Abstract
Background and objectives: Regenerative medicine, with its massive development over the years, has the potential to solve some of the most problematic medical issues, such as functional organ transplantation. The aim of this study was to create a human meniscal shape 3D-printed enriched with human adipose-derived mesenchymal cells. Materials and Methods: Human infrapatellar fat pad was harvested, and mesenchymal cells were isolated. The mesenchymal stem cells were differentiated to the chondrocite lineage and a hydrogel (a nanofibrillar cellulose, sodium alginate, D-mannitol, and Hepes buffer solution combination) cell mixture was bioprinted to create three human-size meniscus structures. The obtained structures were evaluated regarding the cell viability, appropriate size in relation to a native meniscus, and some mechanical characteristics. Results: The human meniscal shape created respected the anatomic characteristic of a native structure. Cell viability of approximately 97% and extracellular matrix formation after the printing process were observed. The mean maximum force for the meniscus with mesenchymal cells was 6.5 N (+/−0.5 N) compared to the mean maximum force for the native meniscus of 10.32 N (+/−0.7 N), which is statistically relevant (p < 0.01). Conclusion: This paper presents the potential of bioprinting viable cell structures that could in the future present enough mechanical strength to replace a human organ, such as a meniscus. There are still limitations regarding the ink and the printing process, but we are confident that these problems will soon be solvable.
1. Introduction
Menisci are important structures in the biomechanical activity of the knee joints. In many cases, due to trauma or simply through degenerative changes, the meniscus is torn and acts as a destructive force over the cartilage covering the articular surfaces of the knee [1]. Treatment mostly consists of meniscectomy of the torn portion, but in order to preserve the normal function of the joint, an intact meniscus is needed [2]. The idea of a meniscal transplant has long been proposed, but a mechanical and biological identical structure has not yet been created [3].
Regenerative medicine is a field of research that has gained much popularity in the last years with the development of new technologies, such as 3D printing. Three-dimensional printing in correlation with biomaterials loaded with human cells can reproduce human structures that could in the future be used to treat real patients [4].
Human mesenchymal stem cells can be harvested from many tissue sources, but the most accessible one is fat tissue. The minimal criteria that define the mesenchymal stem cells (MSCs) set by the International Society for Cellular Therapy include the presence of CD105, CD73, and CD90 surface marker; the lack of CD45, CD34, CD14, CD31, and HLA-DR markers; and their capacity to adhere to plastic and to differentiate into osteogenic, chondrogenic, and adipogenic lineage. These cells can be transformed into any differentiated cell type and can then be integrated in bioinks and used to create different structures [5,6].
There are different techniques to bioprint structures, and many materials used as scaffolds. The main approaches to print bioinks are extrusion, inkjet, laser, and acoustic. The inkjet method was the first method to be used and was a variation of classical printers; thus, it is easily available, affordable, and has a high printing speed. It can be used on a continuous inkjet or a drop-on-demand mode. However, there are also disadvantages like the variation of droplet sizes and the need to use low-viscosity ink. Another drawback of this method is the potential clogging of the nozzle [7,8].
The extrusion method consists of building pressure on a closed container either through a screw or a pneumatic cylinder and generating a continuous filament of bioink. The micro-nozzle enables a variety of viscosities and consistencies of bioinks to be used. The method provides high speed, capability of producing complex structures, and a high cell viability. The main disadvantage is the low resolution and deformation [5,7].
Laser technology is the most expensive, accurate, and complex method of all. The laser pulses create a high-pressure bubble, which drives the ink to the recipient plate. Being a nozzle-free method, it is clog-free and also has a high precision rate [9].
