Abstract
Cork-ring widths have been extensively used in dendroecological studies assessing the relationship between cork growth patterns and climate (precipitation and temperature). Generally, cork growth is assumed as a proxy for stem diameter growth to address cork oak (Quercus suber L.) growth sensitivity to climate and cork yield modeling. Cork growth represents a large part of stem radial increment in this species due to the enhanced activity of phellogen when compared to the cambium activity; thus, similar inter-annual variations of cork-ring widths and tree diameter growth might be expected. However, so far, the influence of rainfall and temperature on stem diameter growth has scarcely been addressed; moreover, it is still not clear whether tree size relates, and in what way, to the variations in radial growth of cork and stem diameter and whether these reflect (proportional) quantitative variations in stem basal area growth. In this study, we computed the annual growth of cork and of stem diameter at breast-height in data series of 47 trees, from 2000 to 2012, corresponding to a full cork production cycle. Results showed a tight link between cork-ring width and stem diameter growth indices. However, while cork growth strongly correlated with climate conditions in autumn–winter prior to the growing season, stem diameter growth correlated with climate conditions of the current growing season, and, more importantly, it was tree size-related. The extrapolation from cork-ring increments to stem basal area growth is likely to progressively underestimate tree growth and biomass increment in larger cork oaks and to further bias it due to climate change effects in the Mediterranean region.
1. Introduction
Climate changes are expected to cause more extreme drought conditions in southern Europe [1]. The evergreen woodlands of cork oak (Quercus suber L.) of southwestern Iberia (in Portugal and Spain) are climatically sensitive and are among the most vulnerable forest ecosystems [2]. Despite its strategies to tolerate drought [3,4], the species risks failing to cope with increasing temperatures and longer and more frequent summer droughts in the near future that will most likely aggravate water stress conditions and will eventually influence cork oak growth rates and patterns [5].
Climate change effects on cork oak growth, at tree or stand level, have been exclusively and extensively addressed—though not fully explored—in numerous dendroecological studies on the relationship between interannual fluctuations of cork-ring widths and climate parameters and their impact on the cork growth rates and yield [5,6,7]. The widely held assumption of these studies is that cork-ring width (or cork growth) at breast height is a reliable predictor of tree stem diameter (and basal area) growth, since cork represents a large part of cork oak’s radial increment [8], and the contribution of a “non-cork” (wood) growth to that increment is ignored. On one hand, such a consensus derives from the difficulty and scarcity of measurements of wood growth, which is slow and results in narrow and undefined tree rings [9], while cork growth shows wide and clearly defined rings; on the other hand, few studies have addressed stem radial growth sensitivity to climate [8,10,11].
In harvested cork oaks, stem diameter growth has two main components—wood growth and cork (bark) growth—resulting from the independent activities of two meristems, the cambium, producing xylem cells inward, and the phellogen, producing cork cell outward, respectively. In response to cork harvesting, cork growth rates are increased by 400% on the first 2 years, in relation to rates at the end of cork production cycles of 9–12 years, while wood growth rates decrease by more than 50% [6,8,12,13]. It has been reported that cork growth is artificially enhanced at the expense of wood growth [12,13], eventually affecting stem diameter growth, but so far, the stem diameter growth in harvested cork oaks has been poorly addressed [8,10]. Moreover, climatic influence on cork-ring width is relatively poorly expressed [5,6,7]—only strongly linked to the previous autumn–winter rainfall. Thus, cork growth variations may be insufficient for an adequate interpretation of climate change effects on stem diameter growth, considering that the activity of the phellogen is less sensitive to climatic influences than that of the cambium [8] and particularly unsuitable because cork-ring area growth responses are possibly biased by the size-related stem radial growth dynamics [11].
In the present study, we measured the annual stem radial growth and cork-ring width (cork growth) of 47 mature cork oaks over a 12-year production cycle. By de-trending the growth curves, we focused on the independent inter-annual fluctuations of stem diameter growth and cork growth indices, assessing their high-frequency sensitivity to climate. Furthermore, tree size-related variations in sensitivity to climate of both radial growths were addressed. The extrapolation to cork-ring area increments and to stem basal area increments was examined, and we hypothesize that Quercus suber’s cork growth is a proxy for stem diameter growth, which, in turn, responds to climate and to tree size (age) as physiological changes in the aging cambium affect stem diameter growth patterns. This approach was expected to add valuable information to stem growth and biomass modeling aiming at a sustainable production of cork (bark) throughout the tree’s life.
2. Materials and Methods
2.1. Study Area and Climate
The study was conducted in a permanent plot named Carro Quebrado (38°50′9″ N–8°49′2″ W, 20 m a.s.l.) established in a cork oak woodland belonging to the state-owned farm Companhia das Lezírias at the Tagus River basin in the southwestern part of mainland Portugal (Figure 1).
