Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composite Block
2.2. Tooth Preparation
2.3. Adhesive Procedures on Dental Substrates
2.4. Micro-Tensile Bond Strength Testing
2.5. Failure Mode Analysis
2.6. SEM Analysis of the Etching Effect on Enamel and Dentin Smear Layer
3. Results
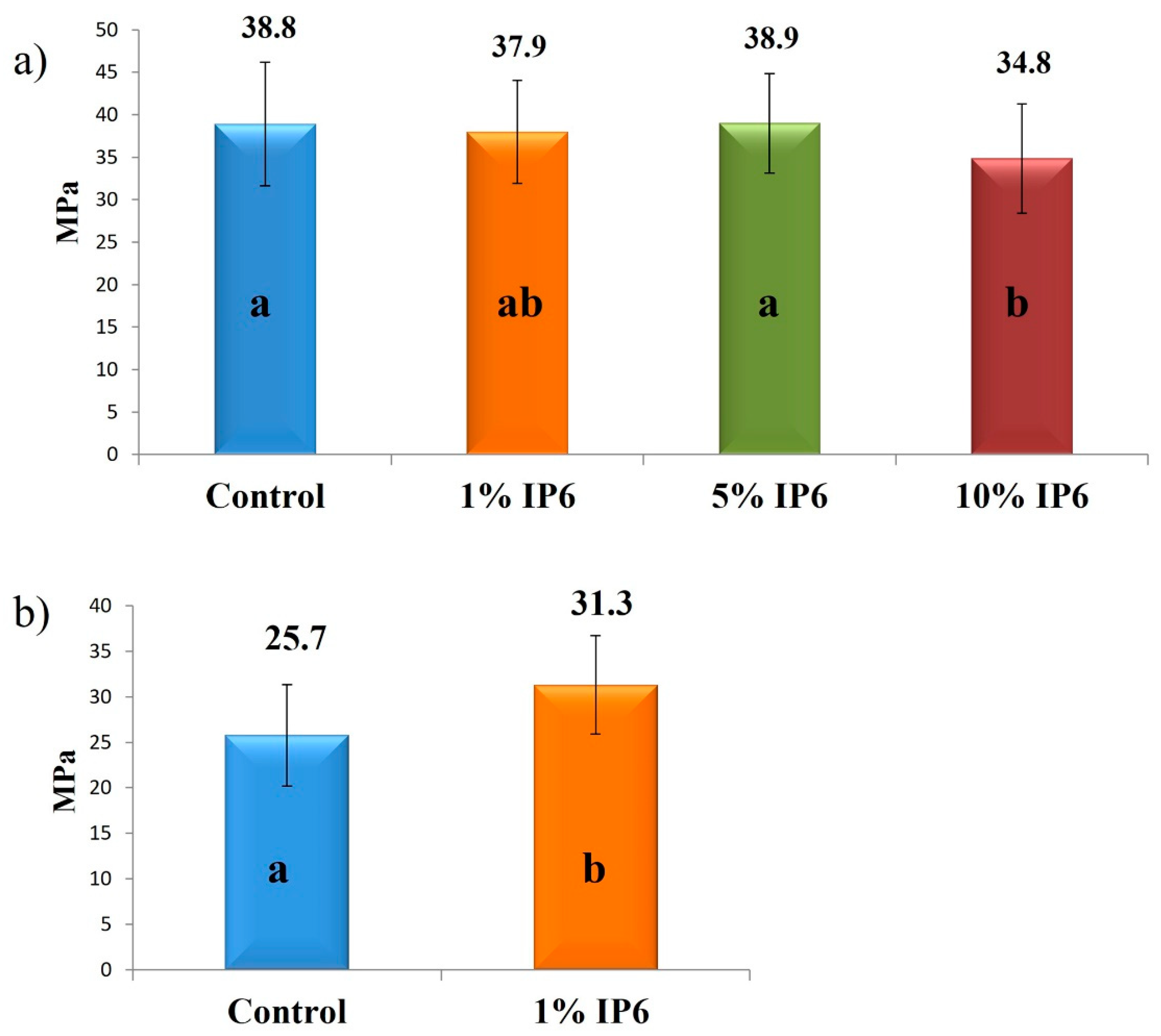
3.1. Micro Tensile Bond Strength
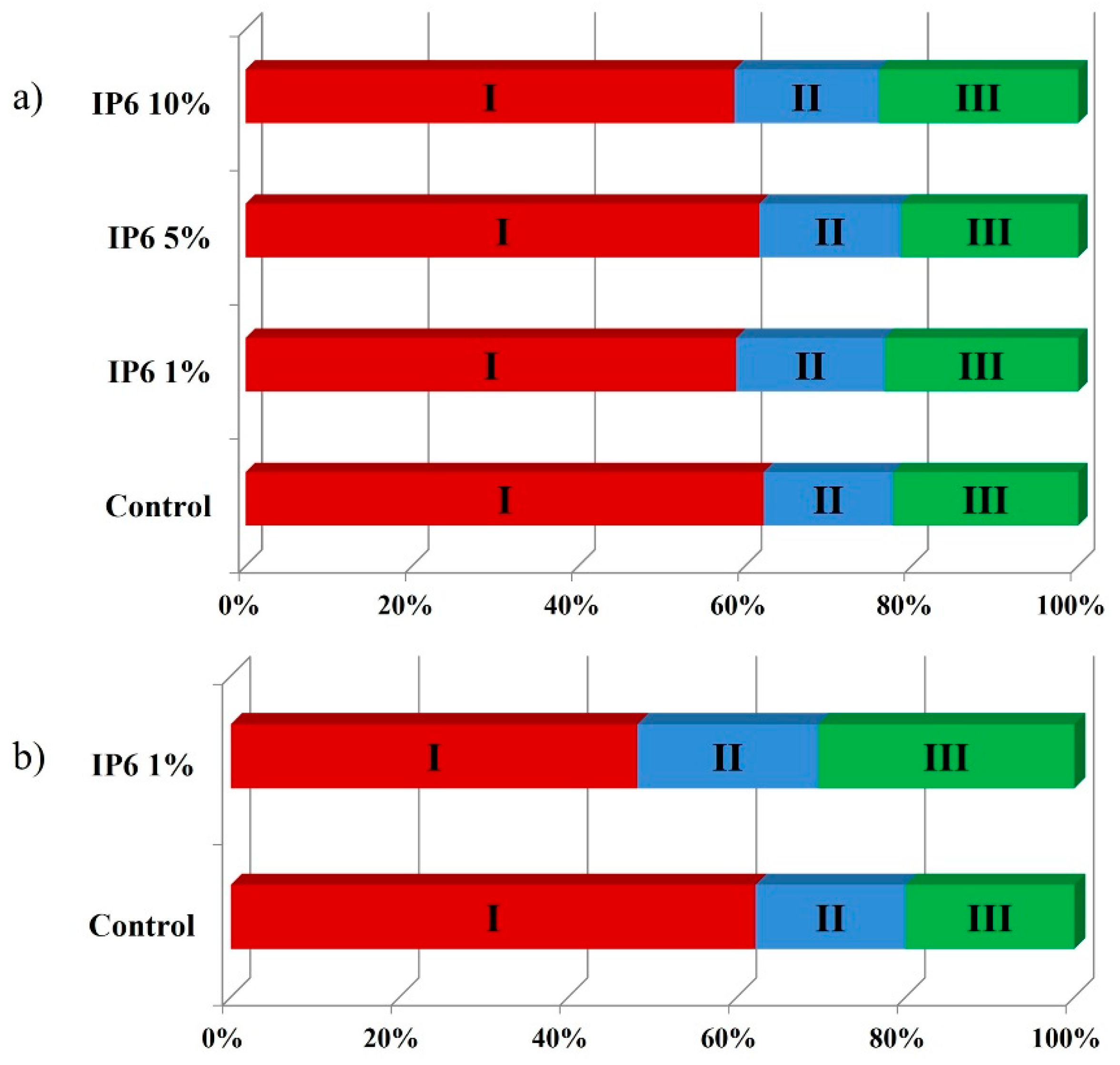
3.2. Failure Mode
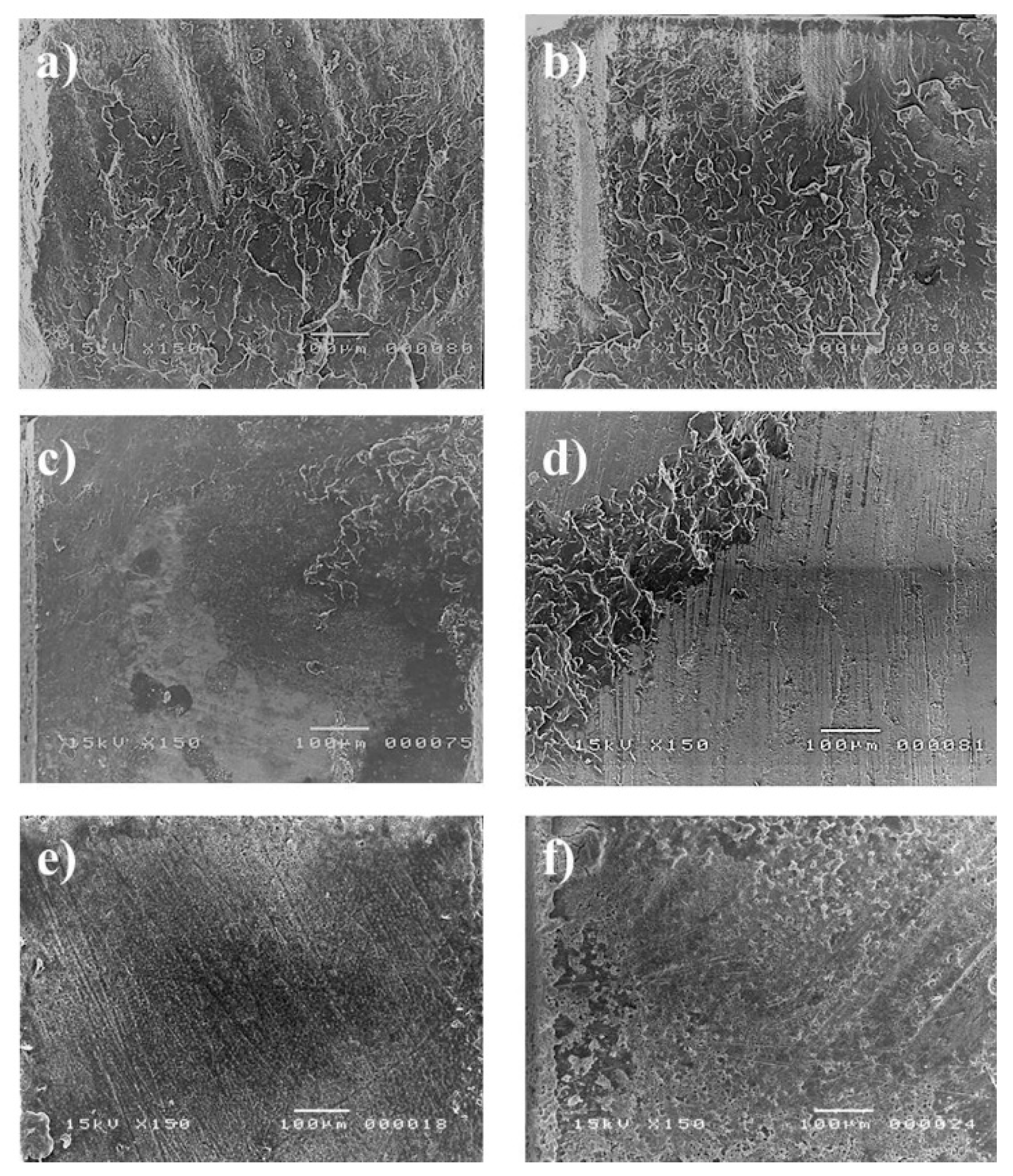
3.3. Effect of Etchant on Smear Layer Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Umino, A.; Nikaido, T.; Tsuchiya, S.; Foxton, R.M.; Tagami, J. Confocal laser scanning microscopic observations of secondary caries inhibition around different types of luting cements. Am. J. Dent. 2005, 18, 245–250. [Google Scholar]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fukushima, T.; Inoue, Y.; Arita, S.; Miyazaki, K. Effect of water, saliva and blood contamination on bonding of metal brackets with a 4-META/MMA/TBB resin to etched enamel. Am. J. Dent. 1999, 12, 299–304. [Google Scholar] [PubMed]
- Shimada, Y.; Seki, Y.; Uzzaman, M.A.; Sattabanasuk, V.; Sasafuchi, Y.; Foxton, R.M.; Otsuki, M.; Tagami, J. Monkey pulpal response to an MMA-based resin cement as adhesive luting for indirect restorations. J. Adhes. Dent. 2005, 7, 247–251. [Google Scholar] [PubMed]
- Coli, P.; Brännström, M. The marginal adaptation of four different bonding agents in Class II composite resin restorations applied in bulk or in two increments. Quintessence Int. 1993, 24, 583–591. [Google Scholar] [PubMed]
- Nakabayashi, N.; Nakamura, M.; Yasuda, N. Hybrid Layer as a Dentin-Bonding Mechanism. J. Esthet. Restor. Dent. 1991, 3, 133–138. [Google Scholar] [CrossRef]
- Takayuki, H.; Yoshizo, M.; Atsushi, M.; Hajime, M.; Emilio, S.H.; Bart, V.M.; Hirofumi, Y.; Takuo, K. A 15-year clinical comparative study of the cumulative survival rate of cast metal core and resin core restorations luted with adhesive resin cement. Int. J. Prosthodont. 2010, 23, 379–405. [Google Scholar]