The bioink should be biocompatible, as any material used in relation to human body must be non-toxic, non-immunogenic, non-thrombogenic, and lastly non-carcinogenic. An important characteristic is cytocompatibility. It is therefore essential to preserve cell function, promote cell proliferation, and enable extracellular matrix formation (ECM). The current options regarding bioinks include hydrogels, decellularized ECM, and microcarriers. They can also be divided into natural bioinks (collagen, gelatin, agarose, alginate, fibrin, hyaluronic acid, etc.) and synthetics (polytethylene glycol, nanocomposites, multimaterials, etc.) [6,8]. Nanocellulose was successfully mixed with alginate sulfate while preserving the advantageous properties of both components. Nanocellulose is an ideal material to enable the printability of low-viscosity materials due to its good biocompatibility and innate mechanical properties.
So far, many efforts have been made to expand and clarify the indications for bioprinting technologies [9,10]. There have been attempts to print kidneys, blood vessels, ears, cartilage, nerves, and other tissues. The main barriers are the discovery of a suitable bioink with perfect biocompatibility and mechanical strength to achieve the biological function [8,11].
The aim of this study was to bioprint a human-shaped meniscus loaded with infrapatellar adipose-derived mesenchymal stem cells, which is, as far as we know, the first study of this mixture, and then to test the cell viability and some mechanical properties of the obtained structure. Additionally, the correlation between MRI studies, the natural organ shape, and the bioprinted structure was investigated.
2. Experimental Part
2.1. Scaffold Design
The model to be printed recreated a human lateral meniscus. Erbagci studied 170 human MRIs and concluded that the average values for a lateral meniscus are:
- Medium circumference of 91.7 mm.
- Anterior horn:
- ○
- Medium width of 8.8 mm.
- ○
- Medium height of 4.33 mm.
- Body of the meniscus:
- ○
- Medium width of 8.3 mm.
- ○
- Medium height of 4.9 mm.
- Posterior horn:
- ○
- Medium width of 9.7 mm.
- ○
- Medium height of 5.3 mm.
Based on these medium values, Cinema 4D (Maxon Computer GMBH) software was used to create the CAD model and the file was then transferred to the computer that controlled the bioprinter. The model was also compared with a human external meniscus excised during a knee arthroplasty (Figure 1) procedure regarding the external dimensions of both (Figure 2).
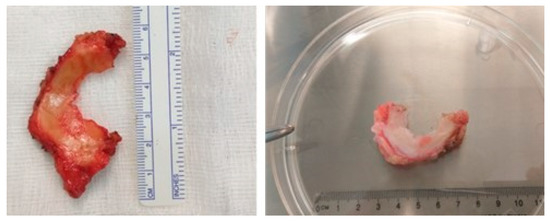
Figure 1.
Human meniscus harvested during a knee arthroplasty procedure.
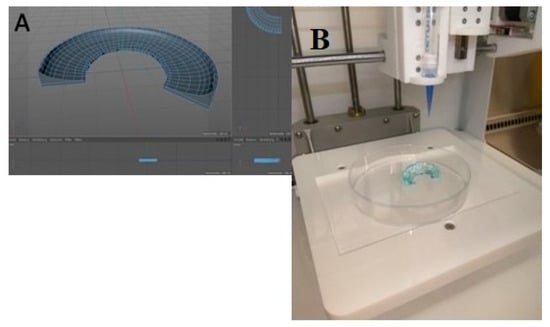
Figure 2.
(A). Aspect during the creation of the software printing model (B). Final aspect of the printed model.
2.2. Bioprinter
The bioprinter used was Cellink Inkredible (Sweden), which used the extrusion technique with the help of two printing heads. On the XY axis, it has a precision of printing of 10 microns and on the Y axis, 2.5 microns. The crosslinking step is optional and is performed under LED ultraviolet light of 354 nm. The viscosity of the gel can vary from 0.001 to 250 Pa-s.
2.3. Bioink
The bioink used was Cellink (Cellink AB Sweden) and is the first universal ink of this company and can be used to print skin, cartilage, and mesenchymal cells. The composition of the ink is made of nanofibrillar cellulose, sodium alginate, D-mannitol, and Hepes buffer solution. It is an opaque, odorless, and sterile gel with a pH of 6.5–7.4 and a viscosity of 1–20,000 Pa-s.
