Viscosupplementation in the Therapy for Osteoarthritic Knee
Abstract
1. Introduction
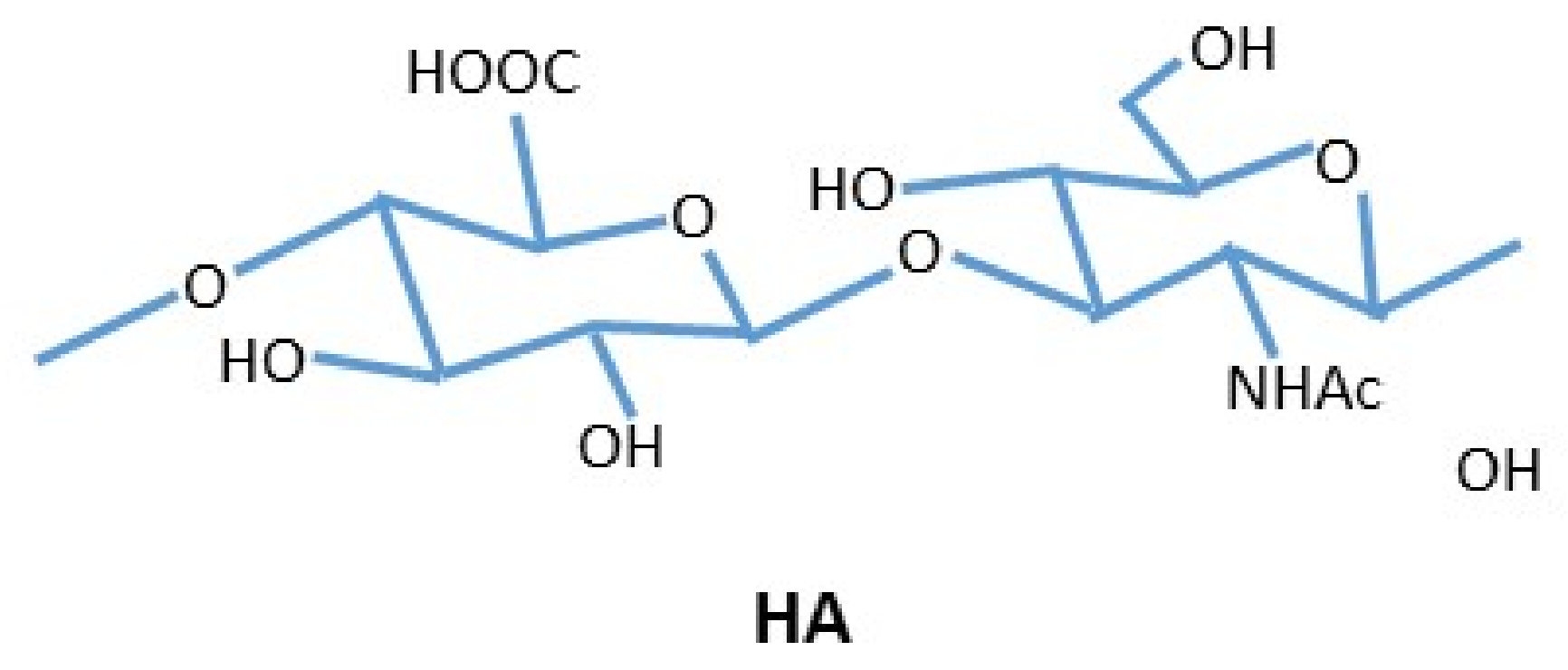
2. Hyaluronic Acid
2.1. Guidelines for the Use of HA
2.2. Hyaluronic Acid Physiology
2.3. Hyaluronic Acid Contraindication and Adverse Effects
2.4. Comparison of HA Products/HA Products Mixed with Other Substances
2.5. HA Administration Method (Frequency, Dose)
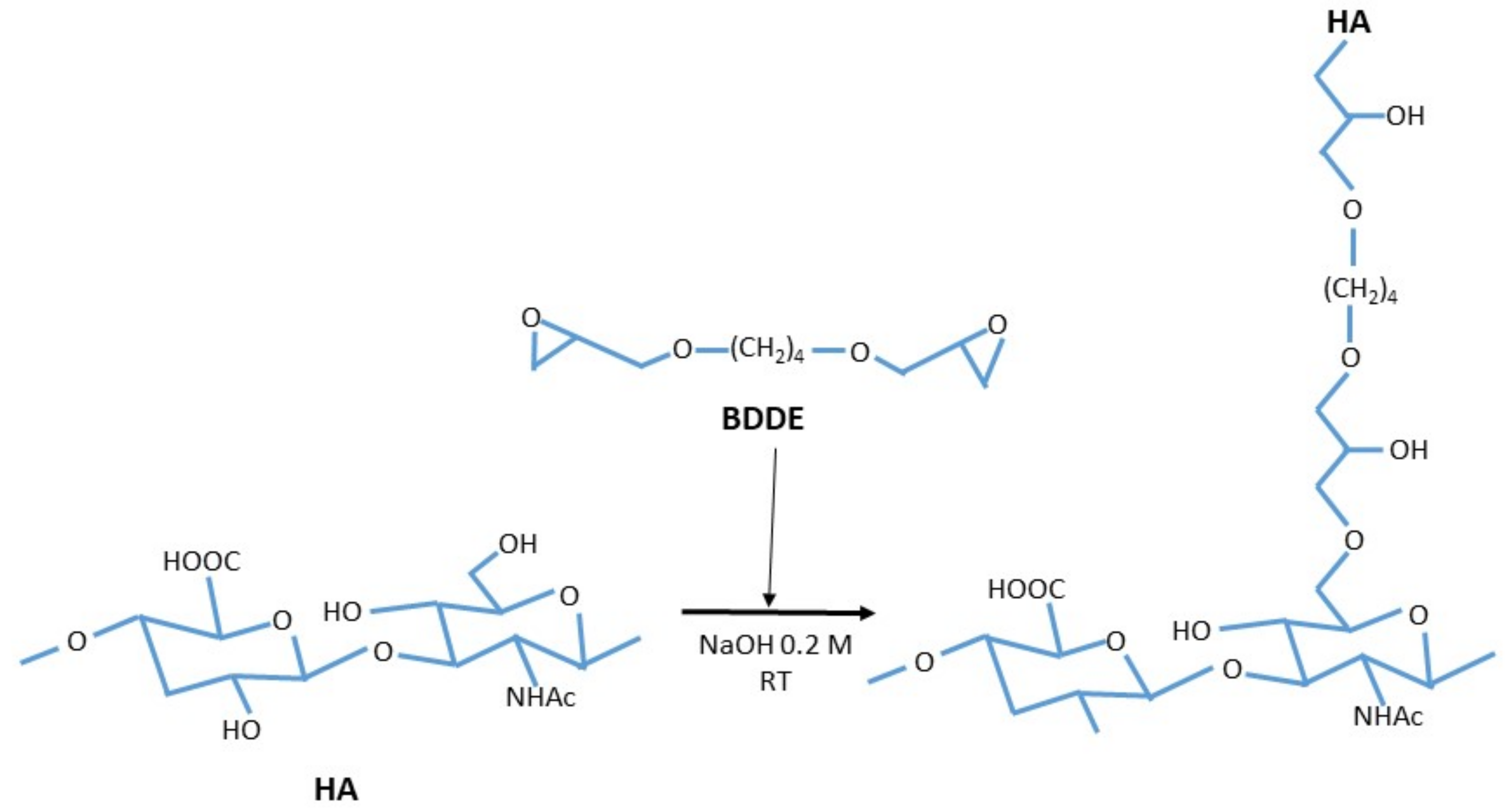
3. Cross-Linked Hyaluronic Acid
3.1. Cross Linked Hyaluronic Acid Physiology
3.2. Cross Linked HA Administration Method (Frequency, Dose)
4. Polynucleotide/Polydeoxyribonucleotide
4.1. PN/PDRN Physiology
4.2. PDRN/PN Administration Method (Frequency, Dose)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.; Helmick, C.; Boring, M.; Brady, T. Vital signs: Prevalence of doctor-diagnosed athritis and arthritis-attributable activity limitation—United States, 2013–2015. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Roman-Blas, J.A.; Bruyère, O.; Cooper, C.; Kanis, J.; Maggi, S.; Rizzoli, R.; Reginster, J.Y. Clinical settings in knee osteoarthritis: Pathophysiology guides treatment. Maturitas 2017, 96, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, L.; Taki, J.; Desjardins, B.; Thevenot, O.; Fransen, M.; Wells, G.A.; Mizusaki Imoto, A.; Toupin-April, K.; Westby, M.; Álvarez Gallardo, I.C.; et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: Strengthening exercise programs. Clin. Rehabil. 2017, 31, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Giardina, S.M.C.; Culmone, A.; Vescio, A.; Turchetta, M.; Cannavò, S.; Pavone, V. Intra-Articular Injections in Knee Osteoarthritis: A Review of Literature. J. Funct. Morphol. Kinesiol. 2021, 6, 15. [Google Scholar] [CrossRef]
- López-Ruiz, E.; Jiménez, G.; Álvarez de Cienfuegos, L.; Antic, C.; Sabata, R.; Marchal, J.A.; Gálvez-Martín, P. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur. Cell Mater. 2019, 37, 186–213. [Google Scholar] [CrossRef] [PubMed]
- Grecomoro, G.; Martorana, U.; Di Marco, C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: A controlled clinical trial versus placebo. Pharmatherapeutica 1987, 5, 137–141. [Google Scholar] [PubMed]
- Leardini, G.; Mattara, L.; Franceschini, M.; Perbellini, A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and 6-methyl prednisolone acetate. Clin. Exp. Rheumatol. 1991, 9, 375–381. [Google Scholar] [PubMed]
- Puhl, W.; Bernau, A.; Greiling, H.; Köpcke, W.; Pförringer, W.; Steck, K.J.; Zacher, J.; Scharf, H.P. Intra-articular sodium hyaluronate in osteoarthritis of the knee: A multicenter, double-blind study. Osteoarthr. Cartil. 1993, 1, 233–241. [Google Scholar] [CrossRef]
- Henderson, E.B.; Smith, E.C.; Pegley, F.; Blake, D.R. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: A randomised single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann. Rheum. Dis. 1994, 53, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Pattrick, M.; Doherty, S.; Doherty, M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthr. Cartil. 1995, 3, 269–273. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Dalén, N.; Englund, G.; Hämäläinen, M.; Jensen, E.M.; Karlsson, K.; Odensten, M.; Ryd, L.; Sernbo, I.; Suomalainen, O.; et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: A randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann. Rheum. Dis. 1996, 55, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Moskowitz, R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: A randomized clinical trial. Hyalgan Study Group. J. Rheumatol. 1998, 25, 2203–2212. [Google Scholar]
- Huskisson, E.C.; Donnelly, S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999, 38, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Block, J.A.; Michalski, J.P.; Moreland, L.W.; Caldwell, J.R.; Lavin, P.T. Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. ORTHOVISC Study Group. Clin. Orthop. Relat. Res. 2001, 385, 130–143. [Google Scholar] [CrossRef]
- Petrella, R.J.; DiSilvestro, M.D.; Hildebrand, C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled clinical trial. Arch. Intern. Med. 2002, 162, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Jubb, R.W.; Piva, S.; Beinat, L.; Dacre, J.; Gishen, P. A one-year, randomised, placebo (saline) controlled clinical trial of 500–730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int. J. Clin. Pract. 2003, 57, 467–474. [Google Scholar]
- Day, R.; Brooks, P.; Conaghan, P.G.; Petersen, M. A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J. Rheumatol. 2004, 31, 775–782. [Google Scholar] [PubMed]
- Neustadt, D.; Caldwell, J.; Bell, M.; Wade, J.; Gimbel, J. Clinical effects of intraarticular injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: A randomized, controlled, multicenter trial. J. Rheumatol. 2005, 32, 1928–1936. [Google Scholar] [PubMed]
- Petrella, R.J.; Petrella, M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J. Rheumatol. 2006, 33, 951–956. [Google Scholar] [PubMed]
- Stitik, T.P.; Blacksin, M.F.; Stiskal, D.M.; Kim, J.H.; Foye, P.M.; Schoenherr, L.; Choi, E.S.; Chen, B.; Saunders, H.J.; Nadler, S.F. Efficacy and safety of hyaluronan treatment in combination therapy with home exercise for knee osteoarthritis pain. Arch. Phys. Med. Rehabil. 2007, 88, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Heimer, M.; Valdeon, A.; Schwartz, H.; Sheldon, E. Effect of a natural extract of chicken combs with a high content of hyaluronic acid (Hyal-Joint) on pain relief and quality of life in subjects with knee osteoarthritis: A pilot randomized double-blind placebo-controlled trial. Nutr. J. 2008, 7, 1–9. [Google Scholar] [CrossRef]
