The Enteric Nervous System and the Microenvironment of the Gut: The Translational Aspects of the Microbiome-Gut-Brain Axis
Abstract
:1. The Immune Microenvironment of the Gut
2. The Heterogeneity of Muscularis Macrophages
3. Neurons and Neurochemistry of the Enteric Nervous System
4. Intraganglionic Macrophages and the Myenteric Plexus Barrier
5. The Neuroimmunological Crosstalk in the Gut: The Central Role of the ENS
6. The Microbiome-Gut-Brain Axis
7. Further Strategies on Microbiome Research
Author Contributions
Funding
Conflicts of Interest
References
- Kmieć, Z.; Cyman, M.; Ślebioda, T.J. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv. Med. Sci. 2017, 62, 1–16. [Google Scholar] [CrossRef]
- Denning, T.L.; Norris, B.A.; Medina-Contreras, O.; Manicassamy, S.; Geem, D.; Madan, R.; Karp, C.L.; Pulendran, B. Functional specializations of intestinal dendritic cell and macrophage subsets that control TH -17 and T regulatory responses is dependent on the T:APC ratio, source of mouse strain and regional localization. J. Immunol. 2011, 18, 733–747. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Kurashima, Y.; Goto, Y.; Kiyono, H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur. J. Immunol. 2013, 43, 3108–3115. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]
- Farache, J.; Koren, I.; Milo, I.; Gurevich, I.; Kim, K.W.; Zigmond, E.; Furtado, G.C.; Lira, S.A.; Shakhar, G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 2013, 38, 581–595. [Google Scholar] [CrossRef] [Green Version]
- Mazzini, E.; Massimiliano, L.; Penna, G.; Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 2014, 40, 248–261. [Google Scholar] [CrossRef] [Green Version]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and function of intestinal macrophages. Front. Immunol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Berin, M.C. Mechanisms of oral tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Lemos, M.P.; Fan, L.; Lo, D.; Laufer, T.M. CD8α + and CD11b + dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J. Immunol. 2003, 171, 5077–5084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahenzli, J.; Köller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Cao, A.T.; Cong, Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin. Cancer Biol. 2013, 23, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandiyan, P.; Bhaskaran, N.; Zou, M.; Schneider, E.; Jayaraman, S.; Huehn, J. Microbiome Dependent regulation of tregs and Th17 cells in mucosa. Front. Immunol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 477–512. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Valdez, P.A.; Danilenko, D.M.; Hu, Y.; Sa, S.M.; Gong, Q.; Abbas, A.R.; Modrusan, Z.; Ghilardi, N.; De Sauvage, F.J.; et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008, 14, 282–289. [Google Scholar] [CrossRef]