- Nurrohman, H.; Nikaido, T.; Takagaki, T.; Sadr, A.; Waidyasekera, K.; Kitayama, S.; Ikeda, M.; Tagami, J. Dentin bonding performance and ability of four MMA-based adhesive resins to prevent demineralization along the hybrid layer. J. Adhes. Dent. 2012, 14, 339–348. [Google Scholar] [CrossRef]
- Kuwano, M.; Mimura, T.; Takaiwa, F.; Yoshida, K.T. Generation of stable “low phytic acid” transgenic rice through antisense repression of the 1D-myo-inositol 3-phosphate synthase gene (RINO1) using the 18-kDa oleosin promoter. Plant Biotechnol. J. 2009, 7, 96–105. [Google Scholar] [CrossRef]
- Barrientos, L.G.; Murthy, P.P.N. Conformational studies of myo-inositol phosphates. Carbohydr. Res. 1996, 296, 39–54. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Borggreven, J.M.P.M.; Driessens, F.C.M. Effect of phytate and hexadecylamine on the permeability of bovine dental enamel. Arch. Oral Biol. 1983, 28, 375–379. [Google Scholar] [CrossRef]
- Nordbö, H.; Rölla, G. Desorption of Salivary Proteins from Hydroxyapatite by Phytic Acid and Glycerophosphate and the Plaque-Inhibiting Effect of the Two Compounds In Vivo. J. Dent. Res. 1972, 51, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Perelló, J.; Sanchis, P.; Isern, B.; Prieto, R.M.; Costa-Bauzá, A.; Santiago, C.; Ferragut, M.L.; Frontera, G. Anticalculus effect of a triclosan mouthwash containing phytate: A double-blind, randomized, three-period crossover trial. J. Periodontal Res. 2009, 44, 616–621. [Google Scholar] [CrossRef]
- Prosser, H.J.; Brant, P.J.; Scott, R.P.; Wilson, A.D. The Cement-forming Properties of Phytic Acid. J. Dent. Res. 1983, 62, 598–600. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Tamura, Y.; Otsuki, M.; Aoki, K.; Tagami, J. Phytic acid: An alternative root canal chelating agent. J. Endod. 2015, 41, 242–247. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Romero, M.J.; Otsuki, M.; Tagami, J. Effect of phytic acid as an endodontic chelator on resin adhesion to sodium hypochlorite-treated dentin. Restor. Dent. Endod. 2020, 45, e44. [Google Scholar] [CrossRef]