- Altman, R.D.; Rosen, J.E.; Bloch, D.A.; Hatoum, H.T.; Korner, P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin. Arthritis Rheum. 2009, 39, 1–9. [Google Scholar] [CrossRef]
- Jørgensen, A.; Stengaard-Pedersen, K.; Simonsen, O.; Pfeiffer-Jensen, M.; Eriksen, C.; Bliddal, H.; Pedersen, N.W.; Bødtker, S.; Hørslev-Petersen, K.; Snerum, L.Ø.; et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: A multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann. Rheum Dis. 2010, 69, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Higuchi, H.; Takagishi, K.; Shinozaki, T.; Kobayashi, T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: Prospective randomized study of sodium hyaluronate and corticosteroid. Orthop. Sci. 2010, 15, 51–56. [Google Scholar] [CrossRef]
- Navarro-Sarabia, F.; Coronel, P.; Collantes, E.; Navarro, F.J.; de la Serna, A.R.; Naranjo, A.; Gimeno, M.; Herrero-Beaumont, G.; AMELIA study group. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: The AMELIA project. Ann. Rheum. Dis. 2011, 70, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chang, C.C.; Lee, C.H.; Chen, S.C.; Lai, C.H.; Tsai, C.L. Intra-articular injections of sodium hyaluronate (Hyalgan) in osteoarthritis of the knee. a randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord. 2011, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- DeCaria, J.E.; Montero-Odasso, M.; Wolfe, D.; Chesworth, B.; Petrella, R.J. The effect of intra-articular hyaluronic acid treatment on gait velocity in older knee osteoarthritis patients: A randomized, controlled study. Arch. Gerontol. Geriatr. 2012, 55, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Housman, L.; Arden, N.; Schnitzer, T.J.; Birbara, C.; Conrozier, T.; Skrepnik, N.; Wei, N.; Bockow, B.; Waddell, D.; Tahir, H.; et al. Intra-articular hylastan versus steroid for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1684–1692. [Google Scholar] [CrossRef]
- Ishijima, M.; Nakamura, T.; Shimizu, K.; Hayashi, K.; Kikuchi, H.; Soen, S.; Omori, G.; Yamashita, T.; Uchio, Y.; Chiba, J.; et al. Research Group of Cartilage Metabolism. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: A multi-center, randomized, open-label, non-inferiority trial. Arthritis Res. Ther. 2014, 16, R18. [Google Scholar] [CrossRef] [PubMed]
- van der Weegen, W.; Wullems, J.A.; Bos, E.; Noten, H.; van Drumpt, R.A. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: A randomized, controlled, double-blind trial. J. Arthroplasty 2015, 30, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.A.V.; Cossich, V.R.A.; Salles-Neto, J.I.; Aguiar, D.P.; de Sousa, E.B. Viscosupplementation improves pain, function and muscle strength, but not proprioception, in patients with knee osteoarthritis: A prospective randomized trial. Clinics 2019, 74, e1207. [Google Scholar] [CrossRef]
- Altman, R.D.; Devji, T.; Bhandari, M.; Fierlinger, A.; Niazi, F.; Christensen, R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: A systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006, 2, CD005321. [Google Scholar] [CrossRef]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Natov, N.S.; Dasi, U.R.; Schmid, C.H.; McAlindon, T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: A systematic review and network meta-analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Cooper, C.; Pelletier, J.P.; Maheu, E.; Rannou, F.; Branco, J.; Luisa Brandi, M.; Kanis, J.A.; Altman, R.D.; Hochberg, M.C.; et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin. Arthritis Rheum. 2016, 45, S3–S11. [Google Scholar] [CrossRef]
- Campbell, K.A.; Erickson, B.J.; Saltzman, B.M.; Mascarenhas, R.; Bach, B.R., Jr.; Cole, B.J.; Verma, N.N. Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatment of Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses. Arthroscopy 2015, 31, 2036–2045. [Google Scholar] [CrossRef]
- Strand, V.; McIntyre, L.F.; Beach, W.R.; Miller, L.E.; Block, J.E. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: A systematic review and meta-analysis of randomized, saline-controlled trials. J. Pain Res. 2015, 8, 217–228. [Google Scholar] [PubMed]
- Bhandari, M.; Bannuru, R.R.; Babins, E.M.; Martel-Pelletier, J.; Khan, M.; Raynauld, J.P.; Frankovich, R.; Mcleod, D.; Devji, T.; Phillips, M.; et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: A Canadian evidence-based perspective. Ther. Adv. Musculoskelet Dis. 2017, 9, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Yang, X.; Ren, Z.; Wang, J.; Dong, H. Comparison of intra-articular hyaluronic acid and methylprednisolone for pain management in knee osteoarthritis: A meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 53, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Bannuru, R.R.; Herrero-Beaumont, G.; Allali, F.; Bard, H.; Migliore, A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin. Arthritis Rheum. 2019, 48, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Nicholls, M.; Shaw, P.; Niazi, F.; Bhandari, M.; Bedi, A. The Impact of Excluding Patients with End-Stage Knee Disease in Intra-Articular Hyaluronic Acid Trials: A Systematic Review and Meta-Analysis. Adv. Ther. 2019, 36, 147–161. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Beaudart, C.; Lengelé, L.; Leclercq, V.; Geerinck, A.; Sanchez-Rodriguez, D.; Bruyère, O.; Reginster, J.Y. Symptomatic Efficacy of Pharmacological Treatments for Knee Osteoarthritis: A Systematic Review and a Network Meta-Analysis with a 6-Month Time Horizon. Drugs 2020, 80, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Gambaro, F.M.; Di Matteo, B.; Kon, E. Injections in the osteoarthritic knee: A review of current treatment options. EFORT Open Rev. 2021, 6, 501–509. [Google Scholar] [CrossRef]
