Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions
Abstract
:1. Introduction
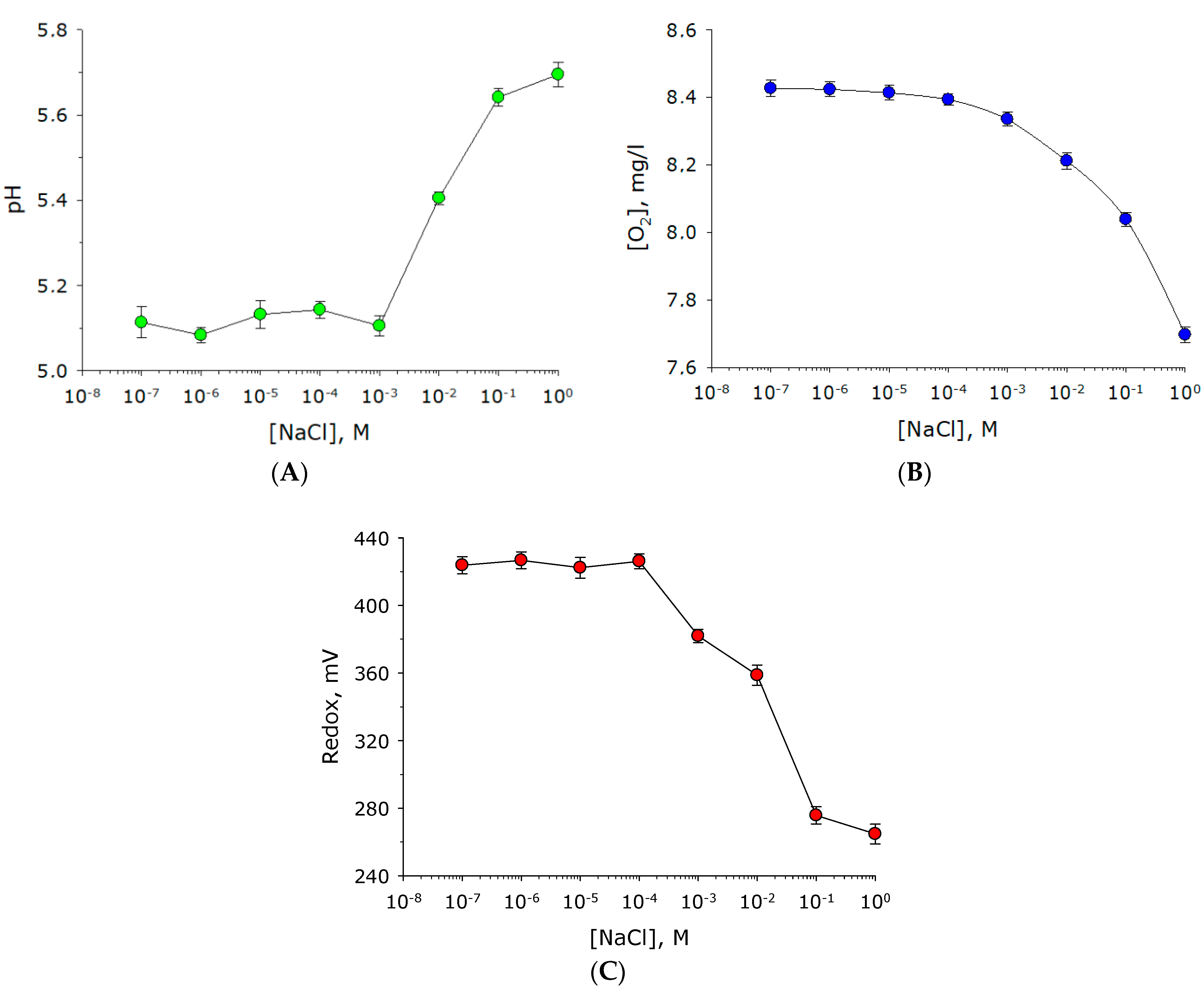
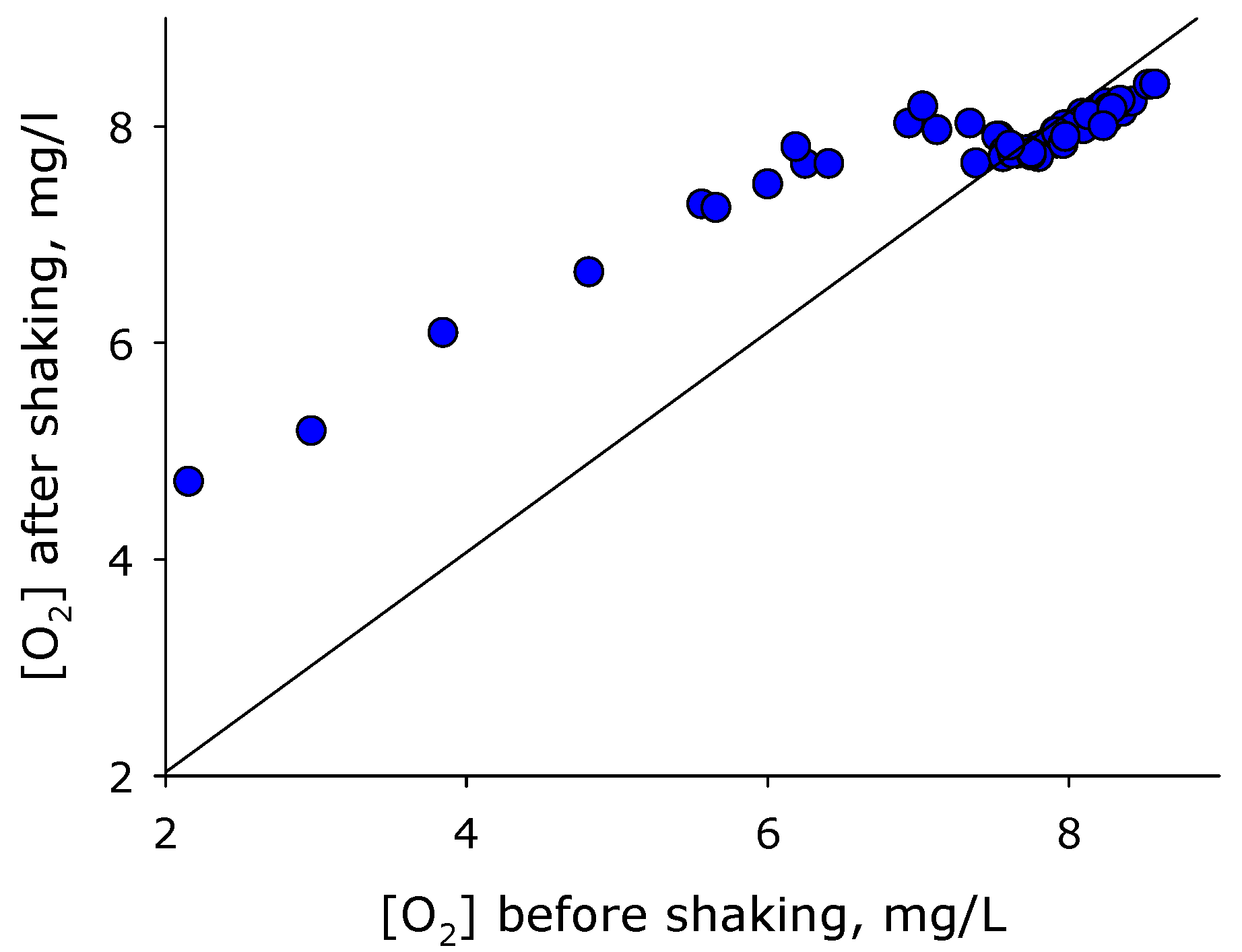
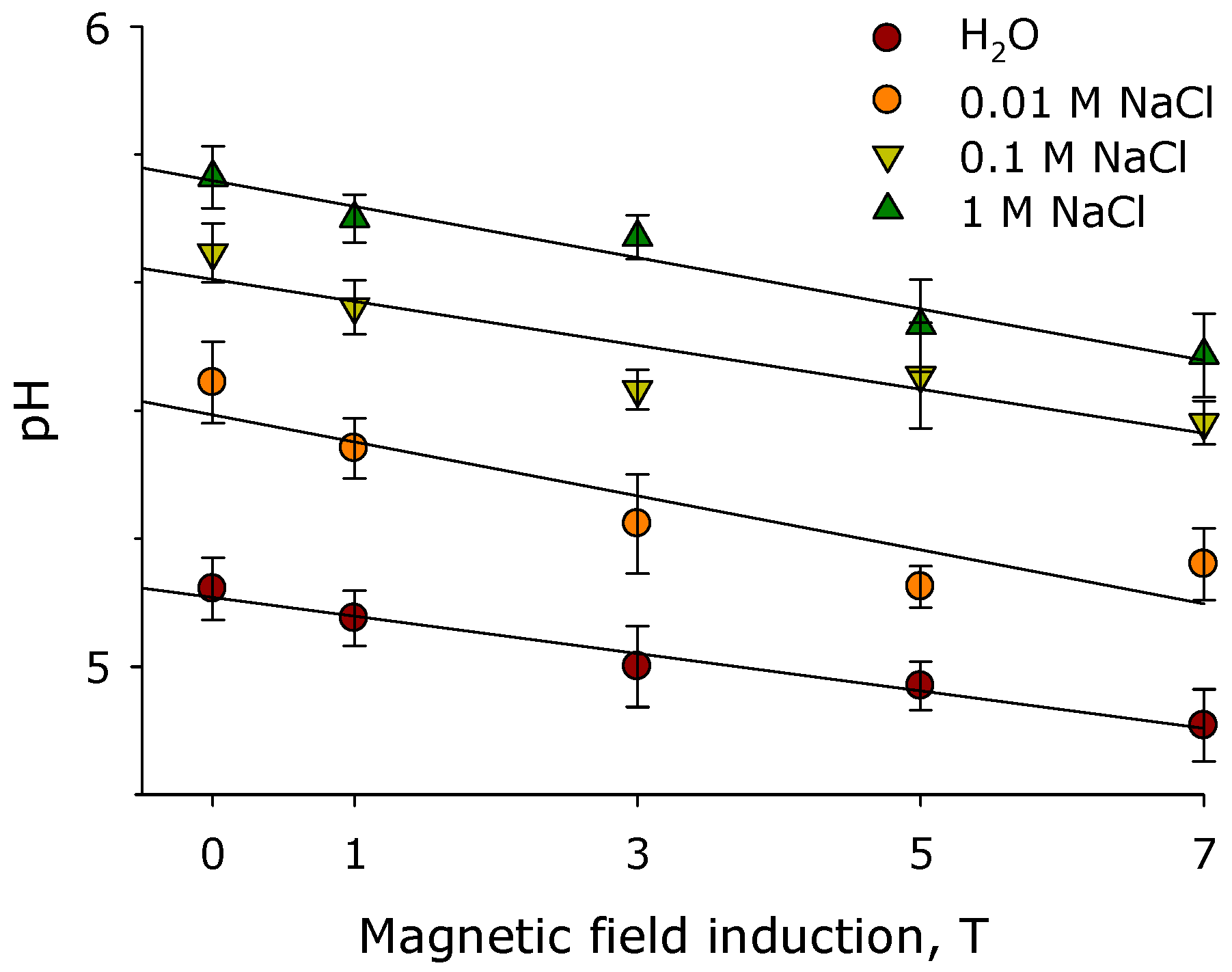
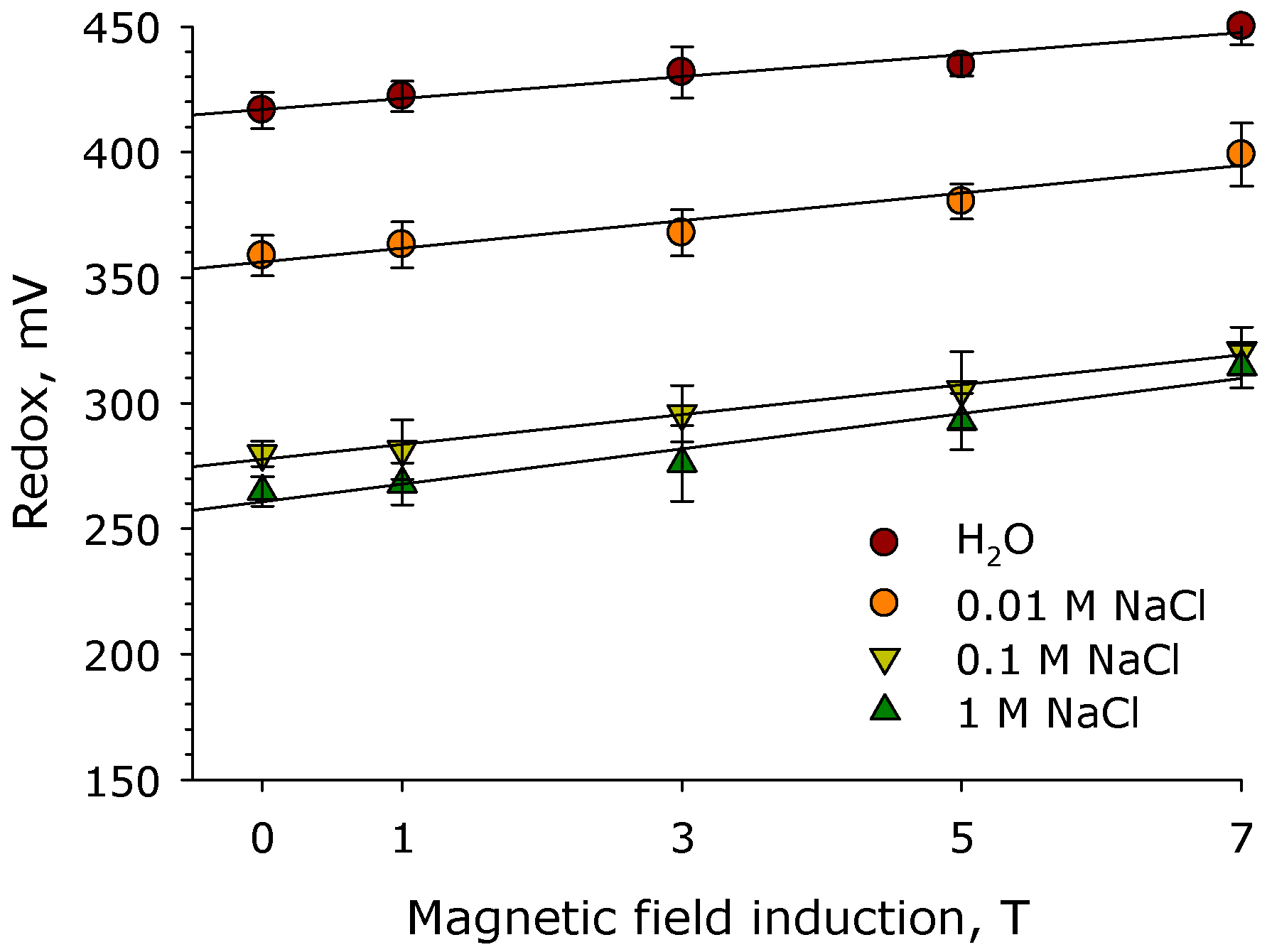
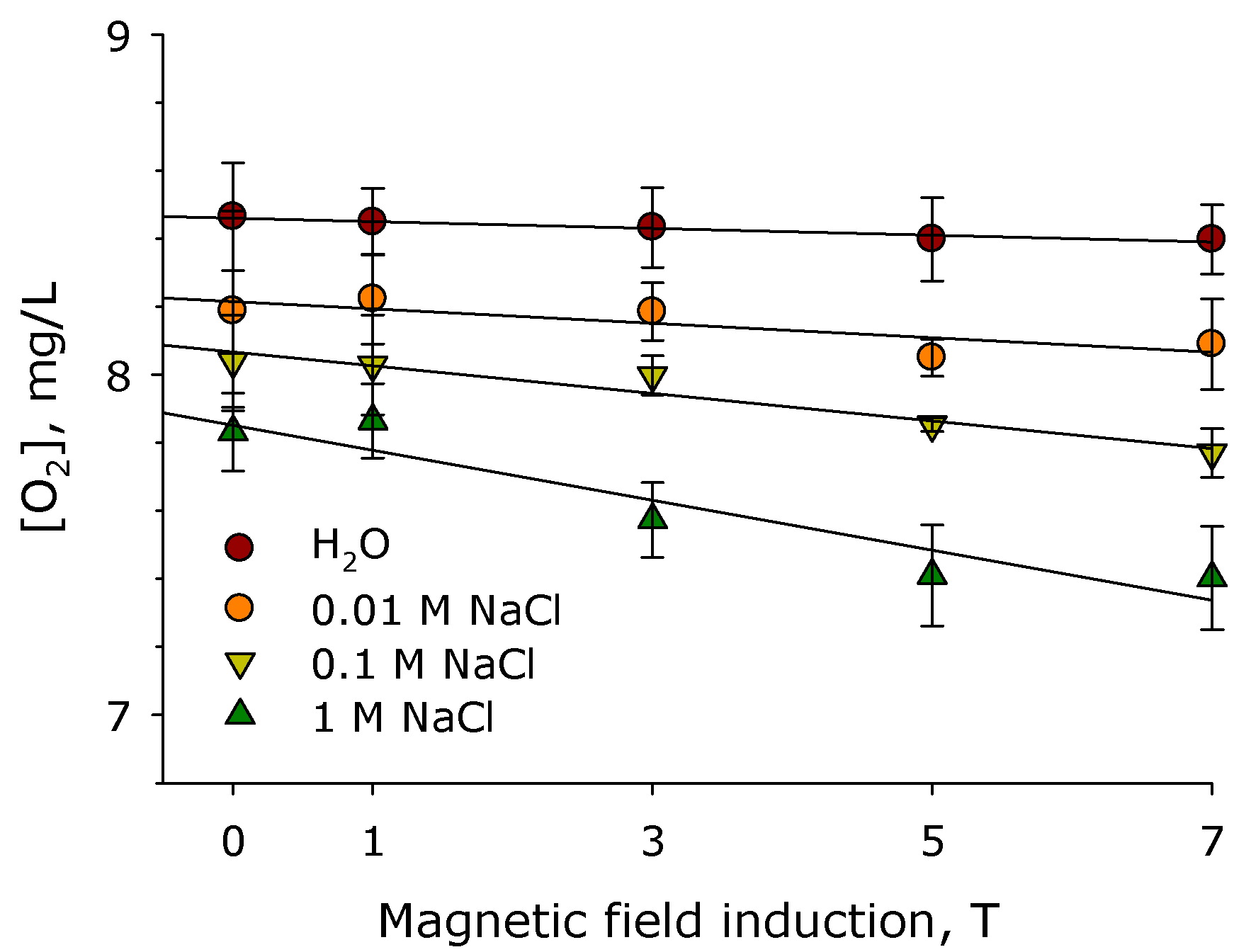
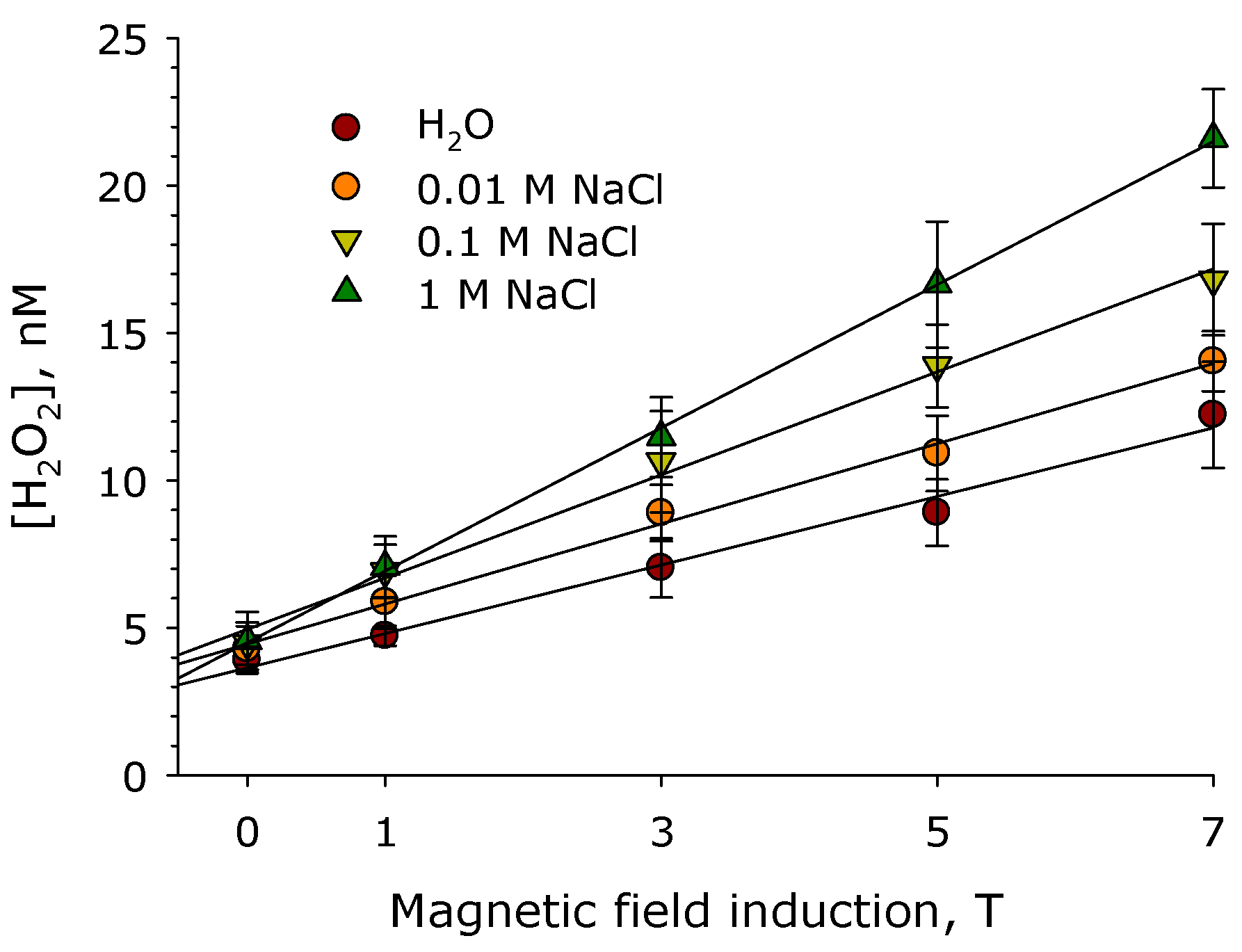
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Mixing with Microfluidics
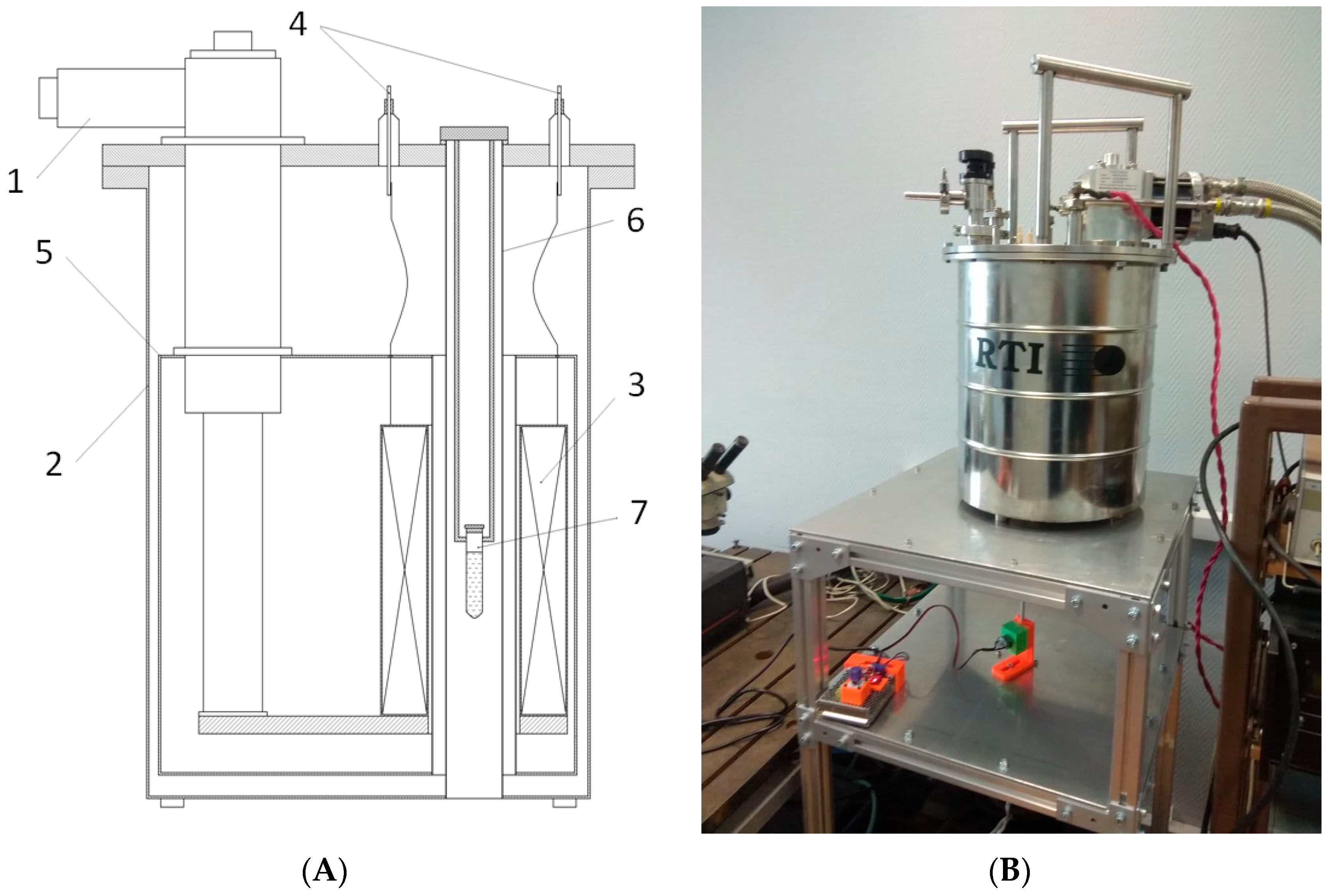
5.3. Exposure of Samples to a Magnetic Field
5.4. Measurement of the Concentration of Hydrogen Peroxide in Aqueous Solutions
5.5. Measurement of the Concentration of Dissolved Molecular Oxygen in Aqueous Solutions
5.6. Measurement of Redox Potential of Aqueous Solutions
5.7. Statistical Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerrato-Carretero, P.; Roncero-Martin, R.; Pedrera-Zamorano, J.D.; Lopez-Espuela, F.; Puerto-Parejo, L.M.; Sanchez-Fernandez, A.; Canal-Macias, M.L.; Moran, J.M.; Lavado-Garcia, J.M. Long-Term Dietary and Physical Activity Interventions in the School Setting and Their Effects on BMI in Children Aged 6–12 Years: Meta-Analysis of Randomized Controlled Clinical Trials. Healthcare 2021, 9, 396. [Google Scholar] [CrossRef]
- Noda, Y.; Suzuki, H.; Kanai, T.; Samejima, Y.; Nasu, S.; Tanaka, A.; Morishita, N.; Okamoto, N.; Hirashima, T. The Association Between Extracellular Water-to-Total Body Water Ratio and Therapeutic Durability for Advanced Lung Cancer. Anticancer. Res. 2020, 40, 3931–3937. [Google Scholar] [CrossRef]