2.4. Bioprinting Process
The printer was sterilized with 70% alcohol and the printing was performed at room temperature and humidity. On an empty Petri dish, the Z axis was manually established until an A4 paper could be introduced between the printing head and the dish. After the calibration, the bioink was introduced into the cartridge and attached to the printing head. The nozzle was 22 G (410 microns). The printing parameters were set to 10–20 mm/s for the piston forwarding and a 0.05 mm distance and the process of printing started.
After the printing process was completed, the cross-linking agent was introduced and covered the whole structure. The cross-linking agent (Crosslinking Agent, Cellink AB Sweden) was a combination of Calcium Chloride, D-mannitol, and Hepes Buffer solution with a pH of 6.5–7.4. After 5 min, the agent was discarded, and a growth medium was introduced.
2.5. Harvesting of the Infrapatellar Fat Pad
We harvested, with the written consent of the patients, the infrapatellar fat pad from 4 patients admitted to the Orthopedic Department from the Military Hospital from Timisoara. The tissue was open harvested from two patients during a knee arthroplasty procedure and arthroscopically in two cases during an anterior cruciate ligament reconstruction surgery (Figure 3).
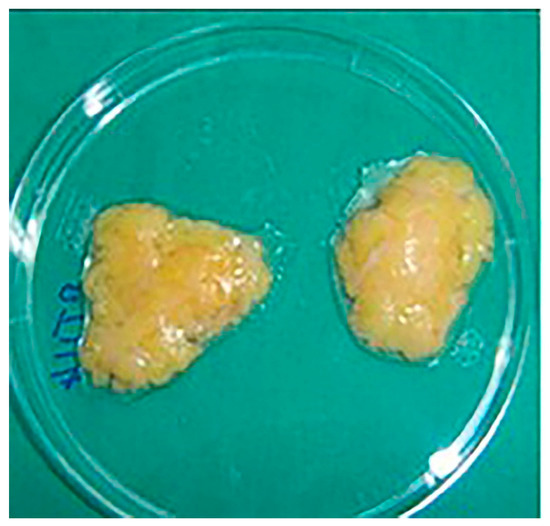
Figure 3.
Samples from the harvested infrapatellar fat pad.
The isolation and differentiation protocol were carried out in the same manner previously published by our group [12]. The tissue harvested was minced with the help of a sterile scalpel and the adipose-derived stem cells were isolated using the explant method based on the plastic adherence properties of the cells. After cell isolation, culture and expansion were performed with alpha-minimum essential medium (MEM; Gibco BRL, Carlsbad, CA, USA), supplemented with 10% Fetal Calf Serum (FCS; PromoCell, Heidelberg, Germany) and 1% Penicillin/Streptomycin solution (10,000 IU/mL; PromoCell, Heidelberg, Germany) medium. Immunocytochemical and flowcytometry analyses were performed to confirm the mesenchymal characteristics of the isolated cells.
The chondrocyte protocol was performed according to Miltenyi Biotech (Bergisch Gladbach, Germany) producer and consisted of a:
- Eagle Dulbecco Modified (DMEM, Sigma-Aldrich, St. Louis, MI, USA).
- Dexamethasone (Sigma-Aldrich) 1 mM.
- Ascobic acid 2 Pfosphate (Sigma-Aldrich) 0.15 mM.
- Sodium pyruvate (Sigma-Aldrich) 0.1 M.
- Proline (Sigma-Aldrich) 35 mM.
- Insulin-Transferrin-Selenium (Sigma-Aldrich) 500×.
- TGF β1 (Sigma-Aldrich) 10 ng/mL.
- 1% Penicillin-Streptomycin (Sigma-Aldrich).
Later, we used the ready-to-use chondrocyte differentiation media, NH ChondroDiff Medium (Miltenyi Biotech) plus 1% Pen-Strep.