- Hong, M.; Liao, Y.; Liang, J.; Chen, X.; Li, S.; Liu, W.; Gao, C.; Zhong, Z.; Kong, D.; Deng, J.; et al. Immunomodulation of human CD19 + CD25 high regulatory B cells via Th17/Foxp3 regulatory T cells and Th1/Th2 cytokines. Hum. Immunol. 2019, 80, 863–870. [Google Scholar] [CrossRef]
- Sattler, S.; Ling, G.S.; Xu, D.; Hussaarts, L.; Romaine, A.; Zhao, H.; Fossati-Jimack, L.; Malik, T.; Cook, H.T.; Botto, M.; et al. IL-10-producing regulatory B cells induced by IL-33 (BregIL-33) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 2014, 50, 107–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol. Motil. 2020, 32, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dora, D.; Ferenczi, S.; Stavely, R.; Toth, V.E.; Varga, Z.V.; Kovacs, T.; Bodi, I.; Hotta, R.; Kovacs, K.J.; Goldstein, A.M.; et al. Evidence of a myenteric plexus barrier and its macrophage-dependent degradation during murine colitis: Implications in enteric neuroinflammation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, T.N.; Houston, S.A.; Wemyss, K.; Bridgeman, H.M.; Barbera, T.A.; Zangerle-Murray, T.; Strangward, P.; Ridley, A.J.L.; Wang, P.; Tamoutounour, S.; et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med. 2018, 215, 1507–1518. [Google Scholar] [CrossRef]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Smidt, I.; Boeckx, B.; Dierckx de Casterlé, I.; et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2018, 175, 400–415.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.M.; Mucida, D.; et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014, 158, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, R.J.; Powley, T.L. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton. Neurosci. 2012, 169, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Qian, A.; Oetjen, L.K.; Yu, W.; Yang, P.; Feng, J.; Xie, Z.; Liu, S.; Yin, S.; Dryn, D.; et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 2018, 49, 107–119.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehmer, A.; Schrödl, F.; Neuhuber, W. Morphological classifications of enteric neurons—100 years after Dogiel. Anat. Embryol. 1999, 200, 125–135. [Google Scholar] [CrossRef]
- Hirst, G.D.S.; Holman, M.E.; Spence, I. Two types of neurones in the Myenteric Plexus of duodenum in the guinea-pig. J. Physiol. 1974, 236, 303–326. [Google Scholar] [CrossRef]
- Nishi, S.; North, R.A. Intracellular recording from the Myenteric Plexus of the guinea-pig ileum. J. Physiol. 1973, 231, 471–491. [Google Scholar] [CrossRef]
- Wood, J.D.; Mayer, C.J. Intracellular study of electrical activity of Auerbach’s Plexus in guinea-pig small intestine. Pflüg. Arch. Eur. J. Physiol. 1978, 374, 265–275. [Google Scholar] [CrossRef]
- Mongardi Fantaguzzi, C.; Thacker, M.; Chiocchetti, R.; Furness, J.B. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009, 336, 179–189. [Google Scholar] [CrossRef]