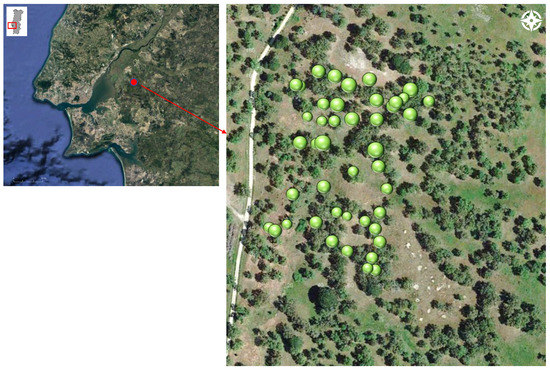
Figure 1.
The study plot of Carro Quebrado in a cork oak woodland at the Tagus River basin, southwest Portugal, and location of the sampled trees (green circles); circle’s size is proportional to the tree’s crown area.
The climate is of Mediterranean type, with some oceanic influence, with a mean annual precipitation of 629 mm, mainly concentrated between late autumn and early spring (wet semester); July is the hottest month, with an average temperature of 22 °C (maximum temperatures reach 29 °C), and January is the coldest, with a mean temperature of 9 °C (data collected for 1961–1990 Vila Franca de Xira weather station, located approximately 11 km NW of the study area).
Climate data consist of monthly precipitation and mean temperature values for 2000–2012 years (the study period). In the study period, a severe drought occurred in the spring and summer of 2005 (between April and September), following a sequence of drought months initiated in April 2004. A moderate drought also occurred in 2009 (mainly in May–August) following a sequence of dry months initiated in October 2008 [7] (please see the data form climate parameters in Figure S1, available as Supplementary data in Supplementary Materials).
The Tagus River spreads in this area, depositing sediments in a 2–10 km wide alluvial plain where the predominant soils are Haplic Arenosols, allowing cork oaks to develop deep roots [14,15]. The Tagus alluvial aquifer is an unconfined shallow aquifer composed of alluvial deposits and alluvial terrace deposits. Groundwater levels increase during the wet semester (October to March), defining the recharge period. From middle/late spring (April–May) onward, the groundwater levels decrease [16].
2.2. Data Measurements
Manual band dendrometers (D1 Dendrometer, with 0.05*π cm resolution, UMS, Munich, Germany) were wrapped around the stems of 47 cork oaks at breast height (1.30 m above ground) immediately after cork harvest in 2000. Dendrometers were in close contact with the stem and were fastened with a spring mechanism that allowed the tape to expand during tree radial growth. This way, dendrometers measured total stem (diameter) growth, i.e., wood growth plus cork (bark) growth, resulting from the independent activities of the cambium and phellogen, respectively.
Selected trees were representative of adult trees in full cork production cycle (mature trees) (3rd–4th cork harvest onward) growing in typical agroforestry systems called montados in the Tagus basin region. Here, sandy soils allow older trees to attain large sizes with large stem diameters (easily reaching 70–80 cm at breast height), thick branches and large crowns [15].
Stem diameter annual increments (Dibh) were measured during the 11 years of the complete cork production cycle (2001–2011). For each tree, the annual stem diameter increment was converted to an annual stem basal area increment (BAi), assuming a circular outline of the stem cross section (Equation (1)):
with as the initial stem diameter at breast height and as its annual increment, i.e., between two consecutive measurements. Stem basal area annual increment (BAi) was used to examine tree growth patterns and trends accounting for tree size. In fact, for the same stem diameter increment (Dibh), trees were expected to have distinct sectional area increments (BAi), depending on their initial stem size () [17].
By the end of the cork production cycle (2012), one cork sample (approximately 10 × 10 cm) was collected from each tree at stem breast height. Each cork sample had a cork-ring width (Crw) data series of 11 complete years (2001–2011). Half years—corresponding to the autumn cork growth following the initial harvest (in 2000) and to the spring cork growth preceding the next harvest (in 2012)—were excluded from the cork-ring widths series. Measurements of Crw were made in the cross section of the cork samples according to the methodology described by [6] using ImageProPlus® image-processing software (Media Cybernetics, Silver Spring, MD, USA) and module length measurements.
Cork-ring width (Crw) data of each tree were used to compute its annual stem basal area increment under cork (BAiuc), assuming a circular outline of the stem cross sections and according to the following equation (Equation (2)):
with as the tree’s stem diameter at breast height in year j and as the cork-ring width of year j. Furthermore, annual cork-ring area increments (Crai) were calculated based on the following formula (Equation (3)):
2.3. Data Analysis
Stem diameter growth and cork-ring width data series were de-trended by fitting polynomial functions [18,19] to maximize the inter-annual fluctuations due to climate. Using this weighted polynomial forecasting technique (SPSS software, version 21.0.0 was used), individual stem diameter growth indices (IDbh) and cork growth (or cork-ring width) indices (ICrw) were computed as residuals (εj, zero mean random error).