The hMSCs harvested from the method before were put in a 50 mL Falcon tube together with 30 mL of MSC growth medium and mixed well. The cell mixture was then poured equally into two petri dishes and incubated at 37 °C and 5% CO2. After 24 h, the cells adhered to the plastic.
The media was replaced with NH ChondroDiff Medium (Miltenyi Biotech) and 1% Pen-Strep. The media was changed every 2–3 days, for the following 24 days.
2.6. Mechanical Test
Compression tests were conducted on 3 natural external meniscus, 3 bioprinted meniscus crosslinked without cells, and 3 bioprinted with adipose-derived mesenchymal stem cells crosslinked. After the printing process and the harvesting procedure, the specimens were kept in a saline solution and the mechanical tests were performed 24 h later. They were retrieved form the saline solution just before the testing was carried out.
We sectioned the central part using a scalpel and we obtained a sample of 8/8/4 mm. We sectioned the central parts of each meniscus (mid-circumferential, mid-radial, and mid-axial region) using a scalpel and we obtained a sample of 8/8/4 mm for each. The sectioning was performed under a microscope with a micrometer (Leica DMS1000). The tests were performed on a Zwick Proline Z005 universal test machine with a maximum force of 5 kN and with compression plates made out of polished titanium to avoid traction between the plates and tissue. The tests were carried out at room temperature of 21 °C. A preload of 0.05 N with a speed of 0.016 mm/s was set. The test speed was 0.01%/s (Figure 4).

Figure 4.
A printed tissue sample during mechanical testing.
The Zwick Proline Z005 machine has high (24-bit) measured-value resolution for maximum test-result accuracy and reproducibility. This means, for example, that even minimal force changes on the specimen can be recorded and displayed accurately. Positioning and repetition accuracy was ±2 µm and the class of machine range was 0.5/1.
Patented Xforce load cells are developed and manufactured by ZwickRoell, and offer outstanding accuracy and high resistance to parasitic influences. Parasitic influences, such as temperature and transverse forces, have significantly less impact on test results than other comparable sensors. Xforce load cells are also very robust and more resistant to factors, such as transverse forces, during compression and flexure tests.
3. Results
3.1. Characteristics of the Harvested Adipose Tissue
Two of the adipose tissue samples were harvested arthroscopically and two by open means. There were three males and one female. The mean weight of the four samples was 11.08 g (+/−2.5 g). The mean age for the open group was 68.5 years and 44 years for the arthroscopic one (Table 1).

Table 1.
Characteristics of the harvested adipose tissue.
3.2. Characteristics of the Excised Human Menisci
The human meniscus excised was measured with a high-precision digital caliper (MT-110051/2/3, MRC Laboratory-instruments, ESSEX, UK) that has limited error of ±0.015 mm. The dimensions were:
- Circumference of 93.2 mm (+/−2.1 mm).
- Anterior horn:
- ○
- Width of 9.3 mm (+/−0.4 mm).
- ○
- Height of 3.97 mm (+/−0.2 mm).
- Body of the meniscus:
- ○
- Width of 7.9 mm (+/−0.6 mm).
- ○
- Height of 4.9 mm (+/−0.2 mm).
- Posterior horn:
- ○
- Width of 9.2 mm (+/−0.3 mm).
- ○
- Height of 5.6 mm (+/−0.1 mm).
3.3. Viability Tests for Mesenchymal Stem Cells
The flow cytometry tests were carried out with a MACSQuant type machine for data acquisition and Flowing Software 2.5.1 for data analysis. All the samples were tested at different passages, from the second passage to the fifth one and the results are presented in Table 2 as mean values for each sample. In Figure 5, the results at the third passage are graphically exposed. It must be emphasized that annexin V binds with phosphatidylserine (PS) when there are destructions of the cellular membrane and propidium iodine (PI) binds with nuclear components when massive membrane and cytoplasmic destructions occur, and thus the cells are considered in late apoptosis, precisely necrosis (Figure 5 and Table 2).

