDG, with two α-linoleic acids (18:3) attached; due to its structure, the identification is certain as no lipids of the same molecular mass could occur. As MGDG analysis was used to a greater extent during the research, the separation of these galactolipids is presented in Figure S1 in the Supplementary Materials.
2.5. Statistical Analysis
Data were analyzed using Statistica 7.1 software (StatSoft, Tulsa, OK, USA). An ANOVA test at α = 0.05 was used to determine the statistical significance of the differences in mean values; if such differences existed, a post hoc Tukey test was performed. All of the results were obtained from analyses of three independent samples.
3. Results and Discussion
3.1. Galactolipids in Processing By-Products
The waste materials were analyzed for their galactolipid content, with a special emphasis on GOPO, which has the greatest pharmacological potential, as was highlighted earlier. The content of the analytes is presented in Table 2.

Table 2.
Content of galactolipids and dry mass in the waste materials analyzed.
The content of analytes varied significantly between the samples, which was partially the effect of using various morphological parts of plants. The subterranean parts of plants did not contain measurable levels of galactolipids, apples and beans were characterized by low levels, and broccoli had a high content of these compounds, as its green florets are abundant in chloroplasts. The rosehip was the richest source of galactolipids among the matrices analyzed, having an MGDGs/DGDGs ratio distinct from other resources.
The rosehip was also characterized by a high content of GOPO, which accounted for almost 70% of galactolipids in the sample. This compound was also detected in broccoli, although its levels were much lower. Additionally, the broccoli wastes were characterized by a high water content, which would hinder the extraction with supercritical carbon dioxide and organic solvents of low polarity. The matrix could be dried prior to the process, although, depending on the technique selected, this would lead to the thermal decomposition of analytes (convection-drying) or generate substantial costs (freeze-drying). Therefore, only the rosehip pomace was selected for further experiments. Additionally, as levels of DGDGs in the material are much lower than those of MGDGs and require additional chromatographic analysis, only levels of MGDGs were evaluated in the later parts of the work.
The galactolipid content in plants can be related to their physiological function because they are essential components of chromoplast membranes [1]. The review paper by Christensen et al. confirms this assumption. The authors reported that the levels of both MGDGs and DGDGs in leafy vegetables are by far the highest [2]. Such results were confirmed by other authors such as Lee et al., who reported a total galactolipid content of 100–200 mg kg−1 (of dry weight) in green leafy vegetables (parsley, spinach, and perilla) [20]. The only two literature reports on GOPO levels in rosehip are by Larsen and coworkers, who recorded a level of 270 mg kg−1 in dried fruits [9], and Chrubasik at al., who reported 300 mg kg−1 in a diet supplement based on dried rosehip [21], which is comparable to green vegetables. Our data provide a total galactolipid content of approximately 60 mg kg−1 in rosehip and less than 40 mg kg−1 in broccoli; the results are consistent with the literature considering the humidity of the samples and interspecies variations.
3.2. Supercritical Fluid Extraction (SFE)
In the first series of the extraction runs, nine sets of process parameters were evaluated—temperatures of 35, 55, and 75 °C were combined with pressures of 10, 20, and 40 MPa. None of the extraction variants was able to obtain a measurable yield of galactolipids. The possible explanation was an insufficient polarity of pure carbon dioxide; therefore, another set of experiments was conducted using ethanol as a cosolvent. The literature data highlight that higher pressures strongly improve the solvating properties of carbon dioxide; therefore, only 40 MPa was applied to limit the number of experiments [14]. Table 3 presents tested parameters and summarizes the results of this part of the work. The cosolvent flow values was selected to make up 0%, 10%, and 20% of total flow.

Table 3.
Impact of supercritical fluid extraction parameters on the efficiency of MGDGs extraction from rosehip pomace. All the experiments were performed at the pressure of 40 MPa. The letters denote statistical significance of the differences (p ≤ 0.05).