To address and compare the sensitivity to climate, IDbh and ICrw were plotted against mean and monthly precipitation and temperature, and the bootstrapped correlation coefficients were computed. Heatmaps for the level of significance of Pearson’s correlation coefficients were built.
Selected climate parameters (with the highest bootstrapped Pearson’s correlation coefficients) were then predictors (fixed effects) of IDbh and ICrw in linear mixed-effects models, which explicitly integrated among-tree variations (considered as random effects) related to the initial tree stem diameter Dbh immediately after cork harvesting (in 2000). Four tree’s stem diameter classes were established based on those defined by [20] to account for the trees’ growth dynamics with aging: Dbh_1 (Dbh ≤ 35 cm); Dbh_2 (35 cm < Dbh ≤ 55 cm); Dbh_3 (55 cm < Dbh < 70 cm); and Dbh_4 (Dbh ≥ 70 cm).
Model fitting was initiated by adding random effects of tree, first through random intercept alone and then by random intercept and slope. The generalized linear mixed-effect models were formulated according to the equation:
where Yij denotes each response variable, related with tree growth patterns IDbh and ICrw of year jth in tree ith; Xji is the selected climatic parameter t in the stem of tree ith, in year jth; α0 and α1 are the parameters of the fixed part of the model; βTreei and μTreei are the random parameters associated with the between-trees variations in stem diameter; and εji is the residual error, with mean zero and variance σε and independent from random effects. To adjust the linear mixed-effects models, the maximum likelihood algorithm (ML) of the lmer function, available in the lme4 library of the R software (version 4.0.3), was used. The best model produced the lowest values of Akaike’s information criterion (AIC). For the selected linear mixed models, the estimates of covariance parameters are presented (please see Tables S1 and S2 in the Supplementary Materials).
Yji = α0 + (α1 + βTreei) × Xji + μTreei + εji
3. Results
3.1. Stem Diameter and Cork Growth Chronologies
The annual increment of stem diameter (Dibh) matched the cork-ring width (Crw) time-series in their general decreasing trend (Figure 2). Median Dibh were highly correlated with Crw (r = 0.75, p-value < 0.005). In the first complete growth ring (2001), trees reached the highest Dibh, 1.44 cm, undoubtedly based on the highest Crw, 4.89 mm, which represented about 67% of diameter growth. On average, Dibh and Crw reached, respectively, 57% and 47% of their initial growth rates by the last year (2011).
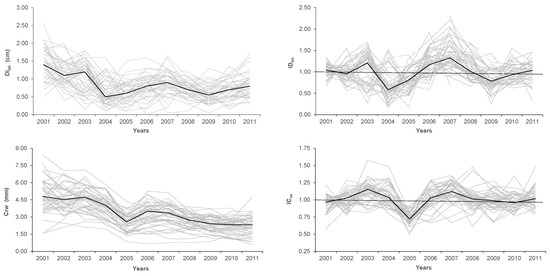
Figure 2.
Time-series (2001–2011) plots of stem diameter growth (Dibh) and cork-ring width (Crw) (on the left) and of stem diameter growth index (IDbh) and cork growth (or cork-ring width) index (ICrw) (on the right). All the sampled 47 trees are represented by the grey lines; the black curve represents the trees’ median.
Median IDbh were relatively less strongly correlated with ICrw (r = 0.51, p-value < 0.05). The 2004–2005 drought years had a similar negative effect on the Dibh and Crw of all trees (Figure 2). However, while the lowest Dibh (0.62 cm yr−1) occurred in 2004, the lowest cork growth (Crw = 2.57 mm yr−1) occurred one year later, in 2005. IDbh reached values of 0.58 in 2004 and 0.81 in 2005 and was much more sensitive to drought than ICrw, with 0.72 only in 2005. Moreover, in the drought year of 2009, trees reduced their IDbh to nearly 80% of a normal year (IDbh = 0.78), while ICrw did not show any decrease (ICrw = 0.99).
3.2. Precipitation and Temperature Relationships with Stem Diameter Growth and Cork Growth Indices
ICrw was more often and stronger correlated with climate parameters than IDbh (Figure 3). Correlations were mostly positive with precipitation (p-value < 0.05) and stronger with temperature of the autumn–winter preceding the growing season (p-value < 0.01) (Figure 3b), while IDbh showed more and stronger negative correlations with the mean temperature of late spring and autumn (Figure 3a).
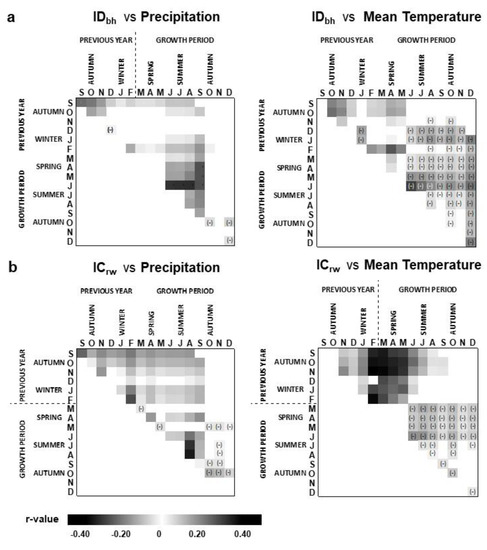
Figure 3.