- Piglowska, M.; Guligowska, A.; Kostka, T. Nutritional Status Plays More Important Role in Determining Functional State in Older People Living in the Community than in Nursing Home Residents. Nutrients 2020, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
- Hevesy, G.; Hofer, E. Elimination of water from the human body. Nature 1934, 134, 879. [Google Scholar] [CrossRef]
- Stoot, L.J.; Cairns, N.A.; Cull, F.; Taylor, J.J.; Jeffrey, J.D.; Morin, F.; Mandelman, J.W.; Clark, T.D.; Cooke, S.J. Use of portable blood physiology point-of-care devices for basic and applied research on vertebrates: A review. Conserv. Physiol. 2014, 2. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Koshland, J.; Daniel, E. Effect of Catalysts on the Hydrolysis of Acetyl Phosphate. Nucleophilic Displacement Mechanisms in Enzymatic Reactions1. J. Am. Chem. Soc. 1952, 74, 2286–2292. [Google Scholar] [CrossRef]
- Gulyaev, Y.V.; Markov, I.A.; Ten, Y.A. Reagentless Modified Water and Its Biological Activity. Phys. Wave Phenom. 2020, 28, 98–102. [Google Scholar] [CrossRef]
- Kononov, M.A.; Pustovoy, V.I.; Svetikov, V.V. Specific Features of the Excitation of Surface Plasmons at the Interface between Metal and Aqueous Solution of Extremely Low Concentration. Phys. Wave Phenom. 2020, 28, 94–97. [Google Scholar] [CrossRef]
- Korenbaum, V.; Chernysheva, T.; Galay, V.; Galay, R.; Ustinov, A.; Zakharkov, S.; Bunkin, N. Possible Effect of Human-Experimenter on Homeopathic-Like Aqueous Preparations. Water 2021, 13, 1475. [Google Scholar] [CrossRef]
- Penkov, N.V. Temporal Dynamics of the Scattering Properties of Deionized Water. Phys. Wave Phenom. 2020, 28, 135–139. [Google Scholar] [CrossRef]