- De Lucia, O.; Jerosch, J.; Yoon, S.; Sayre, T.; Ngai, W.; Filippou, G. One-year efficacy and safety of single or one to three weekly injections of hylan G-F 20 for knee osteoarthritis: A systematic literature review and meta-analysis. Clin. Rheumatol. 2021, 40, 2133–2142. [Google Scholar] [CrossRef]
- Pavone, V.; Vescio, A.; Turchetta, M.; Giardina, S.M.C.; Culmone, A.; Testa, G. Injection-Based Management of Osteoarthritis of the Knee: A Systematic Review of Guidelines. Front. Pharmacol. 2021, 20, 661805. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Seo, I.W.; Shin, Y.S. Intra-Articular Injections of Hyaluronic Acid or Steroids Associated with Better Outcomes Than Platelet-Rich Plasma, Adipose Mesenchymal Stromal Cells, or Placebo in Knee Osteoarthritis: A Network Meta-analysis. Arthroscopy 2021, 37, 292–306. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef]
- Monticone, M.; Frizziero, A.; Rovere, G.; Vittadini, F.; Uliano, D.; LABruna, S.; Gatto, R.; Nava, C.; Leggero, V.; Masiero, S. Hyaluronic acid intra-articular injection and exercise therapy: Effects on pain and disability in subjects affected by lower limb joints osteoarthritis. A systematic review by the Italian Society of Physical and Rehabilitation Medicine (SIMFER). Eur J. Phys. Rehabil Med. 2016, 52, 389–399. [Google Scholar] [PubMed]
- Saltzman, B.M.; Leroux, T.; Meyer, M.A.; Basques, B.A.; Chahal, J.; Bach, B.R.J.R.; Yanke, A.B.; Cole, B.J. The Therapeutic Effect of Intra-articular Normal Saline Injections for Knee Osteoarthritis: A Meta-analysis of Evidence Level 1 Studies. Am. J. Sports Med. 2017, 45, 2647–2653. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.A. AAOS clinical practice guideline: Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 2013, 21, 577–579. [Google Scholar] [PubMed]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lefèvre-Colau, M.M.; Poiraudeau, S.; Rannou, F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 184–189. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Balblanc, J.C.; Richette, P.; Mulleman, D.; Maillet, B.; Henrotin, Y.; Rannou, F.; Piroth, C.; Hilliquin, P.; Mathieu, P.; et al. Osteoarthritis Group of the French Society of Rheumatology. Early effect of hyaluronic acid intra-articular injections on serum and urine biomarkers in patients with knee osteoarthritis: An open-label observational prospective study. J. Orthop. Res. 2012, 30, 679–685. [Google Scholar] [CrossRef]
- Guidolin, D.D.; Ronchetti, I.P.; Lini, E.; Guerra, D.; Frizziero, L. Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthr. Cartil. 2001, 9, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Bert, J.M.; Bert, T.M. Nonoperative treatment of unicompartmental arthritis: From bracing to injection. Clin. Sports Med. 2014, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, V.M.; Goldberg, L. Intra-articular hyaluronans: The treatment of knee pain in osteoarthritis. J. Pain Res. 2010, 3, 51–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khalaj, N.; Abu Osman, N.A.; Mokhtar, A.H.; George, J.; Abas, W.A. Effect of intra-articular hyaluronic injection on postural stability and risk of fall in patients with bilateral knee osteoarthritis. Sci. World J. 2014, 2014, 815184. [Google Scholar] [CrossRef] [PubMed]
- Legré-Boyer, V. Viscosupplementation: Techniques, indications, results. Orthop. Traumatol. Surg. Res. 2015, 101, S101–S108. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Monari, P.; Cammalleri, D.; Pelizzari, L.; Calzavara-Pinton, P. Hyaluronic acid-based products are strictly contraindicated in scleroderma-related skin ulcers. Wounds 2019, 31, 81–88. [Google Scholar]
- Eymard, F.; Chevalier, X.; Conrozier, T. Obesity and radiological severity are associated with viscosupplementation failure in patients with knee osteoarthritis. J. Orthop. Res. 2017, 35, 2269–2274. [Google Scholar] [CrossRef]
- So, K.Y.; Lee, H.Y. Septic Arthritis after Intra-articular Hyaluronic Acid Injections in Patients with Knee Osteoarthritis-A report of two cases. Korean J. Pain. 2002, 15, 80–83. [Google Scholar]
- He, W.W.; Kuang, M.J.; Zhao, J.; Sun, L.; Lu, B.; Wang, Y.; Ma, J.X.; Ma, X.L. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: A meta-analysis. Int. J. Surg. 2017, 39, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, K.; Uebelhart, D. Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial(®)) vs hylan G-F20 (Synvisc(®)) in the treatment of symptomatic knee osteoarthritis. A double-blind, controlled, randomized, parallel-group non-inferiority study. Osteoarthr. Cartil. 2011, 19, 1294–1300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshioka, K.; Katayama, M.; Nishiyama, T.; Harada, K.; Takeshita, S.; Kawamata, Y. Biocompatibility study of different hyaluronan products for intra-articular treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2019, 20, 424. [Google Scholar] [CrossRef]
- Altman, R.D.; Bedi, A.; Karlsson, J.; Sancheti, P.; Schemitsch, E. Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee. Am. J. Sports Med. 2016, 44, 2158–2165. [Google Scholar] [CrossRef]