- Qu, Z.D.; Thacker, M.; Castelucci, P.; Bagyanszki, M.; Epstein, M.L.; Furness, J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res 2008, 334, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Nestor-Kalinoski, A.; Smith-Edwards, K.M.; Meerschaert, K.; Margiotta, J.F.; Rajwa, B.; Davis, B.M.; Howard, M.J. Unique neural circuit connectivity of mouse proximal, middle, and distal colon defines regional colonic motor patterns. Cell. Mol. Gastroenterol. Hepatol. 2021. ahead of print. [Google Scholar] [CrossRef]
- Yoo, B.B.; Mazmanian, S.K. The enteric network: Interactions between the immune and nervous systems of the gut. Immunity 2017, 46, 910. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Sun, Q.; Sun, X.; Chen, D.; Wei, C.; Yu, X.; Liu, C.; Li, Y.; Li, J. Activation of GABAA receptors in colon epithelium exacerbates acute colitis. Front. Immunol. 2018, 9, 987. [Google Scholar] [CrossRef] [Green Version]
- Brookes, S.J.H. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 2001, 262, 58–70. [Google Scholar] [CrossRef]
- Costa, M.; Brookes, S.J.H.; Steele, P.A.; Gibbins, I.; Burcher, E.; Kandiah, C.J. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 1996, 75, 949–967. [Google Scholar] [CrossRef]
- Lomax, A.E.; Furness, J.B. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000, 302, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Schemann, M.; Reiche, D.; Michel, K. Enteric pathways in the stomach. Anat. Rec. 2001, 262, 47–57. [Google Scholar] [CrossRef]
- Li, Z.; Hao, M.M.; Van Den Haute, C.; Baekelandt, V.; Boesmans, W.; Berghe, P. Vanden regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife 2019, 8, 1–27. [Google Scholar] [CrossRef]
- Brehmer, A. Classification of human enteric neurons. Histochem. Cell Biol. 2021, 156, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Schemann, M.; Neunlist, M. The human enteric nervous system. Neurogastroenterol. Motil. 2004, 16 (Suppl. S1), 55–59. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.D.; López, S.H.; Sengupta, R.; Shenoy, A.; Schneider, S.; Wright, C.M.; Feldman, M.; Furth, E.; Valdivieso, F.; Lemke, A.; et al. Robust, 3-dimensional visualization of human colon enteric nervous system without tissue sectioning. Gastroenterology 2020, 158, 2221. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Kunze, W.A.A.; Bertrand, P.P.; Clerc, N.; Bornstein, J.C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 1998, 54, 1–18. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Spencer, N.J.; Smith, T.K. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol. 2004, 558, 577–596. [Google Scholar] [CrossRef]
- May-Zhang, A.A.; Tycksen, E.; Southard-Smith, A.N.; Deal, K.K.; Benthal, J.T.; Buehler, D.P.; Adam, M.; Simmons, A.J.; Monaghan, J.R.; Matlock, B.K.; et al. Combinatorial transcriptional profiling of mouse and human enteric neurons identifies shared and disparate subtypes in situ. Gastroenterology 2021, 160, 755–770.e26. [Google Scholar] [CrossRef]