Heatmaps for the significant bootstrap correlation coefficients (r) between (a) annual stem diameter growth index (IDbh) (top row) and (b) annual cork growth (or cork-ring width) index (ICrw) (bottom row) and monthly precipitation (left column) and mean monthly temperature (right column) (cf. Section 2.1 for climate data). Only correlations with a confidence level significance equal to or higher than to 95% are shown. By default, correlations are positive; negative correlations are indicated with a minus sign (−).
Noticeably, both IDbh and ICrw were significantly correlated (p-value < 0.01) with precipitation (positively) and with temperature (negatively) in summer (June–August) (Figure 3). For IDbh, higher correlations were found with spring–summer precipitation, highest in June–August (r = 0.32), and with spring–summer temperature, highest in June (r = −0.30). For ICrw, higher correlations were found with summer precipitation, highest in August (r = 0.34), and with summer temperature, highest in June (r = −0.22).
3.3. Influence of Tree Size on the Relationship between Climate and Stem Diameter Growth and Cork-Ring Width
Selected mixed-effects linear models (M0–M17) were plotted against the tree size random effect, given by stem diameter at breast height under cork (Dbh) (Table 1). The fixed-effect variables were climate variables which displayed the highest bootstrap correlation coefficients (highly significant, p-value < 0.01). For IDbh: precipitation of September-1 (r = 0.24), June (r = 0.31), June–July (r = 0.32) and June–August (r = 0.32) and mean temperature of October-1 (r = 0.26), February–April (r = 0.28), June (r = −0.30) and January–December (r = −0.24); for ICrw: precipitation of September-1 (r = 0.26), February (r = 0.28), July–August (r = 0.31) and August (r = 0.34) and mean temperature of September-1–March (r = 0.35), October-1–February) (r = 0.36), November-1–February (r = 0.37), November-1–March (r = 0.37), November-1–April (r = 0.35) and February (r = 0.37).

Table 1.
Coefficients and AIC values for linear mixed-effects models of the stem diameter growth index (IDbh) and cork growth (or cork-ring width) index (ICrw), with selected climatic variables and tree size classes (Dbh) as random factor of the 47 trees. The best climate predictors (lowest AIC) are highlighted in bold. Significance of predictors is indicated by: ** (p-value < 0.01). Results of the fixed effects and random effects are presented in the Tables S1 and S2 available as supplementary data in Supplementary Materials.
In all the models, except M4 and M7, IDbh and ICrw increased with monthly precipitation and monthly temperature, and α1 was positive. In the best IDbh model (with the lowest AIC) (M4), IDbh decreased about 0.115 per °C of increase in June temperature. In the best ICrw model (M12), the ICrw increased about 0.120 per °C of monthly winter temperature prior to the growth year (November-1–February) (Table 1).
Both models for IDbh, M0 and M4, respectively with June–July precipitation and June temperature as fixed effects, explained about 10% of the total variation. Random-effect variation, related with tree stem diameter (Dbh classes), was 1% of total variation. This indicates an influence of tree size on the climate-related stem diameter growth index variations. In contrast, both models for ICrw, M8 and M12, respectively with August precipitation and November-1–February temperature as fixed effects, explained 12% (in M8) and 14% (in M12) of the total variation, and no variation was explained by the random effect related to tree stem diameter, which indicates that climate–cork growth relationships were not tree size-related.
In addition, IDbh tree size-related differences were found in the random intercept and random slope (Figure 4). Smaller trees showed steeper slopes of IDbh with summer precipitation (June–July) and with summer (June) temperature (Figure 4, Dbh_1).
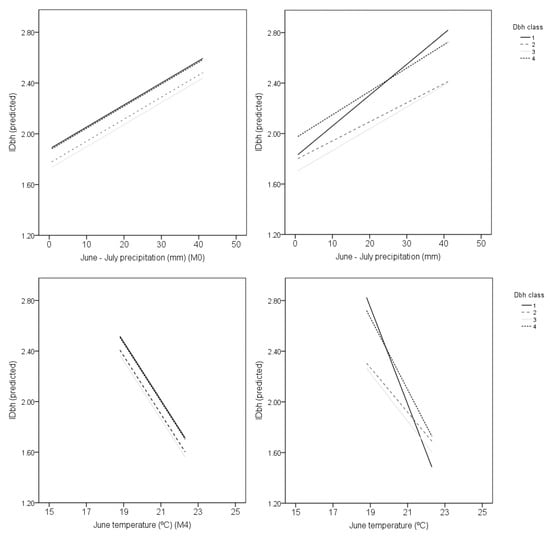
Figure 4.