- Conrozier, T.; Eymard, F.; Afif, N.; Balblanc, J.C.; Legré-Boyer, V.; Chevalier, X.; Happyvisc Study Group. Safety and efficacy of intra-articular injections of a combination of hyaluronic acid and mannitol (HAnOX-M) in patients with symptomatic knee osteoarthritis: Results of a double-blind, controlled, multicenter, randomized trial. Knee 2016, 23, 842–848. [Google Scholar] [CrossRef]
- Borrás-Verdera, A.; Calcedo-Bernal, V.; Ojeda-Levenfeld, J.; Clavel-Sainz, C. Efficacy and safety of a single intra-articular injection of 2% hyaluronic acid plus mannitol in knee osteoarthritis over a 6-month period. Rev. Esp. Cir. Ortop. Traumatol. 2012, 56, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T. Is the Addition of a Polyol to Hyaluronic Acid a Significant Advance in the Treatment of Osteoarthritis? Curr. Rheumatol. Rev. 2018, 14, 226–230. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Gao, J.; Zhao, Y.; Zhao, Y.; Guo, L.; Chen, C.; Duan, Z.; Li, P.; Wei, L. Intra-Articular Injection of Cross-Linked Hyaluronic Acid-Dexamethasone Hydrogel Attenuates Osteoarthritis: An Experimental Study in a Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 411. [Google Scholar] [CrossRef]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A.; et al. Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef]
- Stitik, T.P.; Issac, S.M.; Modi, S.; Nasir, S.; Kulinets, I. Effectiveness of 3 Weekly Injections Compared With 5 Weekly Injections of Intra-Articular Sodium Hyaluronate on Pain Relief of Knee Osteoarthritis or 3 Weekly Injections of Other Hyaluronan Products: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 1042–1050. [Google Scholar] [CrossRef]
- Concoff, A.; Sancheti, P.; Niazi, F.; Shaw, P.; Rosen, J. The efficacy of multiple versus single hyaluronic acid injections: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2017, 18, 542. [Google Scholar] [CrossRef]
- Suppan, V.K.L.; Wei, C.Y.; Siong, T.C.; Mei, T.M.; Chern, W.B.; Nanta Kumar, V.K.; Sheng, K.R.; Sadashiva Rao, A. Randomized controlled trial comparing efficacy of conventional and new single larger dose of intra-articular viscosupplementation in management of knee osteoarthritis. J. Orthop. Surg. 2017, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.H.; Raeissadat, S.A.; Cheraghi, M.; Rahimi-Dehgolan, S.; Ebrahimpour, A. Efficacy of single high-molecular-weight versus triple low-molecular-weight hyaluronic acid intra-articular injection among knee osteoarthritis patients. BMC Musculoskelet Disord. 2020, 21, 550. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.W.; Park, Y.B.; Choi, C.H.; Kyung, H.S.; Lee, J.H.; Yoo, J.D.; Yoo, J.H.; Choi, C.H.; Kim, C.W.; Kim, H.C.; et al. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: A double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet Disord. 2017, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.E.; Atkinson, M.H.; Lussier, A.J.; Schulz, J.I.; Siminovitch, K.A.; Wade, J.P.; Zummer, M. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: A Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr. Cartil. 1995, 3, 213–225. [Google Scholar] [CrossRef]
- Wobig, M.; Dickhut, A.; Maier, R.; Vetter, G. Viscosupplementation with hylan G-F 20: A 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin. Ther. 1998, 20, 410–423. [Google Scholar] [CrossRef]
- Wobig, M.; Bach, G.; Beks, P.; Dickhut, A.; Runzheimer, J.; Schwieger, G.; Vetter, G.; Balazs, E. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: A comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin. Ther. 1999, 21, 1549–1562. [Google Scholar] [CrossRef]
- Karlsson, J.; Sjögren, L.S.; Lohmander, L.S. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology 2002, 41, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Leopold, S.S.; Redd, B.B.; Warme, W.J.; Wehrle, P.A.; Pettis, P.D.; Shott, S. Corticosteroid compared with hyaluronic acid injections for the treatment of osteoarthritis of the knee. A prospective, randomized trial. J. Bone Joint Surg. Am. 2003, 85, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Akermark, C.; Beaulieu, A.D.; Schnitzer, T. Durolane International Study Group. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthr. Cartil. 2004, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Caborn, D.; Rush, J.; Lanzer, W.; Parenti, D.; Murray, C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J. Rheumatol. 2004, 31, 333–343. [Google Scholar] [PubMed]
- Karatay, S.; Kiziltunc, A.; Yildirim, K.; Karanfil, R.C.; Senel, K. Effects of different hyaluronic acid products on synovial fluid NO levels in knee osteoarthritis. Clin. Rheumatol. 2005, 24, 497–501. [Google Scholar] [CrossRef]
- Karatosun, V.; Unver, B.; Gocen, Z.; Sen, A. Comparison of two hyaluronan drugs in patients with advanced osteoarthritis of the knee. A prospective, randomized, double-blind study with long term follow-up. Clin. Exp. Rheumatol. 2005, 23, 213–218. [Google Scholar]
- Jüni, P.; Reichenbach, S.; Trelle, S.; Tschannen, B.; Wandel, S.; Jordi, B.; Züllig, M.; Guetg, R.; Häuselmann, H.J.; Schwarz, H.; et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: A randomized controlled trial. Arthritis Rheum. 2007, 56, 3610–3619. [Google Scholar] [CrossRef]
- Chevalier, X.; Jerosch, J.; Goupille, P.; van Dijk, N.; Luyten, F.P.; Scott, D.L.; Bailleul, F.; Pavelka, K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: A randomised, multicentre, double-blind, placebo controlled trial. Ann. Rheum. Dis. 2010, 69, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Baraf, H.S.B.; Lavin, P.T.; Lim, S.; Hosokawa, H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2012, 20, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Rottigni, V.; Iannitti, T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des. Devel Ther. 2013, 7, 7–12. [Google Scholar]