- Morarach, K.; Mikhailova, A.; Knoflach, V.; Memic, F.; Kumar, R.; Li, W.; Ernfors, P.; Marklund, U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA eequencing. Nat. Neurosci. 2021, 24, 34–46. [Google Scholar] [CrossRef]
- Wright, C.M.; Schneider, S.; Smith-Edwards, K.M.; Mafra, F.; Leembruggen, A.J.L.; Gonzalez, M.V.; Kothakapa, D.R.; Anderson, J.B.; Maguire, B.A.; Gao, T.; et al. ScRNA-seq reveals new enteric nervous system roles for GDNF, NRTN, and TBX3. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1548–1592.e1. [Google Scholar] [CrossRef]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular architecture of the mouse nervous system. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, H. Macrophages in the external muscle layers of mammalian intestines. Histol. Histopathol. 1995, 10, 719–736. [Google Scholar] [PubMed]
- Avetisyan, M.; Rood, J.E.; Lopez, S.H.; Sengupta, R.; Wright-Jin, E.; Dougherty, J.D.; Behrens, E.M.; Heuckeroth, R.O. Muscularis macrophage development in the absence of an enteric nervous system. Proc. Natl. Acad. Sci. USA 2018, 115, 4696–4701. [Google Scholar] [CrossRef] [Green Version]
- Dora, D.; Arciero, E.; Hotta, R.; Barad, C.; Bhave, S.; Kovacs, T.; Balic, A.; Goldstein, A.M.; Nagy, N. Intraganglionic macrophages: A new population of cells in the enteric ganglia. J. Anat. 2018, 233, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.; Micci, M.A.; Leser, J.; Shin, C.; Tang, S.C.; Fu, Y.Y.; Liu, L.; Li, Q.; Saha, M.; Li, C.; et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E3709–E3718. [Google Scholar] [CrossRef] [Green Version]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ydens, E.; Amann, L.; Asselbergh, B.; Scott, C.L.; Martens, L.; Sichien, D.; Mossad, O.; Blank, T.; De Prijck, S.; Low, D.; et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 2020, 23, 676–689. [Google Scholar] [CrossRef]
- Monaco, S.; Gehrmann, J.; Raivich, G.; Kreutzberg, G.W. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J. Neurocytol. 1992, 21, 623–634. [Google Scholar] [CrossRef]
- Gershon, M.D.; Bursztajn, S. Properties of the enteric nervous system: Limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J. Comp. Neurol. 1978, 180, 467–487. [Google Scholar] [CrossRef]
- Kiernan, J.A. Vascular permeability in the peripheral autonomic and somatic nervous systems: Controversial aspects and comparisons with the blood-brain barrier. Microsc. Res. Tech. 1996, 35, 122–136. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s Disease a gliopathy? Rev. Enteric Nerv. Syst. Heal. Dis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spear, E.T.; Mawe, G.M. Enteric neuroplasticity and dysmotility in inflammatory disease: Key players and possible therapeutic targets. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G853–G861. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Abalo, R.; Nurgali, K. Targeting enteric neurons and plexitis for the management of inflammatory Bowel disease. Curr. Drug Targets 2020, 21, 1428–1439. [Google Scholar] [CrossRef]