Table 2.
Percentual values of viable cell populations analyzed with Annexin/PI.
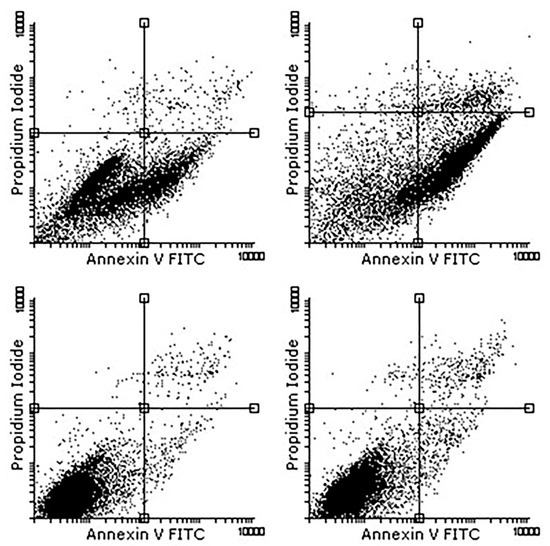
Figure 5.
Annexin V-FITC results.
3.4. Immunophenotype Marker Identification through Flow Cytometry
The flow cytometry analysis was performed on the mesenchymal stem cells isolated from the infrapatellar fat pad as well as for those differentiated into the chondrocyte lineage. The selected markers for stem cell immunophenotype identification were CD90, CD73, CD29, CD44, CD105, and CD117 positive and CD106, CD34, CD146, alfa-SMA, E-caderina, and CD95 negative.
3.5. Cell Viability after Bioprinting
Two days after bioprinting, samples were collected from the culture serum and the number of cells present in the printed structure was estimated. Out of 50 × 106 cells used for printing of each meniscus, 8 × 106 cells were present in the supernatant and out of these, 2 × 105 were dead cells (Figure 6).
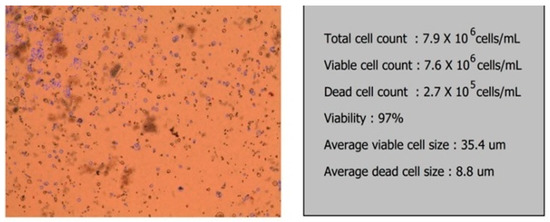
Figure 6.
Viability test for the bioprinted meniscus.
3.6. Mechanical Test Result
The mean compression test results for the bioprinted menisci showed a displacement at the mean maximum force of 4.1 mm (+/−0.3 mm) with different values of the forces. The mean maximum force for the meniscus with mesenchymal cells was 6.5 N (+/−0.5 N) compared to the one without cells, which managed only 6.01 N. The difference has no statistical relevance (p > 0.05) but can be explained by the secretion of extracellular matrix by the MSC cells.
The mean maximum force for the native meniscus was 10.32 N (+/−0.7 N), which was statistically relevant (p < 0.01) using an ANOVA test analysis (Figure 7).
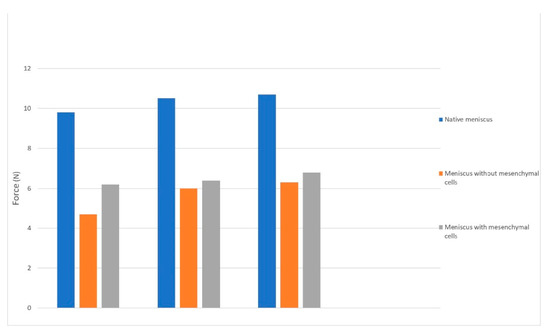
Figure 7.
The force-displacement characteristics chart for the bioprinted menisci.