The use of a cosolvent allowed the extraction of significant amounts of galactolipids and the yields obtained were in the range from 37 to 87%. The flow rate of ethanol appeared to be the crucial parameter for the process efficiency: the best results were observed for the 0.4 mL min−1 share of the co-solvent, while pure carbon dioxide was unable to extract any galactolipids. The two-fold increase of ethanol content (0.2 vs. 0.4 mL min−1) resulted in improvement of the yield obtained higher than 100%; this may suggest that obtaining proper polarity of CO2–ethanol mixture may be more significant than simply adding ethanol to the system.
The possibility of implementation of supercritical fluids for isolation of galactolipids from other matrices was reported by few teams. The paper by Ijima et al. described experiments on extraction of galactolipids from spinach (Spinacia oleracea) leaves by SFE with and without a modifier [22]. Similarly to our results, the use of pure CO2 did not allow MGDG isolation, while ethanol as a cosolvent gave good results. The authors used a different matrix and tested only one parameter set; thus, full comparison of results is not possible. The work by Yang et al. showed that supercritical CO2 and ethanol mixture was able to extract over 80% of lipids from Cyanobacterium Arthrospira, while pure carbon dioxide gave negligible yield [23]. The possibility of application of less orthodox solvent was reported by Nekrasov and coworkers, who successfully extracted galactolipids from fern fronds with dimethyl ether–water–ethanol mixture in the supercritical state [24].
3.3. Ultrasound-Assisted Extraction (UAE)
The experiments on the possible use of UAE in the isolation of galactolipids began with the evaluation of twelve commonly used solvents of various polarities. The rosehip pomace was mixed with them in a ratio of 1:10 (m/v) and subsequently extracted in two variants: with and without sonication. The yields of MGDGs obtained are listed in Table 4.

Table 4.
Impact of sonication on the efficiency of MGDG extraction from rosehip pomace using various solvents. All yields in mg per kg of pomace; sonication time was 5 min. An asterisk next to the solvent name denotes a significant difference between sonicated and control samples, while letters stand for differences between solvents (both at p ≤ 0.05).
Apart from the most and the least polar (water and hexane, respectively), all the solvents were able to extract MGDGs to some extent. The best results were obtained for chlorinated hydrocarbons, yet even dichloromethane, which is much safer for people and the environment than chloroform, can be hardly considered a green solvent. Therefore, the solvent characterized by a lower extraction efficiency, but of lesser environmental and health impact, must be chosen. Ethanol combines low toxicity with a low cost; thus it was chosen for the further experiments. Additionally, its use as a cosolvent during SFE allows a systematic comparison of these two techniques. The sonication made it possible to significantly increase the yield of extraction in all the solvents evaluated, which indicated the potential of the technique.
The next part of the experiments was the investigation of the impact of the sonication time and ratio of pomace and ethanol on the efficiency of the extraction—the results are presented in Figure 2. Two solid–liquid ratios and three sonication times were investigated. The control samples were extracted with ethanol for the same amount of time without any means of a process intensification. As was stated before, the samples were shaken for 1 min prior to removal of the solvent.
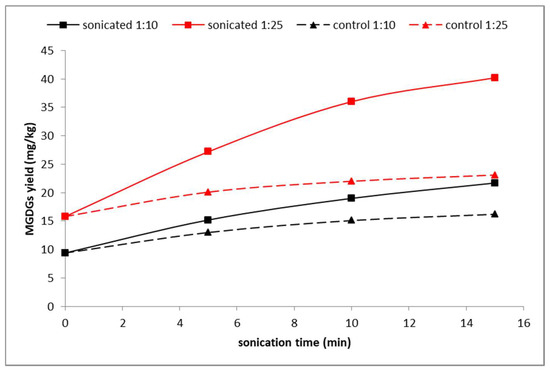
Figure 2.
Impact of the sonication time and the solid–liquid ratio (m/V) on the efficiency of MGDGs extraction from rosehip pomace with ethanol.