- Nagy, N.; Goldstein, A.M. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 2017, 66, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M.A.; Leboeuf, M.; et al. Origin of the lamina propria dendritic cell network. Immunity 2009, 31, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Chalazonitis, A.; Kessler, J.A. Pleiotropic Effects of the bone morphogenetic proteins on development of the enteric nervous system. Dev. Neurobiol. 2012, 72, 843–856. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.M.; Brewer, K.C.; Doyle, A.M.; Nagy, N.; Roberts, D.J. BMP Signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech. Dev. 2005, 122, 821–833. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Abrams, G.D.; Bishop, J.E. Effect of the normal microbial flora on gastrointestinal motility. Proc. Soc. Exp. Biol. Med. 1967, 126, 301–304. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology 2012, 143, 1006–1016.e4. [Google Scholar] [CrossRef] [Green Version]
- Pullinger, G.D.; van Diemen, P.M.; Carnell, S.C.; Davies, H.; Lyte, M.; Stevens, M.P. 6-Hydroxydopamine-mediated release of norepinephrine increases faecal excretion of salmonella enterica serovar typhimurium in pigs. Vet. Res. 2010, 41, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, I.P. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex; Dover Publications: Mineola, NY, USA, 1904. [Google Scholar]
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Schneiderhan, J.; Master-Hunter, T.; Locke, A. Targeting gut flora to treat and prevent disease. J. Fam. Pract. 2016, 65, 34–38. [Google Scholar]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain. Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.V.-A.; Foster, K.R. Why does the microbiome affect behaviour? Nat. Rev. Microbiol. 2018, 16, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 2018, 172, 500–516.e16. [Google Scholar] [CrossRef] [Green Version]
- Slykerman, R.F.; Thompson, J.; Waldie, K.E.; Murphy, R.; Wall, C.; Mitchell, E.A. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017, 106, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Yano, J.M.; Fung, T.C.; Hsiao, E.Y. The microbiome and host behavior. Annu. Rev. Neurosci. 2017, 40, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. Elife 2017, 6, e25887. [Google Scholar] [CrossRef]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Kantak, P.A.; Bobrow, D.N.; Nyby, J.G. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus Rhamnosus GG). Behav. Pharmacol. 2014, 25, 71–79. [Google Scholar] [CrossRef]