- Arden, N.K.; Åkermark, C.; Andersson, M.; Todman, M.G.; Altman, R.D. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr. Med. Res. Opin. 2014, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, K.; Zhang, X.; Zhu, Z.; Yan, S.; Sun, T.; Guo, A.; Jones, J.; Steen, R.G.; Shan, B.; et al. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: A multicenter, randomized, double-blind, 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Res. Ther. 2015, 17, 51. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, P.; Liu, L. Origin and Efficacy of Hyaluronan Injections in Knee Osteoarthritis: Randomized, Double-Blind Trial. Med. Sci. Monit. 2018, 24, 4728–4737. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.; Bierma-Zeinstra, S.M.A.; Bos, P.K.; Niesten, D.D.; Verhaar, J.A.N.; Reijman, M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: A randomised controlled trial. BMC Musculoskelet Disord. 2019, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Takamura, J.; Seo, T.; Strand, V. A Single Intra-Articular Injection of Gel-200 for Treatment of Symptomatic Osteoarthritis of the Knee Is More Effective than Phosphate Buffered Saline at 6 Months: A Subgroup Analysis of a Multicenter, Randomized Controlled Trial. Cartilage 2019, 10, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Bannuru, R.; Malaise, M.; Ea, H.K.; Confavreux, C.; Bentin, J.; Urbin-Choffray, D.; Conrozier, T.; Brasseur, J.P.; Thomas, P.; et al. Hyaluronan derivative HYMOVIS® increases cartilage volume and type ii collagen turnover in osteoarhritic knee: Data from MOKHA study. BMC Musculoskelet Disord. 2019, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Petterson, S.C.; Plancher, K.D. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: A multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.E.; Dursema, H.D.; Pollak, C.T.; Skrabut, E.M. Clearance kinetics of a hylan-based viscosupplement after intra-articular and intravenous administration in animal models. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheum. Suppl. 1993, 39, 3–9. [Google Scholar]
- Wang, Y.; Hall, S.; Hanna, F.; Wluka, A.E.; Grant, G.; Marks, P.; Feletar, M.; Cicuttini, F.M. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: A two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011, 12, 195. [Google Scholar] [CrossRef]
- Khanasuk, Y.; Dechmaneenin, T.; Tanavalee, A. Prospective randomized trial comparing the efficacy of single 6-ml injection of hylan G-F 20 and hyaluronic acid for primary knee arthritis: A preliminary study. J. Med. Assoc. Thai. 2012, 95, 92–97. [Google Scholar]
- Frampton, J.E. Hylan G-F 20 single-injection formulation. Drugs Aging 2010, 27, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Jerosch, J.; Beks, P.; Kemper, F.; Euller-Ziegler, L.; Bailleul, F.; Chevalier, X. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: A pilot study. Arch. Orthop Trauma Surg. 2009, 129, 417–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.K.; Chung, J.Y. Effectiveness of polydeoxyribonucleotide injection versus normal saline injection for treatment of chronic plantar fasciitis: A prospective randomised clinical trial. Int Orthop. 2015, 39, 1329–1334. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Lee, D.H.; Lee, M.Y.; Yoon, S.H. Polydeoxyribonucleotide injection in the treatment of chronic supraspinatus tendinopathy: A case-controlled, retrospective, comparative study with 6-month follow-up. Arch. Phys. Med. Rehabil. 2017, 98, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Irrera, N.; D’Ascola, A.; Avenoso, A.; Nastasi, G.; Campo, G.M.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A(₂A) receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kang, J.J.; Kim, J.; Park, S.; Kim, J.M. Efficacy and safety of intra-articular injections of hyaluronic acid combined with polydeoxyribonucleotide in the treatment of knee osteoarthritis. Ann. Rehabil. Med. 2019, 43, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.R.; Kim, H.J. Anti-inflammatory Effect of DNA Polymeric Molecules in a Cell Model of Osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Di Stefano, A.; Cavezza, T.; Saladino, G.; Bellomo, R. Intrarticular treatment of osteoartropaty knee with polynucleotides: A pilot study with medium-term follow-up. J. Biol. Regul. Homeost. Agents 2013, 27, 543–549. [Google Scholar] [PubMed]
- Zazgyva, A.; Gergely, I.; Russu, O.M.; Roman, C.; Pop, T.S. Polynucleotides versus sodium hyaluronate in the local treatment of knee osteoarthritis. Acta Med. Transilv. 2013, 2, 260–263. [Google Scholar]
- Meccariello, L.; Cioffi, S.; Franzese, R.; Cioffi, R.; Petrucci, C.; Errico, G.; Olivieri, M.; Mugnaini, M. Intraarticular knee joint injection: Hyaluronic acid vs polynucleotides. Euromediterranean Biomed. J. 2013, 8, 35–41. [Google Scholar]