3.7. Quantity and Quality of the Cells from the Bioprinted Meniscus
In the bioprinted meniscus seeded with chondrocytes from adipose-derived stem cells, we noted high cellularity and the fibers of the extracellular matrix were well organized and regular. When performing immunochemistry tests for Aggrecan, which is a chondrocyte marker, we obtained a highly intense coloration, which resembles the natural meniscus morphology and structure (Figure 8).
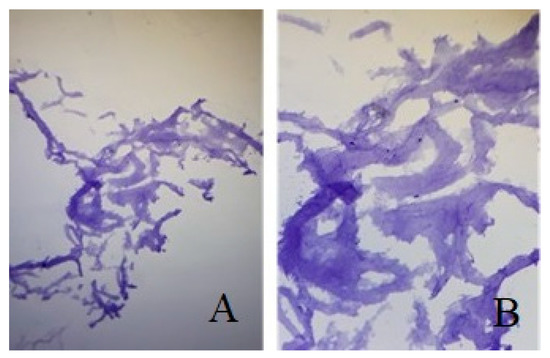
Figure 8.
(A). Bioprinted meniscus with in vitro cultivated adipose-derived stem cells colored with Aggrecan (B). Same Image under 10× magnification.
The structure of the bioprinted meniscus seeded with mature chondrocytes is the optimum combination for tissue engineering procedures. We also tried to further culture these cells for three weeks in a medium chondrocyte serum in vitro, but no other benefits were observed regarding the structural and cellularization of the bioprinted meniscus.
The bioprinted meniscus with simple adipose mesenchymal stem cells or cultivated in chondrocyte-specific medium did not ensure proper cellularity of the printed structure, nor the secretion of extracellular matrix or a fiver pattern able to result in a structure with high force resistance.
4. Discussions
The idea of being able to produce organs that are fully functional and have the capacity of integration and restoration of the normal relations from different systems in the human body is an old one. The idea of replicating a human meniscus tries to solve one of the most important challenging pathologies in orthopedic surgery [13]. This study focussed on bioprinting a viable 3D full-scale meniscus seeded with human infrapatellar fat pad mesenchymal-derived stem cells that could provide a positive high percentage of chondrogenic live cells able to secrete matrix through the hydrogel.
Hockaday et al. [14] managed to print aortic valves through a combination of interstitial cells and a mixture of hydrogels consisting of alginate and poly(ethylene glycol)-diacrylate (PEG-DA). Micro-CT studies of the printed organ revealed that the normal architecture was achieved and the constructed organ was able to encapsulate aortic root sinus smooth muscle cells. Other groups [15] managed to produce a human ectopic artificial liver (HEAL), which, after transplantation into mice, presented humanized liver function, human protein synthesis, human drug metabolism, and drug-induced liver injury. Kim et al. [1] implanted in a rodent model a muscle construct built with human muscle progenitor cells. The construction presented myofiber-like structures and after 8 weeks of implantation into a tibialis anterior muscle defect, it provided 82% muscle function recovery.
The additive manufacturing method of the printer used in correlation with the low heat of the printer head allows a uniform distribution of cells layer after layer, resulting in an equally loaded structure. The accuracy of cell deposition is dependent on the density of the bioink, speed of printing, and the nozzle diameter [4].
The present study has some limitations. Although the shape of the meniscus was precisely recreated, the bioink was distributed layer by layer in a single manner, but it is well known that the natural structure has different patterns of tissue distribution. These patterns offer great strength to the natural meniscus, thus making it able to resist multiple types of forces like compression, traction, shear, and multiple combinations [9]. Additionally, although the printed structure revealed the presence of extracellular matrix formation, the compression test showed significant differences to the native tissue. The hydrogel was used according to the manufacturer’s methodology, but they provide it as a universal ink. Some authors [4,16] have managed to produce ultra-strong hydrogel that has a better performance on mechanical tests, but they have not tested these structures loaded with cells.