- Nassar, R.; Nassar, M.; Vianna, M.E.; Naidoo, N.; Alqutami, F.; Kaklamanos, E.G.; Senok, A.; Williams, D. Antimicrobial activity of phytic acid: An emerging agent in endodontics. Front. Cell Infect. Microbiol. 2021, 11, 753649. [Google Scholar] [CrossRef]
- Muana, H.L.; Nassar, M.; Dargham, A.; Hiraishi, N.; Tagami, J. Effect of smear layer removal agents on the microhardness and roughness of radicular dentin. Saudi Dent. J. 2021, 33, 661–665. [Google Scholar] [CrossRef]
- Nassar, R.I.; Nassar, M. Antimicrobial effect of phytic acid on Enterococcus faecalis. Int. Arab. J. Antimicrob. Agents 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Aizawa, M.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. Effect of phytic acid used as etchant on bond strength, smear layer, and pulpal cells. Eur. J. Oral Sci. 2013, 121, 482–487. [Google Scholar] [CrossRef]
- Kong, K.; Islam, M.S.; Nassar, M.; Hiraishi, N.; Otsuki, M.; Yiu, C.K.Y.; Tagami, J. Effect of phytic acid etchant on the structural stability of demineralized dentine and dentine bonding. J. Mech. Behav. Biomed. Mater. 2015, 48, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Hiraishi, N.; Nassar, M.; Otsuki, M.; Yiu, C.K.Y.; Tagami, J. Effect of phytic acid etchant on resin–dentin bonding: Monomer penetration and stability of dentin collagen. J. Prosthodont. Res. 2017, 61, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Nassar, R.; Maki, H.; Al-Yagoob, A.; Hachim, M.; Senok, A.; Williams, D.; Hiraishi, N. Phytic Acid: Properties and Potential Applications in Dentistry. Front. Mater. 2021, 8, 638909. [Google Scholar] [CrossRef]