Representation of the selected mixed-effects model fitting with stem diameter growth index (IDbh) as the response function: M0 (upper plots) and M4 (lower plots), for summer precipitation (June–July) and summer temperature (June) (fixed effects) only, with random intercept (on the left), and with random slope and random intercept (on the right). Random effects are related to tree size stem diameter (Dbh). The four Dbh classes are represented: Dbh_1, Dbh ≤ 35 cm; Dbh_2, 35 cm < Dbh ≤ 55 cm; Dbh_3, 55 cm < Dbh < 70 cm and; Dbh_4, Dbh ≥ 70 cm.
3.4. Cross-Sectional Area Increments of Cork and Stem Diameter
In the time window of a cork production cycle, the increments of cork (cork-ring width, Crw) and stem diameter at breast height (Dibh) (Figure 2) extrapolated to cork-ring area increments (Crai) and to the basal area increments (BAi), respectively, showed average values of Crw ranging between 3 and 4 mm, and of Dibh, ranging between 7 and 10 mm. This radial growth represented higher proportional cross-sectional areas that were highly correlated (r = 0.71, p-value < 0.01) and more than 2.5 times higher in the largest trees (Dbh_4) than in the smallest ones (Dbh_1) (Figure 5). Moreover, tree size-related curves for these cross-sectional area increments clearly showed that the lowest values of Crai and BAi of the smaller trees reached more than 30% and 60% of stem basal area, respectively. Thus, in smaller trees (e.g., Dbh_1), low values of cross-sectional stem diameter area increments (i.e., values below the normalized stem basal area increments of 0.5) represented more than half of their stem basal area. In contrast, larger trees (e.g., Dbh_4) presented high values of cross-sectional area increments (i.e., values above the normalized stem basal area increments of 1.5), less than half of their stem basal area (40%) (Figure 5b).
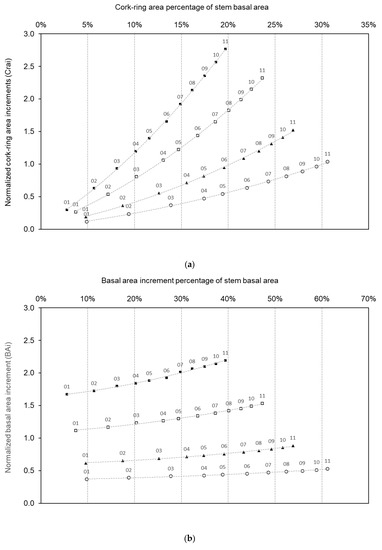
Figure 5.
Variation of normalized cork-ring width areas (a) and basal area increments (b) with the percentage of stem basal area, along the cork production cycle (11 years) for all the sampled trees, clustered by Dbh classes: Dbh 1 (smallest trees; white circles); Dbh 2 (black triangles); Dbh 3 (white squares); and Dbh_4 (largest trees; black squares).
Results also showed clear inter-annual variations and, specifically, a reduction of the cross-sectional area increments related with climate, namely under the influence of drought years (e.g., 2005 or 2009) (Figure 5). Trees’ BAi showed a clear deceleration of growth in those years, and the BAi of larger trees (Dbh_4) was greatly reduced when compared to smaller trees. These higher decreases of the cross-sectional area increment in the larger trees, however, corresponded to relatively lower percentages of the stem basal area affected when compared to smaller trees.
4. Discussion
The annual cork growth values found in this study (3–4 mm), were within the range of cork-ring widths reported at national level (2.2–4.8 mm) [19] and for southwestern Spain (1.9–5.3 mm) [18]. Moreover, for average stem diameter growth rates of 7–10 mm yr−1, the estimated “non-cork” (wood) mean annual increments (1–2 mm), were in line with the range of values previously reported for cork oak raw wood-ring widths [6,9] and for tree (wood)-ring width values reported for other oak species (1.0–2.5 mm) [21].
Along the 11 complete years of the cork production cycle, cork and stem showed similar variation patterns of radial growth (Figure 2). Cork growth revealed decreasing trends as expected from the immediately enhanced activity of the traumatic phellogen formed after cork harvesting and subsequent reduction [8]. The similarity of the stem diameter and cork growth curves indicates that the latter represents a large part of the radial increment but that the “non-cork” (wood) growth responds, as well, directly or indirectly, to cork harvesting. We know that taking measurements of stem diameter growth (encompassing wood and cork growths) and corresponding measurements of cork growth would allow us to derive wood growth. Wood growth is very difficult to measure in cork oak due to the extreme difficulty in obtaining tree-ring chronologies. These chronologies are only possible to achieve through stem cross-sections following tree felling, which is not allowed due to strict regulations enforced to protect cork oaks in cork-producing countries [6,9].