- Giarratana, L.S.; Marelli, B.M.; Crapanzano, C.; De Martinis, S.E.; Gala, L.; Ferraro, M.; Marelli, N.; Albisetti, W. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: The efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee 2014, 21, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Dallari, D.; Sabbioni, G.; Del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Fini, M. Efficacy of Intra-Articular Polynucleotides Associated with Hyaluronic Acid Versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Clin. J. Sport Med. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.N.; Lee, Y.J.; Sim, S.E.; Ko, Y.R.; Shim, J.W.; Lee, K.S.; Joo, M.; Park, H.J. Pilot Study to Evaluate the Efficacy of Polynucleotide Sodium Compared to Sodium Hyaluronate and Crosslinked Sodium Hyaluronate in Patients with Knee Osteoarthritis. J. Clin. Med. 2021, 10, 1138. [Google Scholar] [CrossRef]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; Piccolo, N.D.; Rani, N.; Govoni, M.; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, double-blind comparison of a fixed co-formulation of intra-articular polynucleotides and hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: Two-year follow-up. Res. Sq. 2021, 1, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, M.P.; Christjanson, L.; Deforge, S.; Deluca, B.; Gysbers, J.W.; Hindley, S.; Jovetich, M.; Middlemiss, P.; Takhal, S. Extracellular purine nucleosides stimulate cell division and morphogenesis: Pathological and physiological implications. Med. Hypotheses 1992, 37, 232–240. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Cho, R.K.; In, Y. Zhang of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef] [PubMed]
| Publication Year [Ref.] | Study Design | Comparison Agent | Patients Number (Treat/Control) | Injection Times | Result |
|---|---|---|---|---|---|
| 1987 [8] | RCT | saline | 34(17/17) | 3 | Significant difference compared to control group in all variables of spontaneous pain intensity, tactile sensation, load, and pain when walking at 60 days first post-injection |
| 1991 [9] | RCT | MPA | 40(20/20) | 3 | When comparing the HA group and the MPA group, the analgesic effect was the same during the treatment period, but the analgesic effect at 45 days after the end of treatment all the pain monitoring parameters presented significant differences in favor of the HA-treated group |
| 1993 [10] | RCT | 0.25 mg HA/2.5 mL | 209(102/107) | 5 | LI showed a significant superiority of the Verum-treated patients after the third injection until 9 weeks. The consumption of paracetamol did not reveal significant differences between the treatment groups |
| 1994 [11] | RCT | saline | 91(45/46) | 5 | No significant benefit compared to placebo group |
| 1995 [12] | RCT | TA | 63(32/31) | 5 | Significantly less pain was experienced by the HA group during the 6 months follow-up period |
| 1996 [13] | RCT | saline | 189(96/93) | 5 | Patients with knee OA over 60 years of age and high degree of knee OA have a greater therapeutic effect of IA HA injection |
| 1998 [14] | RCT | saline | 495(248/247) | 5 | HA group, VAS decreased by 2 or more (5~26 weeks). In the HA group about half of the group had mild pain or no pain at week 26 (about 30% of the naproxen group). There was also less pain when walking. |
| 1999 [15] | RCT | saline | 50(25/25) | 5 | Pain during walking, VAS, LI: Proved that the HA group was superior with a significant difference at 5 weeks and 6 months F/U |
| 2001 [16] | RCT | saline | 226(113/113) | 3 | WOMAC, pain on standing were improved from Weeks 7 to 27 |
| 2002 [17] | RCT | saline, NSAID | 120(30/30/30/30) | 3 | IA HA + p.o. placebo vs. IA HA + p.o. NSAID vs. IA placebo + p.o. NSAID vs. IA placebo + p.o. placebo: For resting pain relief, HA seems to be as effective as NSAIDs. For pain with physical activity and functional performance, HA may be superior to placebo alone or NSAIDs alone. |
| 2003 [18] | RCT | saline | 408(204/204) | 3 (2 cycle: total 6) | No superior effect compared to placebo group (in mean joint space width) |
| 2004 [19] | RCT | saline | 240(120/120) | 5 | WOMAC pain, stiffness improved compared to placebo group |
| 2005 [20] | RCT | arthrocentesis | 374(128/120/124) | 4 | HAX4 (O4), HAX3+ Arthrocentesis 1 time (O3A1), or 4 arthrocenteses (control group, A4). More O4 patients improved their WOMAC pain scores by more than 40% compared to A4. |
| 2006 [21] | RCT | saline | 106(53/53) | 3 | WOMAC, VAS improved compared to placebo group |
| 2007 [22] | Clinical study | Home exercise program | 60(20/20/20) | 5 | HA 5 times vs. HA 3 times vs. HA 3 times + exercise: HA 3 times + exercise group significantly faster onset of pain relief compared with other group: WOMAC, VAS improved in all groups |
| 2008 [23] | RCT | saline | 20(10/10) | 8 | WOMAC, VAS improved compared to placebo group |
| 2009 [24] | RCT | saline | 588(293/295) | 3 | WOMAC, pain, QOL improved compared to placebo group |
| 2010 [25] | RCT | saline | 48(24/24) | 3 | WOMAC, VAS improved but there was no statistically significant difference in functional and symptom improvement with respect to placebo |
| 2010 [26] | RCT | CS | 51(26/25) | 5 | VAS improved both groups; measured joint fluid levels of HA, biomarker: IA HA may have protective effects on the articular cartilage by increasing the HA concentration in synovial fluid, inhibitory effects on the catabolism |
| 2011 [27] | RCT | saline | 306(153/153) | 5 (4 cycle: total 20) | Received four cycles of five IA HA or placebo injections: repeated cycles of IA HA not only improve knee OA symptoms during the in-between cycle period but also exert a marked carry-over effect for at least 1 year after the last cycle. |
| 2011 [28] | RCT | saline | 200(100/100) | 5 | WOMAC, pain 50-foot walking test improved compared to placebo group |
| 2012 [29] | RCT | saline | 30(15/15) | 3 | Old age (mean age: 72), The overall effect of HA on gait velocity in older knee OA patients was not significant compared to placebo. |