- Grynspan, F.; Cheryan, M. Calcium phytate: Effect of ph and molar ratio on in vitro solubility. J. Am. Oil Chem. Soc. 1983, 60, 1761–1764. [Google Scholar] [CrossRef]
- Camps, J.; Pashley, D.H. Buffering action of human dentin in vitro. J. Adhes. Dent. 2000, 2, 39–50. [Google Scholar]
- Selle, P.H.; Cowieson, A.J.; Cowieson, N.P.; Ravindran, V. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 2012, 25, 1–17. [Google Scholar] [CrossRef]
- Nezu, T.; Winnik, F.M. Interaction of water-soluble collagen with poly(acrylic acid). Biomaterials 2000, 21, 415–419. [Google Scholar] [CrossRef]
- Forgione, D.; Nassar, M.; Seseogullari-Dirihan, R.; Thitthaweerat, S.; Tezvergil-Mutluay, A. The effect of phytic acid on enzymatic degradation of dentin. Eur. J. Oral Sci. 2021, 129, e12771. [Google Scholar] [CrossRef]
- Lopes, G.C.; Thys, D.G.; Klaus, P.; Oliveira, G.M.S.; Widmer, N. Enamel acid etching: A review. Compend. Contin. Educ. Dent. 2007, 28, 18–24. [Google Scholar]
- Shinchi, M.J.; Soma, K.; Nakabayashi, N. The effect of phosphoric acid concentration on resin tag length and bond strength of a photo-cured resin to acid-etched enamel. Dent. Mater. 2000, 16, 324–329. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Toledano, M.; Aguilera, F.S.; Mannocci, F.; Pashley, D.H.; Tay, F.R.; Watson, T.F.; Osorio, R. Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using ethanol wet-bonding. Dent. Mater. 2010, 26, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, R.; Lohbauer, U.; Roggendorf, M.J.; Naumann, M.; Taschner, M. Selective enamel etching reconsidered: Better than etch-and-rinse and self-etch? J. Adhes. Dent. 2008, 10, 339–344. [Google Scholar]
- Nanci, A. Enamel: Composition, Formation, and Structure. Ten Cate’s Oral Histology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 288–369. [Google Scholar]