- Dickerson, F.; Severance, E.; Yolken, R. The microbiome, immunity, and Schizophrenia and Bipolar Disorder. Brain. Behav. Immun. 2017, 62, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.D.N.; Viscogliosi, E.; Delhaes, L. The lung mycobiome: An emerging field of the human respiratory microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The role of lung and gut microbiota in the pathology of asthma. Immunity 2020, 52, 241. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Chehrazi-Raffle, A.; Placencio-Hickok, V.; Guan, M.; Hendifar, A.; Salgia, R. The gut microbiome and response to immune checkpoint inhibitors: Preclinical and clinical strategies. Clin. Transl. Med. 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, Y.; Wang, Y.; Xu, L.; Guo, Y.; Wang, Y.; Wang, L.; Guo, C. Oral administration of bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived Interleukin 12. Oncoimmunology 2021, 10, 1868122. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with Anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Rook, G.A. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA 2013, 110, 18360–18367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, A.; Bekeschus, S.; Bröker, B.M.; Schleibinger, H.; Razavi, B.; Assadian, O. Maintaining health by balancing microbial exposure and prevention of infection: The hygiene hypothesis versus the hypothesis of early immune challenge. J. Hosp. Infect. 2013, 83 (Suppl. S1), S29–S34. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to Anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.M.; DeFazio, J.R.; Hyoju, S.K.; Sangani, K.; Keskey, R.; Krezalek, M.A.; Khodarev, N.N.; Sangwan, N.; Christley, S.; Harris, K.G.; et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nat. Commun. 2020, 11, 2354. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Lleal, M.; Sarrabayrouse, G.; Willamil, J.; Santiago, A.; Pozuelo, M.; Manichanh, C. A Single faecal microbiota transplantation modulates the microbiome and improves clinical manifestations in a rat model of colitis. EBioMedicine 2019, 48, 630. [Google Scholar] [CrossRef] [Green Version]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narula, N.; Kassam, Z.; Yuan, Y.; Colombel, J.F.; Ponsioen, C.; Reinisch, W.; Moayyedi, P. Systematic review and meta-analysis: Fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
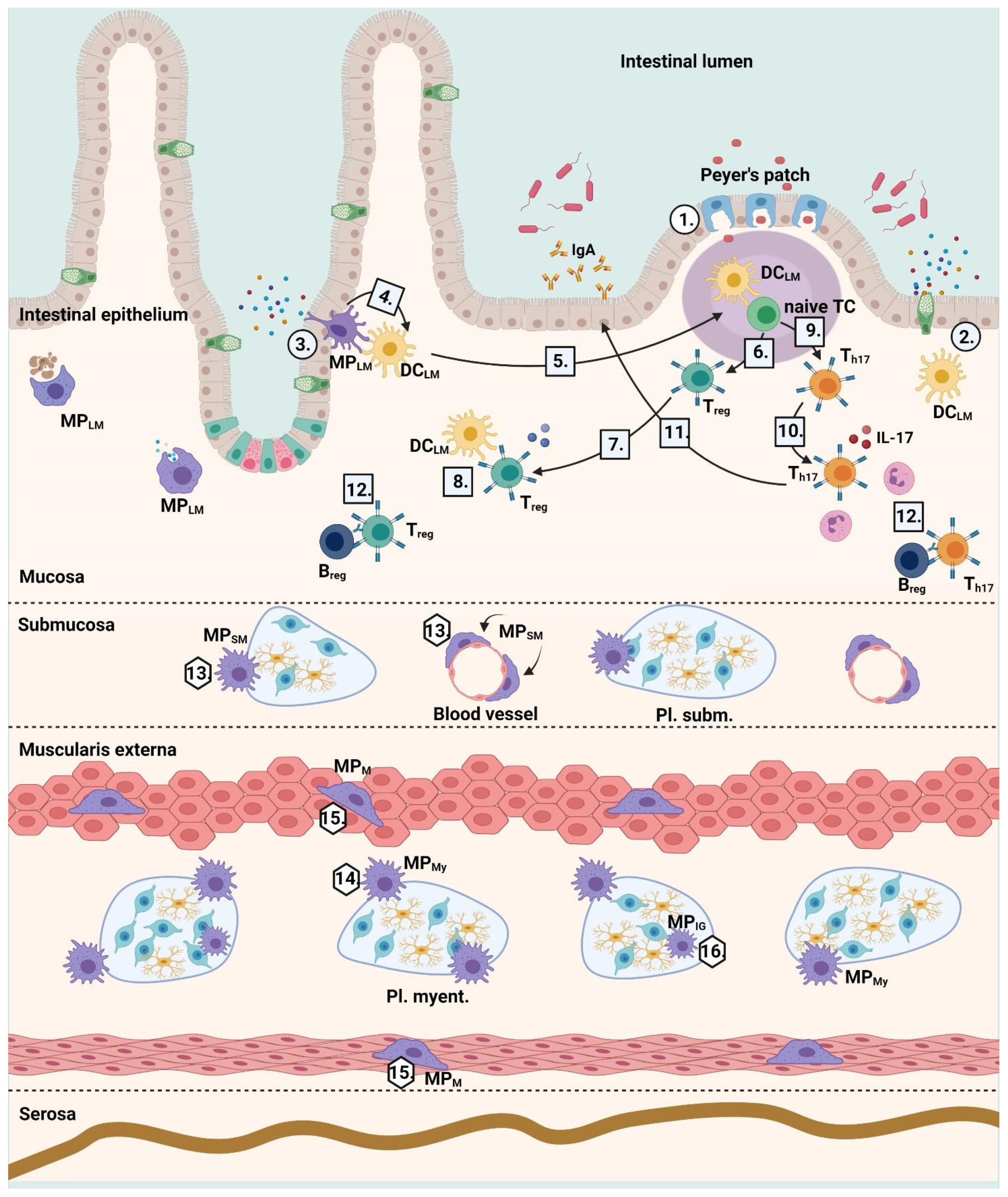
| Cell Type | DCLM | MPLM | MPSM | MPM | MPMy | MPIG |
|---|---|---|---|---|---|---|
| Markers | CD45 MHCII CD11b F4/80 CX3CR1 +/− CD11c CD103 +/− connexin-43 | CD45 MHCII CD11b F4/80 CX3CR1low/+ connexin-43 | CD45 MHCII CD11b F4/80 CX3CR1 low/+ CD11c low/+ | CD45 MHCII CD11b F4/80 CX3CR1 Retn1a Mrc1 CD163 TRPV4 BMP2 | MPM markers glycine-, nicotinic acetylcholine-, adrenergic-, purine receptors | MPM markers Iba1, CSF1R |
| Morphology | amoeboid | amoeboid | amoeboid | bipolar star shaped | star shaped | star shaped |
| Presence | Lamina propria mucosae | Lamina propria mucosae | Submucosa | Muscularis | Myenteric plexus | Within the ECM barrier of myenteric ganglions |