- Stunzhas, P.A. Mechanochemical Instability of Water and Its Applications. Phys. Wave Phenom. 2020, 28, 111–115. [Google Scholar] [CrossRef]
- Joshi, K.M.; Kamat, P.V. Effect of magnetic field on the physical properties of water. J. Ind. Chem. Soc 1966, 43, 620–622. [Google Scholar]
- Quickenden, T.I.; Betts, D.M.; Cole, B.; Noble, M. Effect of magnetic fields on the pH of water. J. Phys. Chem. 1971, 75, 2830–2831. [Google Scholar] [CrossRef]
- Gonet, B. Influence of Constant Magnetic Fields on Certain Physiochemical Properties of Water. Bioelectromagnetics 1985, 6, 169–175. [Google Scholar] [CrossRef]
- Ueno, S.; Iwasaka, M.; Kitajima, T. Redistribution of dissolved oxygen concentration under magnetic fields up to 8 T. J. Appl. Phys. 1994, 75, 7174–7176. [Google Scholar] [CrossRef]
- Pang, X.F.; Deng, B.; Tang, B. Influences of magnetic field on macroscopic properties of water. Mod. Phys. Lett. B 2012, 26, 13. [Google Scholar] [CrossRef]
- Seyfi, A.; Afzalzadeh, R.; Hajnorouzi, A. Increase in water evaporation rate with increase in static magnetic field perpendicular to water-air interface. Chem. Eng. Process.-Process. Intensif. 2017, 120, 195–200. [Google Scholar] [CrossRef]
- Parsons, S.A.; Wang, B.L.; Judd, S.J.; Stephenson, T. Magnetic treatment of calcium carbonate—Effect of pH control. Water Res. 1997, 31, 339–342. [Google Scholar] [CrossRef]