- Hobson, R.S.; McCabe, J.F. Relationship between enamel etch characteristics and resin-enamel bond strength. Br. Dent. J. 2002, 192, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Ogliari, A.O.; Yoshihara, K.; Zanchi, C.H.; Correr-Sobrinho, L.; Sinhoreti, M.A.; Correr, A.B.; Watson, T.F.; et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent. Mater. 2014, 30, e317–e323. [Google Scholar] [CrossRef]
- Zafar, M.S.; Ahmed, N. The effects of acid etching time on surface mechanical properties of dental hard tissues. Dent. Mater. J. 2015. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.; Lv, L.; He, X.; Li, W.; Guo, L. Effect of phosphoric acid concentration used for etching on the microtensile bond strength to fluorotic teeth. Medicine 2018, 97, e12093. [Google Scholar] [CrossRef]
- Trzcionka, A.; Narbutaite, R.; Pranckeviciene, A.; Maskeliūnas, R.; Damaševičius, R.; Narvydas, G.; Połap, D.; Mocny-Pachońska, K.; Wozniak, M.; Tanasiewicz, M. In vitro analysis of quality of dental adhesive bond systems applied in various conditions. Coatings 2020, 10, 891. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, M.; Islam, M.S.; A C, S.A.; El-Damanhoury, H.M.; Sauro, S.; Hiraishi, N. Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study. Appl. Sci. 2021, 11, 11976. https://doi.org/10.3390/app112411976
Nassar M, Islam MS, A C SA, El-Damanhoury HM, Sauro S, Hiraishi N. Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study. Applied Sciences. 2021; 11(24):11976. https://doi.org/10.3390/app112411976
Chicago/Turabian StyleNassar, Mohannad, Md. Sofiqul Islam, Smriti Aryal A C, Hatem Mostafa El-Damanhoury, Salvatore Sauro, and Noriko Hiraishi. 2021. "Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study" Applied Sciences 11, no. 24: 11976. https://doi.org/10.3390/app112411976
APA StyleNassar, M., Islam, M. S., A C, S. A., El-Damanhoury, H. M., Sauro, S., & Hiraishi, N. (2021). Resin-Based Cement Applied to Enamel and Dentin Pre-Treated with Phytic Acid: An In Vitro Study. Applied Sciences, 11(24), 11976. https://doi.org/10.3390/app112411976