The discrepancy of the lowest stem diameter growth in 2004, when a “normal” enhanced cork growth was still measured (Figure 2), might be explained by trees’ investment on the new traumatic phellogen activity to produce cork layers in the first four post-harvest years [6,22,23], which forces a drastic shift in carbon allocation for that purpose at the expense of wood radial growth [6,12,13,20,24] and might severely impact stem diameter growth. Moreover, the 2005 drought was a prolonged drought initiated in 2004 (cf. Section 2.1) with a moderate drought in April–September [16]. This spring–summer drought seemed to affect stem diameter growth more than cork growth, which, in turn, was much more influenced by the previous winter–autumn temperatures and precipitation (Figure 3). For these reasons, it is noteworthy that, in 2004, the stem diameter growth decrease was mostly due to a reduction of “non-cork” (wood) growth rather than of cork growth. Furthermore, if it only depended on “non-cork” growth, stem radial growth would have a better recovery and returned to normal growth in 2005. However, drought might have harshly compromised cork growth and, consequently, stem diameter growth.
In this study, similar but fewer and weaker correlations between climatic variables and stem diameter growth were found when compared with cork growth (Figure 3). However, in the drought year of 2009, increments of stem diameter were smaller, and deviations were more pronounced in stem diameter than in cork indices (Figure 2). This apparent paradox indicates that, by the end of the cork-production cycle, when trees have already reduced their energy allocation to cork growth after the cork harvest disturbance and returned to normal cork growth rates [16], the variation (decrease) of stem diameter increment was mostly due to a decreased in “non-cork” (wood) growth and suggests that the cambium activity is highly sensitive to climate factors, in agreement with what has been reported for tree-rings in Quercus ilex and other (deciduous) oaks [25,26,27]. In 2009, there was a moderate drought between May and August (cf. Section 2.1), which probably had a negative impact on cambium activity, much more influenced by the climatic factors prevailing in the current growth season (Figure 3). Trees might use (less) current photosynthates to support this cambial activity [27] because the “non-cork” (wood) growth probably competes with other carbon sinks namely with reproduction efforts [7,11,28,29].
Our findings suggest that the decreasing trend of stem diameter growth derived mostly from the decreased phellogen activity and cork growth after their harvest-induced boost, which outpaces the “non-cork” growth in a first phase, corresponding to the years immediately after cork harvesting; in a second phase, cork growth rates decrease due to the normal (reduced) phellogen activity, and after 5-6 complete years of cork growth [23], the trend patterns of stem diameter derives mostly from cambium activity and “non-cork” (wood) growth, which have a less buffered relationship with climate parameters.
Summer weather (June–August) strongly influenced both cork and stem diameter growths but had a stronger effect on the latter, which was positively correlated with precipitation and negatively correlated with mean temperature, suggesting that trees underwent some period of water stress that affected stem diameter growth. These results support previous findings in the study region reporting that trees use groundwater during summer to maintain cork growth [16]. Thus, water stress conditions during the dry summer months would mainly affect the “non-cork” (wood) component of the stem diameter growth. Moreover, cambium activity, in contrast with that of the renewed traumatic phellogens, might change with tree aging [30], supporting our results on the influence of tree size on the relationship between stem diameter growth and climate (Table 1; Figure 4). Indeed, larger (or older) trees that develop deep roots and reach groundwater [6,16] cope with water stress conditions by developing an intense growth flush in spring, probably relying on current photosynthesis to produce relatively wider wood-rings, as reported by [31] for Quercus robur, and, particularly in the study region, by extending the growing season into late autumn [5]. This way, wood growth and stem diameter growth would be less affected. On the other hand, smaller (or younger) trees will not easily reach the deeper water table in summer and will be much more affected by water stress. Trees cope with drought by lowering respiration rates during water stress periods and by decreasing carbon allocation to stem diameter growth and, particularly, to wood growth, similarly to the strategies of species such as Quercus ilex facing droughts [3,27,32]. Adding to tree size, other factors such as trees’ intra-specific competition or land management [33], might affect cork and stem radial growths and should not be ignored in further and locally adjusted attempts to use cork growth as a reliable proxy for stem diameter growth in Quercus suber.
By using tree radial (linear) increments to calculate stem basal area increments, this study indicates that cork-ring widths at breast height are useful, though flawed, surrogates of stem basal area growth to estimate trees growth through modeling at tree or stand level. Similar cork-ring increments between trees can result in distinct cross-sectional increment areas: larger trees take huge efforts to fix and allocate carbon to produce cork layers of the same size, when compared to the smaller trees [11,34]. On the other hand, in smaller trees, the cross-sectional increment area corresponds to the highest percentage of stem basal area reaching 62% (Figure 5). This makes them much more sensitive (steeper slopes for cork growth decrease with temperature and precipitation in summer, Figure 4) to inter-annual variations of precipitation and temperature, and particularly to drought episodes, than larger trees in cork oak woodlands [11], similarly to other forest species [35].