- Ayrapetyan, S.N.; Grigorian, K.V.; Avanesian, A.S.; Stamboltsian, K.V. Magnetic fields alter electrical properties of solutions and their physiological effects. Bioelectromagnetics 1994, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Al Helal, A.; Soames, A.; Gubner, R.; Iglauer, S.; Barifcani, A. Influence of magnetic fields on calcium carbonate scaling in aqueous solutions at 150 degrees C and 1 bar. J. Colloid Interface Sci. 2018, 509, 472–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, I.B.; Neto, J.C.Q.; Petri, D.F.S. The effect of magnetic field on ion hydration and sulfate scale formation. Colloids Surf. Physicochem. Eng. Asp. 2015, 465, 175–183. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, J.D.; Liu, Z.A.; Zhao, E.J.; Yang, X.; Shao, X.J. Effect on the calcium carbonate scale in circulating cooling water: Constant magnetic and pulsed magnetic fields. Desalination Water Treat. 2018, 124, 125–133. [Google Scholar] [CrossRef]
- Dempsey, M.F.; Condon, B.; Hadley, D.M. MRI safety review. Semin. Ultrasound CT MRI 2002, 23, 392–401. [Google Scholar] [CrossRef]
- Glaser, R. Biophysics: An Introduction, 2nd ed.; Springer Science & Business Media: Heidelberg, Germany, 2012; p. 407. [Google Scholar]
- Lai, H.C.; Singh, N.P.; IOP. Medical Applications of Electromagnetic Fields. In Proceedings of the Conference on Electromagnetic Phenomena and Health—A Continuing Controversy, London, UK, 10 September 2008. [Google Scholar]
- Wang, J.; Xiang, B.; Deng, J.X.; Freed, D.H.; Arora, R.C.; Tian, G.H. Inhibition of Viability, Proliferation, Cytokines Secretion, Surface Antigen Expression, and Adipogenic and Osteogenic Differentiation of Adipose-Derived Stem Cells by Seven-Day Exposure to 0.5 T Static Magnetic Fields. Stem Cells Int. 2016, 2016, 7168175. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Melnikov, G.Y.; Makarova, E.B.; Fadeyev, F.A.; Sedneva-Lugovets, D.V.; Shabadrov, P.A.; Volchkov, S.O.; Mekhdieva, K.R.; Safronov, A.P.; Armas, S.F.; et al. Effects of Constant Magnetic Field to the Proliferation Rate of Human Fibroblasts Grown onto Different Substrates: Tissue Culture Polystyrene, Polyacrylamide Hydrogel and Ferrogels gamma-Fe(2)O(3)Magnetic Nanoparticles. Nanomaterials 2020, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Raylman, R.R.; Clavo, A.C.; Wahl, R.L. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics 1996, 17, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Zefirov, N.S. Chemical encyclopedia. Soviet Encycl. 1998, 5, 3355. [Google Scholar]
- Chernikov, A.V.; Gudkov, S.V.; Shtarkman, I.N.; Bruskov, V.I. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar]
- Zobell, C.E. Studies on redox potential of marine sediments. AAPG Bull. 1946, 30, 477–513. [Google Scholar]
- Chibowski, E.; Hotysz, L.; Szczes, A. Time dependent changes in zeta potential of freshly precipitated calcium carbonate. Colloids Surf. Physicochem. Eng. Asp. 2003, 222, 41–54. [Google Scholar] [CrossRef]
- Holysz, L.; Szczes, A.; Chibowski, E. Effects of a static magnetic field on water and electrolyte solutions. J. Colloid Interface Sci. 2007, 316, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Arps, J.J. The effect of temperature on the density and electrical resistivity of sodium chloride solutions. J. Pet. Technol. 1953, 5, 17–20. [Google Scholar] [CrossRef]