Another limitation is the shape isolated to be mechanically tested. We chose to compare only a small part of the printed meniscus (shape described) with a homogenous portion of the native meniscus. It has been clearly demonstrated by many authors that the collagen fibers of the human menisci have different patterns of distribution to be able to resist multiple (compressions, shear, torsion) and combined forces [4,17,18]. The mid portion of the native meniscus has the most constant consistency of the whole structure and is mostly capable of withstanding axial compression in a normal environment [9]. As the mechanical part was only a secondary objective of this study, we did not perform further tests.
Compression strength in combination with infrapatellar adipose-derived stem cells was low compared to the natural meniscus. The construct could be improved using ECM [19], maybe of porcine origin like Romanazzo et al. used, to obtain a stronger construct. Unlike us, they tested porcine origin cells and thus the human ones in contrast to the porcine ECM should be tested in the future [20].
The quantity of the harvested tissue for us was 10.91 g for the open procedures and 11.25 g for the arthroscopically performed surgeries. There were no significant differences between the two groups and this correlates with the findings of Tangchitphisut et al. [21].
The cell viability after the printing process of 97% of the total cells corresponds to what other authors published in a recent study [22,23]. The difference between Filardo’s study and ours is the cell source. In our study, we utilized mesenchymal cells isolated from the infrapatellar fat pad whilst the Italian group used cells isolated from bone marrow. It is well known that adipose-derived stem cells are not age dependent and provide excellent differentiation capabilities towards the main lineages [6,12,23].
Although the printing process was carried out considering MRI values, the bioprinted menisci respected the human anatomy and were in perfect correlation with the tissues extracted during surgeries. We proved, like others, that bioprinting inks loaded with cells can recreate a desired shape, maintain it, and provide a uniform distribution of cells in the construct [21].
The extrusion technique for bioprinting provides, on average, a cell viability of 89.46%. Our results exceed these mean values. These results could be the result of the low printing speed and the low viscosity considering the maximum allowed by the technique (30–63 × 107 mPa s) [7]. The high zero-shear viscosity of the bioink, associated with its rapid capability to re-establish the high viscosity after extrusion, helps to improve the spatial resolution of the printed objects and maintain a high shape fidelity during the bioprinting process. These important rheological properties are illustrated by the end results of the 3D cell-laden construct. The cell-laden constructs showed an excellent shape and size stability after bioprinting and 3D culture for up to 3 weeks, demonstrating the stability of the bioink network after crosslinking. A homogeneous cell distribution was observed after embedding the cells into the bioink, indicating a successful mixing process and capability of this bioink to keep the cells in suspension due to its high zero-shear viscosity [8].
Considering the inferior mechanical performance obtained with the hydrogel used in this study, our future directions will focus on improving the resistance but maintaining the optimum environment for proper cell proliferation and activity [24]. Another step forward will be to implant the newly developed structure on an animal model to also evaluate the in vivo evolution and joint function.
5. Conclusions
Nowadays, the use of bioprinting techniques holds great promise in solving medical problems. Although the printed meniscus did not resist the same forces as a native one, we proved that the printing process does not affect the quality and quantity of the cells in the final structure. There is still some research to be done regarding the bioink as the same formula is used for cartilage and skin, which obviously have different compositions. The results make us believe that it is a matter of time until we will manage to produce structures identical in morphology and function as the native one and you can only hope that these structures will eventually be used as in vivo solutions.