5. Conclusions
Our results suggest that cork-ring width is not a fully reliable proxy for cork oak stem diameter growth of trees subjected to cork harvesting cycles. Tree stem diameter growth and the cork-ring widths showed similar patterns, with a decreasing trend along the successive years. However, while stem diameter growth mostly resulted from cork growth in the first years after cork harvesting, by the end of the cork production cycle, it resulted mostly from a “non-cork” (wood) growth. Stem diameter growth is primarily affected by climate conditions prevailing in the growing season, while cork growth and phellogen activity rely on climate conditions prevailing in autumn, prior to the growing season, which support a cork growth flush in spring. Furthermore, the strength of the relationship between stem diameter growth and temperature and precipitation in summer is influenced by tree size (age), while cork growth is not. The increasing severity and frequency of droughts are likely to differently influence tree radial growth rates and patterns, stem basal area growth and biomass modeling. These responses of individual trees, at the local scale, might reflect larger discrepancies in cork oak woodlands at regional scales, and our findings highlight the importance of tailoring cork woodlands forest management, taking the cork harvesting pressure into account.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app112411998/s1, Figure S1: Monthly precipitation (P) (full grey bars) and mean temperature (T) (black line) for the period 2000–2012 for the study area. Climate data for the period under study refer to the weather stations of Vila Franca de Xira, located approximately 11 km NW of the study area, for the monthly precipitation and mean temperature, and of Sto Estevão, located in the study area, for the monthly precipitation. Mean total annual precipitation (Pannual) and mean annual temperature (Tmean) are indicated in the graph. Severe drought of 2005 and moderate drought of 2009 are indicated in light grey; Table S1: Results of the fixed effects of the linear models shown in Table 1 for stem diameter growth index (IDbh) and cork growth index (ICrw). and; Table S2: Results of the random effects of the selected linear models shown in Table 1 for stem diameter growth index (IDbh) and cork growth index (ICrw). Figure S2: Residuals against the estimates of IDbh in the models M0 and M4 (Table 1).
Author Contributions
A.C. conceived the study, developed the concept of the paper, wrote the paper and, together with P.C., discussed and commented on the obtained results. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the IsoCork project (Climate effects on cork growth assessed by isotope fingerprinting) funded by the Foundation for Science and Technology of the Portuguese Ministry of Education and Science (FCT–MEC) (EXPL/AGR/FOR/1220/2012) and funding from the project PDR2020-101-031071 funded by the Portuguese Ministry of Agriculture and Rural development. Augusta Costa’s work was funded by FCT–MEC Research Contract CEEINST/00012/2018- ENGFL.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be shared upon reasonable request.
Acknowledgments
Authors acknowledge the collaboration of Companhia das Lezírias, S.A. (Benavente, Portugal) for the development of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Garzón, M.B.; De Dios, R.S.; Ollero, H.S. Effects of climate change on the distribution of Iberian tree species. Appl. Veg. Sci. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Cherubini, P.; Gartner, B.L.; Tognetti, R.; Bräker, O.U.; Schoch, W.; Innes, J. Identification, measurement and interpretation of tree rings in woody species from mediterranean climates. Biol. Rev. 2003, 78, 119–148. [Google Scholar] [CrossRef] [PubMed]
- Kurz-Besson, C.; Otieno, D.; Vale, R.L.D.; Siegwolf, R.; Schmidt, M.; Herd, A.; Nogueira, C.; David, T.; David, J.S.; Tenhunen, J.; et al. Hydraulic Lift in Cork Oak Trees in a Savannah-Type Mediterranean Ecosystem and its Contribution to the Local Water Balance. Plant Soil 2006, 282, 361–378. [Google Scholar] [CrossRef]
- Costa, A.; Barbosa, I.; Roussado, C.; Graça, J.; Spiecker, H. Climate response of cork growth in the Mediterranean oak (Quercus suber L.) woodlands of southwestern Portugal. Dendrochronologia 2016, 38, 72–81. [Google Scholar] [CrossRef]
- Oliveira, G.; Costa, A. How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps. For. Ecol. Manag. 2012, 270, 257–272. [Google Scholar] [CrossRef]
- Ghalem, A.; Barbosa, I.; Bouhroua, R.T.; Costa, A. Climate signal in cork-rings chronologies: Case-studies on southwestern Portugal and north-western Algeria. Tree Ring Res. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Costa, A.; Pereira, H.; Oliveira, Â. Influence of climate on the seasonality of radial growth of cork oak during a cork production cycle. Ann. For. Sci. 2002, 59, 429–437. [Google Scholar] [CrossRef]
- Leal, S.; Nunes, E.; Pereira, H. Cork oak (Quercus suber L.) wood growth and vessel characteristics variations in relation to climate and cork harvesting. Eur. J. For. Res. 2008, 127, 33–41. [Google Scholar] [CrossRef]
- Costa, A.; Pereira, H.; Oliveira, A. Variability of radial growth in cork oak adult trees under cork production. For. Ecol. Manag. 2003, 175, 239–246. [Google Scholar] [CrossRef]
- Mendes, M.P.; Cherubini, P.; Plieninger, T.; Ribeiro, L.; Costa, A. Climate effects on stem radial growth of Quercus suber L.: Does tree size matter? Forestry 2019, 92, 73–84. [Google Scholar] [CrossRef]
- Machado, D.P. Contribuição para o estudo da formação da cortiça no sobreiro. Rev. Agronómica 1935, 23, 75–104. [Google Scholar]
- Knapic, S.; Louzada, J.L.; Leal, S.; Pereira, H. Radial variation of wood density components and ring width in cork oak trees. Ann. For. Sci. 2007, 64, 211–218. [Google Scholar] [CrossRef]
- Almeida, C.; Mendonça, J.J.L.; Jesus, M.R.; Gomes, A.J. Sistemas Aquíferos de Portugal: Aluviões do Tejo (T7); Instituto Nacional da Água, Sistema Nacional de Informação de Recursos Hídricos: Lisbon, Portugal, 2000.