- Scatchard, G.; Hamer, W.J.; Wood, S.E. Isotonic solutions. I. The chemical potential of water in aqueous solutions of sodium chloride, potassium chloride, sulfuric acid, sucrose, urea and glycerol at 25. J. Am. Chem. Soc. 1938, 60, 3061–3070. [Google Scholar] [CrossRef]
- Shatkay, A.; Lerman, A. Individual activities of sodium and chloride ions in aqueous solutions of sodium chloride. Anal. Chem. 1969, 41, 514–517. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of Mechanical Shaking on the Physicochemical Properties of Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, I.A. Specific Features of the Concentration Dependences of Impurities in Condensed Media. Phys. Wave Phenom. 2020, 28, 83–87. [Google Scholar] [CrossRef]
- Yablonskaya, O.; Voeikov, V.; Buravleva, E.; Trofimov, A.; Novikov, K. Physicochemical Effects of Humid Air Treated with Infrared Radiation on Aqueous Solutions. Water 2021, 13, 1370. [Google Scholar] [CrossRef]
- Iwasaka, M.; Ueno, S. Structure of water molecules under 14 T magnetic field. J. Appl. Phys. 1998, 83, 6459–6461. [Google Scholar] [CrossRef]
- Cai, R.; Yang, H.W.; He, J.S.; Zhu, W.P. The effects of magnetic fields on water molecular hydrogen bonds. J. Mol. Struct. 2009, 938, 15–19. [Google Scholar] [CrossRef]
- Ding, Z.R.; Zhao, Y.J.; Chen, F.L.; Chen, J.Z.; Duan, S.X. Magnetization mechanism of magnetized water. Acta Phys. Sin. 2011, 60, 432–439. [Google Scholar]