| Role | antigen presentation, TC activation | phagocytosis of antigens | supporting of blood vessels and enteral neurons regulating intestinal secretion tissue homeostasis | regulation of gastrointestinal motility | development of the myenteric plexus, survival of enteric neurons | phagocytosis of apoptotic ganglionic cells |
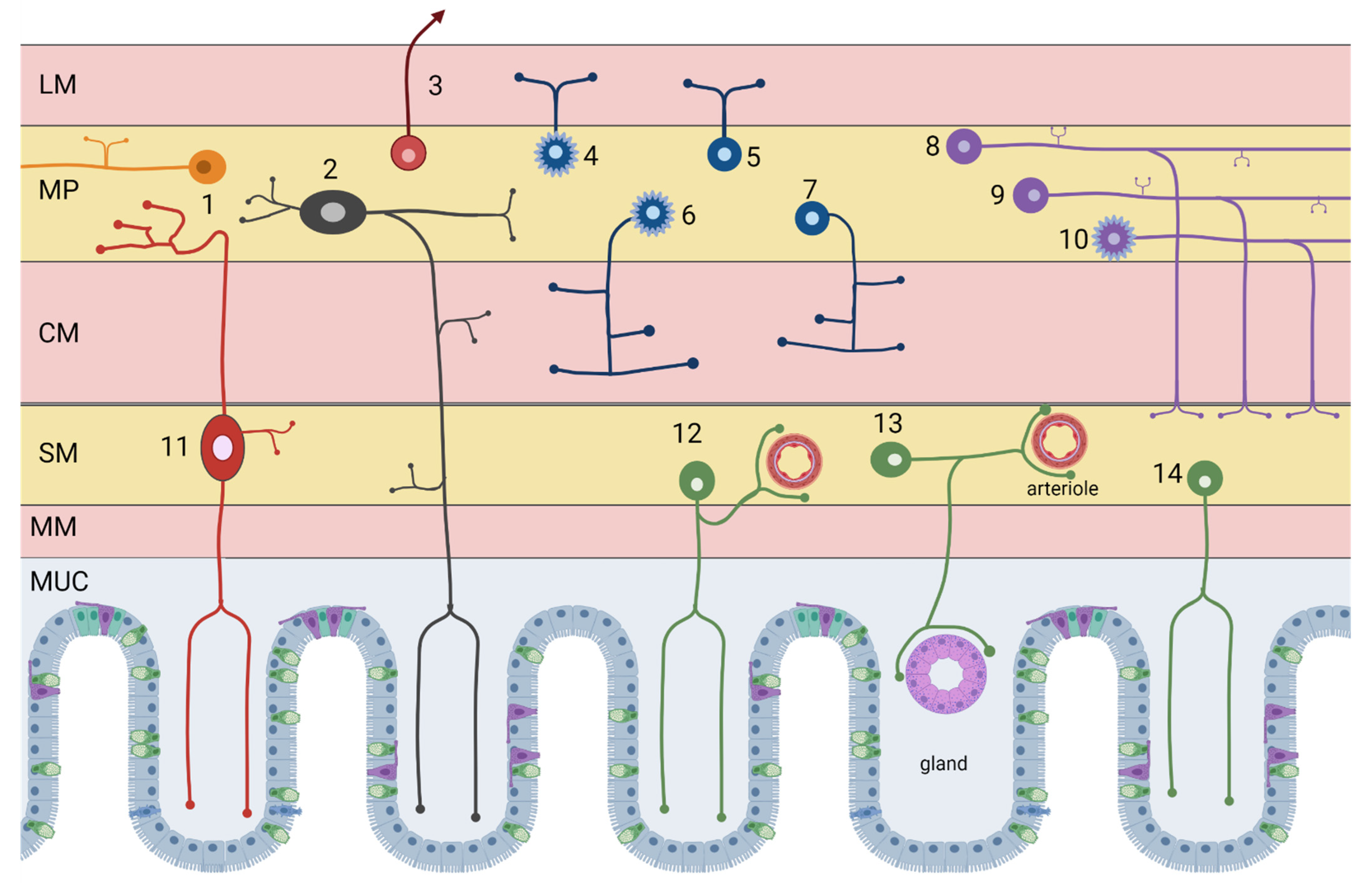
| # ID | Neuron Type | Incidence | Neurotransmitters |
|---|---|---|---|
| Myenteric neurons | |||
| 6 | Excitatory circular smooth muscle motor neurons | 12% | Acetylcholine/GABA (enkephalin, tachykinins) |
| 4 | Excitatory longitudinal smooth muscle motor neurons | 25% | Acetylcholine/Calretinin (tachykinins) |
| 7 | Inhibitory circular smooth muscle motor neurons | 16% | NOS/VIP (PACAP) |
| 5 | Inhibitory longitudinal smooth muscle motor neurons | ~3% | NOS/VIP/GABA |
| 1 | Ascending interneurons (local reflex circuit) | 5% | Acetylcholine/Calretinin (tachykinins) |
| 8 | Descending interneurons (local reflex circuit) | 5% | Acetylcholine/NOS/VIP |
| 9 | Descending interneurons (secretomotor reflex circuit) | 3% | Acetylcholine/Serotonin |
| 10 | Descending interneurons (migrating myoelectric complex) | 4% | Acethylcholine/Somatostatin |
| 2 | Myenteric intrinsic primary afferent neurons (IPAN) | 27% | Acetylcholine/Calbindin (tachykinins) |
| 3 | Intestinofugal neurons | 1% | Acetylcholine/VIP (CCK, enkephalin) |
| Submucosal neurons | |||
| 12 | Non-cholinergic secretomotor/vasodilator neurons | 45% | VIP/Galanin |
| 13 | Cholinergic secretomotor/vasodilator neurons | 15% | Acetylcholine/Calretinin |
| 14 | Cholinergic secretomotor non-vasodilator neurons | 29% | Acetylcholine/NPY/CCK (somatostatin, CGRP) |
| 11 | Submucosal intrinsic primary afferent neurons | 11% | Acetylcholine/Calbindin (tachykinins) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogor, F.; Kovács, T.; Lohinai, Z.; Dora, D. The Enteric Nervous System and the Microenvironment of the Gut: The Translational Aspects of the Microbiome-Gut-Brain Axis. Appl. Sci. 2021, 11, 12000. https://doi.org/10.3390/app112412000
Mogor F, Kovács T, Lohinai Z, Dora D. The Enteric Nervous System and the Microenvironment of the Gut: The Translational Aspects of the Microbiome-Gut-Brain Axis. Applied Sciences. 2021; 11(24):12000. https://doi.org/10.3390/app112412000
Chicago/Turabian StyleMogor, Fruzsina, Tamás Kovács, Zoltan Lohinai, and David Dora. 2021. "The Enteric Nervous System and the Microenvironment of the Gut: The Translational Aspects of the Microbiome-Gut-Brain Axis" Applied Sciences 11, no. 24: 12000. https://doi.org/10.3390/app112412000
APA StyleMogor, F., Kovács, T., Lohinai, Z., & Dora, D. (2021). The Enteric Nervous System and the Microenvironment of the Gut: The Translational Aspects of the Microbiome-Gut-Brain Axis. Applied Sciences, 11(24), 12000. https://doi.org/10.3390/app112412000