| 2012 [30] | RCT | MPA | 391(130/129/132) | 3 | HAX1 vs. HAX2 vs. MPS 40 mg 1 time: Both IA HA regimens were effective in relieving pain: did not show a difference vs. IA MPA |
| 2014 [31] | Randomized, open-label | NSAID | 184(98/86) | 5 | HAX5 vs. NSAIDs 3 times per day for 5 weeks: early efficacy of IA HA is suggested to be not inferior to that of NSAIDs, and that the safety of the early phase of IA HA is superior to that of NSAIDs for patients with knee OA. |
| 2015 [32] | RCT | saline | 196(98/98) | 3 | HA was not superior to placebo. |
| 2019 [33] | RCT | HA + CS CS | 44(16/16/12) | 3 | In HA alone group, WOMAC, knee extensor and flexor strength improved, but not proprioception |
| Publication Year [Ref.] | Study Design | Pro/Cons | Result |
|---|---|---|---|
| 2006 [35] | Systemic review | pro | 5 to 13 weeks post injection period which showed improvement for pain and function. Comparable efficacy was noted against NSAIDs and longer-term benefits were noted in comparisons against IA CS |
| 2010 [36] | Systemic review | pro | HA is more effective than the placebo group without major side effects. It is similar to CS in terms of effect. |
| 2011 [37] | Meta-analysis | pro | IA HA is efficacious by 4 weeks, reaches its peak effectiveness at 8 weeks and exerts a residual detectable at 24 weeks. |
| 2015 [38] | Meta-analysis | pro | Acetaminophen, diclofenac, ibuprofen, naproxen, celecoxib, IA CS, IA HA, oral & IA placebo. Comparing 2 or more; most efficacious treatment is IA HA, the least efficacious treatment is acetaminophen |
| 2015 [39] | Systemic review | pro | ESCEO 2014 Short-term weekly HA injections are effective for up to 6 months. |
| 2015 [40] | Meta-analysis | pro | IA HA vs. NSAIDs, IA CSs, IA PRP, or IA placebo; The current highest level of evidence suggests that IA HA is a viable option for knee OA. |
| 2015 [41] | Meta-analysis | pro | IA HA vs. IA placebo; IA HA is safe and efficacious through 26 weeks in patients with symptomatic knee OA. |
| 2017 [42] | Systemic review | pro | statistically significant improvements in pain, function and stiffness up to 26 weeks were found with IA HA therapy compared with IA placebo or controls |
| 2018 [43] | Meta-analysis | pro | IA-HA vs. IA CS Both HA and CS injections were effective therapies for patients with knee OA. |
| 2019 [44] | Meta-analysis | pro | IA HA provides a moderate symptomatic benefit to knee OA patients and without major safety concerns |
| 2019 [45] | Systemic review | pro | OARSI guideline (2019) In the absence of comorbidity, the recommendation level is 2, and in the presence of cardiovascular and gastrointestinal comorbidity, the recommendation level is 1B. |
| 2019 [46] | Meta-analysis | pro | Efficacy of IA HA: early-moderate OA vs. end-stage OA Regardless of OA stage, IA HA provided significant pain relief compared to the saline group, but the level of pain relief was lower in end-stage OA patients. |
| 2019 [47] | Systemic review | pro | ESCEO 2019 Weak recommendation to the use of IAHA in patients who have contraindications to NSAIDs, or if the patient is still symptomatic despite the use of NSAIDs. |
| 2020 [48] | Meta-analysis | pro | Six months of treatment IA HA and the combination of IA HA and triamcinolone improve pain and/or physical function in patients suffering from knee OA. |
| 2021 [6] | Systemic review | pro | The use of IA HA is suggested for refractory patients who do not respond to treatment with drugs such as NSAIDs and acetaminophen. |
| 2021 [49] | Systemic review | pro | Even when not supported by high evidence consensus, intra-articular CS and HA injections have gained precise indications for symptomatic relief and clinical improvement in OA. |
| 2021 [50] | Meta-analysis | pro | Hylan G-F 20 at both frequencies (either as single or 1–3 weekly injections) are efficacious and generally well tolerated for long-term use. |
| 2021 [51] | Systemic review | pro | HA reported good outcomes both for pain reduction and functional improvement. |
| 2021 [52] | Meta-analysis | pro | In the meta-analysis, support the appropriate use of steroids and HAs for patients with Knee OA. For pain relief and AE, steroids are most likely the best treatment, followed by HA |
| 2012 [53] | Systemic review | cons | IA HA has fewer benefits and increases the risk of serious side effects. |
| 2016 [54] | Meta-analysis | cons | IA HA vs. physical therapy; Physical therapy agents seemed to have greater effects than IA HA on disability and pain. |
| 2017 [55] | Meta-analysis | cons | IA-saline injection yields a statistically and clinically meaningful improvement up to 6 months after the injection in patients with knee OA. placebo effect for IA saline injections affects outcomes during comparative studies for the IA HA group |
| 2019 [56] | Systemic review | cons | IA HA and IA CS can provide meaningful benefits to an appreciable number of patients, emerging evidence indicates that the apparent effectiveness of these treatments is largely a result of placebo effect. |
| Publication Year | Study Design | Comparison Agent | Patients Number (Treat/Control) | Injection Times | Result |
|---|---|---|---|---|---|
| 1995 [87] | RCT | NSAID | 102(34/31/37) | 3 | Hylan G-F 20 was better than continuous NSAID therapy for VAS, joint function except activity restriction at 26 weeks |
| 1998 [88] | RCT | saline | 117(57/60) | 3 | VAS, pain during weight-bearing, resting, painful movement-dramatic improvement during 24 weeks |