- Wang, Y.F.; Zhang, B.; Gong, Z.B.; Gao, K.X.; Ou, Y.J.; Zhang, J.Y. The effect of a static magnetic field on the hydrogen bonding in water using frictional experiments. J. Mol. Struct. 2013, 1052, 102–104. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wei, H.N.; Li, Z.W. Effect of magnetic field on the physical properties of water. Results Phys. 2018, 8, 262–267. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.Y.; Li, F.; Yuan, J.S. Study of Raman Spectroscopy on the Structure of NH4Cl Aqueous Solution Under Strong Magnetic Field. Spectrosc. Spectr. Anal. 2021, 41, 116–121. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Chernikov, A.V.; Gudkov, S.V.; Masalimov, Z.K. Thermal activation of the reducing properties of seawater anions. Biophysics 2003, 48, 942–949. [Google Scholar]
- Bruskov, V.I.; Chernikov, A.V.; Ivanov, V.E.; Karmanova, E.E.; Gudkov, S.V. Formation of the Reactive Species of Oxygen, Nitrogen, and Carbon Dioxide in Aqueous Solutions under Physical Impacts. Phys. Wave Phenom. 2020, 28, 103–106. [Google Scholar] [CrossRef]
- Lyakhov, G.A.; Shcherbakov, I.A.; Shermeneva, M.A. Layering Phase Transition in a Liquid Solution with Cross Hydrogen Bonds: Bond Number Density as the Second Order Parameter. Phys. Wave Phenom. 2020, 28, 236–240. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Karp, O.E.; Bruskov, V.I.; Andreev, S.N.; Bunkin, N.F.; Gudkov, S.V. Formation of long-lived reactive products in blood serum under heat treatment and low-intensity laser irradiation, their role in hydrogen peroxide generation and DNA damage. Indian J. Biochem. Biophys. 2019, 56, 214–223. [Google Scholar]
- Baimler, I.V.; Lisitsyn, A.B.; Gudkov, S.V. Water Decomposition Occurring During Laser Breakdown of Aqueous Solutions Containing Individual Gold, Zirconium, Molybdenum, Iron or Nickel Nanoparticles. Front. Phys. 2020, 8, 600. [Google Scholar] [CrossRef]