The amphiphilic character of galactolipids was visible during the selection of the appropriate solvent. Both highly polar (water) and non-polar (hexane) solvents were unsuitable for the extraction. The impact of the sonication time on the yield presented in Figure 2 is in accordance with the extraction curves reported by other authors [26,27,28]. Sonication leads to the disintegration of cells on the surface of the matrix particles, which at the beginning of the process leads to a significant increase in yield, although longer treatment does not have such an effect, as the majority of the cells are already broken. The comparison of the extraction curves suggests that differences between the sonicated and the control samples are mainly the result of improvement of the mass transfer. The bigger gap between sonicated and control yields in the case of 1:25 ratio is supposedly the result of a limited solubility of galactolipids in ethanol. The sonication of the rosehip samples led to a substantial improvement in the yields obtained (up to 74%) with a relatively low cost of implementation. The abovementioned facts indicate that the method could be suitable to improve technological aspects as well as the sustainability of the process. Evidently, the implementation of UAE for an industrial-scale operation would require optimization as such processes are not easily scaled up.
3.4. Comparison of the Techniques and General Remarks
In order to compare efficiency of the extraction methods, an additional UAE run using the same processing time (1 h) and the solid–liquid ratio (4.8 m/V) was performed along with control sample. The results are presented in Figure 3.
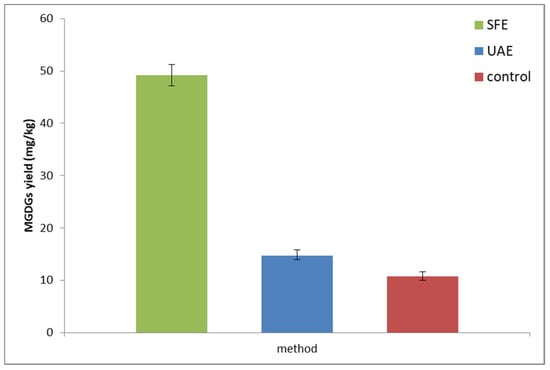
Figure 3.
The comparison of MGDGs extraction efficiency using different techniques with the same processing time (1 h) and solid–liquid ratio (4.8, m/V). SFE was conducted at a temperature of 55 °C and at 40 MPa. There were significant differences (p ≤ 0.05) between all the samples tested.
The use of such low solid–liquid ratio significantly hindered the extraction process, as the limited solubility of MGDGs played more crucial role. Nevertheless, about 36% improvement in efficiency was observed after use of the sonication. SFE yields were approx. 5-fold higher than the control sample, proving the superiority of this technique. On the other hand, the use of SFE is connected with high initial costs of the machinery, while UAE can be considered as a very low-priced and easy-to-implement technology. Nevertheless, we believe that the presented data are a satisfactory proof of suitability of green extraction techniques for isolation of galactolipids from waste materials of fruit and vegetable processing.
Rosehip preparations are often sold in the form of capsules filled with milled fruits instead of extract. Such an approach is undoubtedly a much cheaper alternative; however, it is disadvantageous from a bioavailability point of view. The highest intestinal absorption of galactolipids would probably be achieved after ingestion of encapsulated or micronized extracts, while absorption after administration of the milled tissue would probably be very low [29,30].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app112412088/s1. Figure S1: The chromatograms of MGDGs standard mixture on various m/z values.