Author Contributions
All authors have equal contributions to the current work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki (nr. 14.05.2015; 14 May 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.; Seol, Y.; Ko, I.; Kang, H.; Lee, Y.; Yoo, J.; Atala, A.; Lee, S. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.J.; Wanivenhaus, F.; Burge, A.J.; Warren, R.F.; Rodeo, S.A. The human meniscus: A review of anatomy, function, injury, and advances in treatment. Clin. Anat. 2015, 28, 269–287. [Google Scholar] [CrossRef]
- Frank, R.M.; Cole, J.B. Meniscus transplantation. Curr. Rev. Musculoskelet. Med. 2015, 8, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colter, D.C.; Class, R.; Digirolamo, C.M.; Prockop, D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213–3218. [Google Scholar] [CrossRef]
- Irvine, S.; Venkatraman, S. Bioprinting and differentiation of stem cells. Molecules 2016, 21, 1188. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Yu, Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Zimeng, Z.; Ruochen, L.; Herman, Z.; Li, Z.; Jingjing, Q.; Shiren, W. 3D Printing Super Strong Hydrogel for Artificial Meniscus. ACS Appl. Polym. Mater. 2019, 1, 202. [Google Scholar]
- Mao, A.; Mooney, D. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Calzada, G.; Hernandez-Martínez, A.R.; Cruz-Soto, M. Development of meniscus substitutes using a mixture of biocompatible polymers and extra cellular matrix components by electrospinning. Mater. Sci. Eng. C 2016, 1, 893–905. [Google Scholar] [CrossRef]
- Hurmuz, M.; Bojin, F.; Ionac, M.; Tatu, F.; Puscasiu, D.; Tatu, C. Plastic Adherence Method for Isolation of Stem Cells Derived from Infrapatellar Fat Pad. Mater. Plast. 2016, 53, 553–556. [Google Scholar]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [Green Version]
- Tsang, V.L.; Chen, A.A.; Cho, L.M.; Jadin, K.D.; Sah, R.L.; DeLong, S.; West, J.L.; Bhatia, S.N. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007, 21, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Urechescu, H.; Pricop, M.; Pricop, C.; Mateas, M.; Simon, N.; Galatanu, S. Thermoplastic Materials Used for Fabrication of Maxillary Obturator Prostheses Experimental compression and traction tests. Mater. Plast. 2017, 54, 477–480. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Tatu, R.F.; Marsavina, L.; Voiconi, T.; Hurmuz, M.; Tatu, C.; Ungurean, C.; Rosu, S. Reinforcement of Tibial Fixation in Anterior Cruciate Ligament Reconstruction Using a Polyester Multi Stranded Long Chain Polyethylene Core Suture Material. Mater. Plast. 2014, 51, 460–462. [Google Scholar]
- Suhun, C.; Sung-Sahn, L.; Yeong-Jin, C.; Da Hee, H.; Ge, G.; Joon Ho, W.; Dong-Woo, C. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2020, 267, 120466. [Google Scholar]
- Romanazzo, S.; Vedicherla, S.; Moran, C.; Kelly, D.J. Meniscus ECM-functionalised hydrogels containing infrapatellar fat pad-derived stem cells for bioprinting of regionally defined meniscal tissue. J. Tissue Eng. Regen. Med. 2018, 12, e1826–e1835. [Google Scholar] [CrossRef]
- Tangchitphisut, P.; Srikaew, N.; Numhom, S.; Tangprasittipap, A.; Woratanarat, P.; Wongsak, S.; Tawonsawatruk, T. Infrapatellar Fat Pad: An Alternative Source of Adipose-Derived Mesenchymal Stem Cells. Arthritis 2016, 2016, 4019873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatu, R.F.; Anuşca, D.N.; Groza, S.; Marusciac, L.; Bojin, F.M.; Tatu, C.; Hurmuz, M.; Păunescu, V. Morphological and functional characterization of femoral head drilling-derived mesenchymal stem cells. Rom. J. Morphol. Embryol. 2014, 55, 1415–1422. [Google Scholar] [PubMed]
- Filardo, G.; Petretta, M.; Cavallo, C.; Roseti, L.; Durante, S.; Albisinni, U.; Grigolo, B. Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone Joint Res. 2019, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Baylon, E.G.; Crowder, H.A.; Gold, G.E.; Levenston, M.E. Non-ionic CT contrast solutions rapidly alter bovine cartilage and meniscus mechanics. Osteoarthr. Cartil. 2020, 28, 1286–1297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).