| 1999 [89] | RCT | LMW HA | 70(38/32) | 3 | VAS, pain during weight-bearing, painful movement: Hylan G-F 20 patients significantly better results compare with LMW HA patients |
| 2002 [90] | RCT | Artzal saline | 210(70/70/70) | 3 | WOMAC, weight-bearing pain, resting pain, maximum pain: no significant differences in outcome between any of the three study groups during the first 26 weeks Significantly longer duration of clinical benefit for hyaluronan treatment than for placebo. |
| 2003 [91] | RCT | betamethasone | 100(50/50) | 3 | VAS, WOMAC improved: no significant differences in outcome between two group |
| 2004 [92] | RCT | saline | 346(172/174) | 1 | WOMAC and quality of life were improved in both; greater response to NASHA than placebo was observed at week 6 |
| 2004 [93] | RCT | TA | 215(113/102) | 3 | WOMAC and VAS were improved in both; Hylan G-F 20 resulted in a longer duration of effect than TA |
| 2005 [94] | RCT | HA | 40(20/20) | 3 | Joint fluid aspiration: NO levels were reduced in both groups VAS, WOMAC improved: no significant differences in outcome between two group |
| 2005 [95] | RCT | Orthovisc | 184(92/92) | 3 | VAS, WOMAC improved during 52 weeks: no significant differences in outcome between two group |
| 2007 [96] | RCT | MMW HA, LMW HA | 660(220/220/220) | 3 | WOMAC improved: no significant differences in outcome between 3 group |
| 2010 [97] | RCT | saline | 232(115/117) | 1 | WOMAC and VAS were improved; Hylan G-F 20 statistically significantly greater improvements |
| 2012 [98] | RCT | saline | 379(251/128) | 1 | WOMAC and VAS were improved; GEL-200 statistically significantly greater improvements |
| 2013 [99] | RCT | DCF, CN | 61(20/21/20) | 1 | WOMAC improved: no significant differences in outcome between 3 group |
| 2014 [100] | RCT | saline | 218(108/110) | 1 | no significant differences in outcome; statistically significant improvement in pain relief at 6 weeks among patients without clinical effusion at baseline |
| 2015 [101] | RCT | Artz | 349(175/174) | 1 | Durolane 1 time vs. Artz 5 times WOMAC improved in both group; single injection of Durolane is non-inferior to 5 injections of Artz over 18 and 26 weeks for pain, physical function, global self-assessment, and knee stiffness |
| 2018 [102] | RCT | Hylan G-F 20 (ADH) | 258(129/129) | 1 | CCH vs. ADH VAS, WOMAC, Lequesne improved; CCH injection was superior to ADH injection. |
| 2019 [103] | Open label, RCT | Usual care | 156(77/79) | 3 | KOOS, VAS improved in both group; IA HMW-HA added to usual care is effective for knee OA in patients in the working age. |
| 2019 [104] | RCT | saline | 311(152/159) | 1 | WOMAC, VAS, stiffness improved; GEL-200 statistically significantly greater improvements |
| 2019 [105] | Open label, prospective | non | 46 | 2 | decrease of cartilage catabolism, MRI cartilage volume and thickness increased |
| 2019 [106] | RCT | saline | 369(185/184) | 1 | WOMAC, VAS improved; Monovisc statistically significantly greater improvements |
| 2020 [85] | RCT | Hyalgan | 90(45/45) | 3 | WOMAC, VAS, LI improved in both group; Athromac statistically significantly greater improvements at WOMAC stiffness. |
| Publication Year [Ref] | Agent | Study Design | Comparison Agent | Patients Number (Treat/Control) | Injection Times | Result |
|---|---|---|---|---|---|---|
| 2010 [118] | PN | RCT | HA | 60(30/30) | 5 | VAS, KOOS, NSAID consumption improved: no significant differences in outcome between two group |
| 2013 [119] | PN | Open label | non | 95 | Radiologic OA: 1 Severe OA: 3 | KOOS, NRS, ROM improved. |
| 2013 [120] | PN | RCT | HA | 30(15/15) | 3 | VAS, KOOS improved in both group; PN statistically significantly greater improvements at KSS. |
| 2013 [121] | PN | Retrospective | HA | 60(30/30) | 4 | VAS, KOOS improved in both group; PN statistically significantly greater improvements after 6 month. |
| 2014 [122] | PN | RCT | HA | 72(36/36) | 3 | VAS, KOOS improved in both group; PN showed a significant difference after 2 weeks and HA after 18 weeks |
| 2020 [123] | PN | RCT | HA | 100(50/50) | 3 | PN + HA vs. HA VAS, KSS improved in both group: PN + HA group significantly greater improvements at knee function, pain. |
| 2021 [124] | PN | Retrospective | HA, CLHA | 15(5/5/5) | 3 | Function or weight-bearing pain, PN decreased the most by 6 weeks, and there was no difference between groups in VAS, K-WOMAC, EuroQoL, and painDETECT |
| 2021 [125] | PN | RCT | HA | 98(49/49) | 3 | PN + HA vs. HA WOMAC, KSS improved in both group: PN + HA group significantly greater improvements at knee function, pain after 2 years. |
| 2019 [116] | PDRN | RCT | HA | 30(15/15) | 3 | PDRN + HA vs. HA WOMAC, KSS improved in both group: PDRN + HA group significantly greater improvements at VAS, WOMAC. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Park, H.J.; Rho, M.C.; Joo, J. Viscosupplementation in the Therapy for Osteoarthritic Knee. Appl. Sci. 2021, 11, 11621. https://doi.org/10.3390/app112411621
Park J, Park HJ, Rho MC, Joo J. Viscosupplementation in the Therapy for Osteoarthritic Knee. Applied Sciences. 2021; 11(24):11621. https://doi.org/10.3390/app112411621
Chicago/Turabian StylePark, Junghyun, Hue Jung Park, Min Cheol Rho, and Jin Joo. 2021. "Viscosupplementation in the Therapy for Osteoarthritic Knee" Applied Sciences 11, no. 24: 11621. https://doi.org/10.3390/app112411621
APA StylePark, J., Park, H. J., Rho, M. C., & Joo, J. (2021). Viscosupplementation in the Therapy for Osteoarthritic Knee. Applied Sciences, 11(24), 11621. https://doi.org/10.3390/app112411621