Author Contributions
Conceptualization, Ł.W. and S.S.; methodology, Ł.W. and K.M.; formal analysis, Ł.W. and M.W.; investigation, Ł.W. and M.W.; writing—original draft preparation, Ł.W. and M.W.; writing—review and editing, K.M. and S.S.; visualization, Ł.W.; supervision, K.M. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hölzl, G.; Dörmann, P. Structure and function of glycoglycerolipids in plants and bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Galactolipids as potential health promoting compounds in vegetable foods. Recent Pat. Food Nutr. Agric. 2009, 1, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Weatherby, K.; Carter, D. Chromera velia: The missing link in the evolution of parasitism. Adv. Appl. Microbiol. 2013, 85, 119–144. [Google Scholar] [PubMed]
- Wang, R.; Furomoto, T.; Motoyama, K.; Okazaki, K.; Kondo, A.; Fukui, H. Possible anti-tumor promoters in Spinacia oleracea (spinach) and comparison of their contents among cultivars. Biosci. Biotechnol. Biochem. 2002, 66, 248–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuriyama, I.; Musumi, K.; Yonezawa, Y.; Takemura, M.; Maeda, N.; Iijima, H.; Hada, T.; Yoshida, H.; Mizushina, Y. Inhibitory effects of glycolipids fraction from spinach on mammalian DNA polymerase activity and human cancer cell proliferation. J. Nutr. Biochem. 2005, 16, 594–601. [Google Scholar] [CrossRef]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina—Rose hip pharmacological ingredients and molecular mechanics counteracting osteoarthritis—A systematic review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef]
- Warholm, O.; Skaar, S.; Hedman, E. The effects of a standardized herbal remedy made from a subtype of Rosa canina in patients with osteoarthritis: A double-blind, randomized, placebo—controlled clinical trial. Curr. Therap. Res. 2003, 64, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Larsen, E.; Kharazmi, A.; Christensen, L.P.; Christensen, S.B. An antiinflammatory galactolipid from Rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J. Nat. Prod. 2003, 66, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Zábranská, M.; Vrkoslav, V.; Sobotníková, J.; Cvačka, J. Analysis of plant galactolipids by reversed-phase high-performance liquid chromatography/mass spectrometry with accurate mass measurement. Chem. Phys. Lipids 2012, 165, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Sep. Sci. Technol. 2016, 51, 1472–1479. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S.; Jędrzejczak, R. The application of supercritical carbon dioxide and ethanol for the extraction of phenolic compounds from chokeberry pomace. Appl. Sci. 2017, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of triterpenic acids and phytosterols from apple pomace with supercritical carbon dioxide: Impact of process parameters, modelling of kinetics, and scaling-up study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, Ł.; Połaska, M.; Marszałek, K.; Skąpska, S. Photosensitizing furocoumarins: Content in plant matrices and kinetics of supercritical carbon dioxide extraction. Molecules 2020, 25, 3805. [Google Scholar] [CrossRef]
- Lee, S.J.; Song, Y.; Chung, M.Y.; Kim, I.H.; Kim, B.H. Isolation and compositional analysis of galactoglycerolipids from perilla [Perilla fructescens (L.) Britton] leaves and comparison to the galactoglycerolipids from spinach and parsley. J. Food Sci. 2020, 85, 4271–4280. [Google Scholar] [CrossRef]
- Chrubasik, C.; Wiesner, L.; Black, A.; Müller-Ladner, U.; Chrubasik, S. A one-year survey on the use of a powder from Rosa canina lito in acute exacerbations of chronic pain. Phytother. Res. 2008, 22, 1141–1148. [Google Scholar] [CrossRef]
- Iijima, H.; Musumi, K.; Hada, T.; Maeda, N.; Yonezawa, Y.; Yoshida, H.; Mizushina, Y. Inhibitory effect of monogalactosyldiacylglycerol, extracted from spinach using supercritical CO2, on mammalian DNA Polymerase activity. J. Agric. Food Chem. 2006, 54, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported supercritical CO2 enhances extraction of γ-linolenic acid from the cyanobacterium Arthrospira (Spirulina) platensis and bioactivity evaluation of the molecule in zebrafish. Mar. Drugs 2019, 17, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasov, E.V.; Tallon, S.J.; Vyssotski, M.V.; Catchpole, O.J. Extraction of lipids from New Zealand fern fronds using near-critical dimethyl ether and dimethyl ether-water-ethanol mixtures. J. Supercrit. Fluids 2021, 170, 105137. [Google Scholar] [CrossRef]
- Welton, T.; Reinchardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Wienheim, Germany, 2003. [Google Scholar]
- Da Porto, C.; Natolino, A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018, 137–143. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Sergio, C.S.A.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Natolino, A.; Da Porto, C. Kinetic models for conventional and ultrasound assistant extraction of polyphenols from defatted fresh and distilled grape marc and its main components skins and seeds. Chem. Eng. Res. Des. 2020, 156, 1–12. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Park, Y. Controlling lipid bioavailability through physiochemical and structural approaches. Crit. Rev. Food Sci. Nutr. 2009, 49, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Pellegrini, N. An integrated look at the effect of structure on nutrients bioavailability in plant foods. J. Sci. Food Agric. 2019, 99, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).