- Costa, A.; Madeira, M.; Oliveira, Â.C. The relationship between cork oak growth patterns and soil, slope and drainage in a cork oak woodland in Southern Portugal. For. Ecol. Manag. 2008, 255, 1525–1535. [Google Scholar] [CrossRef][Green Version]
- Mendes, M.P.; Ribeiro, L.; David, T.S.; Costa, A. How dependent are cork oak (Quercus suber L.) woodlands on groundwater? A case study in southwestern Portugal. For. Ecol. Manag. 2016, 378, 122–130. [Google Scholar] [CrossRef]
- West, P.W. Use of diameter increment and basal area increment in tree growth studies. Can. J. For. Res. 1980, 10, 71–77. [Google Scholar] [CrossRef]
- Caritat, A.; Gutiérrez, E.; Molinas, M. Influence of weather on cork-ring width. Tree Physiol. 2000, 20, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Lopes, F.; Pereira, H. Caractérisation de la croissance et de la qualité du liège dans une région de production. Ann. For. Sci. 2000, 57, 187–193. [Google Scholar] [CrossRef][Green Version]
- Natividade, J.V. Subericultura; Direcção Geral dos Serviços Florestais e Aquícolas: Lisbon, Portugal, 1950. [Google Scholar]
- Johnson, S.E.; Abrams, M.D. Age class, longevity and growth rate relationships: Protracted growth increases in old trees in the eastern United States. Tree Physiol. 2009, 29, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Costa, A.; Nunes, L.C.; Spiecker, H.; Graça, J. Insights into the Responsiveness of Cork Oak (Quercus suber L.) to Bark Harvesting. Econ. Bot. 2015, 69, 171–184. [Google Scholar] [CrossRef]
- Seabra, L. Aspectos gerais da cicatrização no sobreiro. An. Inst. Super. Agron. 1939, X, 89–97. [Google Scholar]
- Tessier, L.; Nola, P.; Serre-Bachet, F. Deciduous Quercus in the Mediterranean region: Tree-ring/climate relationships. New Phytol. 1994, 126, 355–367. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees 2004, 18, 83–92. [Google Scholar]
- Gea-Izquierdo, G.; Martín-Benito, D.; Cherubini, P.; Cañellas, I. Climate-growth variability in Quercus ilex L. west Iberian open woodlands of different stand density. Ann. For. Sci. 2009, 66, 802. [Google Scholar] [CrossRef]
- Andivia, E.; Fernández, M.; Vázquez-Piqué, J.; González-Pérez, A.; Tapias, R. Nutrients return from leaves and litterfall in a mediterranean cork oak (Quercus suber L.) forest in southwestern Spain. Eur. J. For. Res. 2010, 129, 5–12. [Google Scholar] [CrossRef]
- Costa, A.; Barbosa, I.; Miguel, C.; Graça, J. Variation of cork porosity along the stem in harvested cork oak (Quercus suber L.) trees. Ann. For. Sci. 2021, 78, 52. [Google Scholar] [CrossRef]
- Costa, A.; Barbosa, I.; Pestana, M.; Miguel, C. Modelling bark thickness variation in stems of cork oak in south-western Portugal. Eur. J. For. Res. 2020, 139, 611–625. [Google Scholar] [CrossRef]
- Rozas, V. Dendrochronology of pedunculate oak (Quercus robur L.) in an old-growth pollarded woodland in northern Spain: Tree-ring growth responses to climate. Ann. For. Sci. 2005, 62, 209–218. [Google Scholar] [CrossRef]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- Faias, S.P.; Paulo, J.A.; Firmino, N.; Tomé, M. Drivers for annual cork growth under two understory management alternatives on a podzolic cork oak stand. Forests 2019, 10, 133. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Ruger, N.; et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 2014, 507, 7490. [Google Scholar] [CrossRef] [PubMed]
- Vanoni, M.; Bugmann, H.; Nötzli, M.; Bigler, C. Quantifying the effects of drought on abrupt growth decreases of major tree species in Switzerland. Ecol. Evol. 2016, 6, 3555–3570. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).