- Baymler, I.V.; Gudkov, S.V.; Sarimov, R.M.; Simakin, A.V.; Shcherbakov, I.A. Concentration Dependences of Molecular Oxygen and Hydrogen in Aqueous Solutions. Dokl. Phys. 2020, 65, 5–7. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Binhi, V.N.; Matveeva, T.A.; Penkov, N.V.; Gudkov, S.V. Unfolding and Aggregation of Lysozyme under the Combined Action of Dithiothreitol and Guanidine Hydrochloride: Optical Studies. Int. J. Mol. Sci. 2021, 22, 2710. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar] [CrossRef]
| Samples | Content | pH | Redox, mV | [O2], mg/L |
|---|---|---|---|---|
| Sample 1 | 2 M NaCl solution mixed with highly diluted NaCl | 5.65 ± 0.01 | 290.2 ± 2.9 * | 7.88 ± 0.01 |
| Sample 2 | 2 M NaCl solution mixed with highly diluted H2O | 5.65 ± 0.02 | 291.6 ± 4.3 * | 7.86 ± 0.02 |
| Sample 3 | NaCl without mixing | 5.63 ± 0.01 | 275.1 ± 2.0 | 7.84 ± 0.02 |
| Sample 4 | H2O mixed with highly diluted NaCl (10−12 M) | 5.13 ± 0.04 | 424.9 ± 8.1 * | 8.43 ± 0.03 * |
| Sample 5 | H2O mixed with highly diluted H2O | 5.04 ± 0.07 | 428.2 ± 8.8 * | 8.45 ± 0.03 * |
| Sample 6 | H2O without mixing | 5.15 ± 0.05 | 416.5 ± 5.1 * | 8.42 ± 0.04 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarimov, R.M.; Simakin, A.V.; Matveeva, T.A.; Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Troitskii, A.V.; Shcherbakov, I.A. Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions. Appl. Sci. 2021, 11, 11466. https://doi.org/10.3390/app112311466
Sarimov RM, Simakin AV, Matveeva TA, Gudkov SV, Lyakhov GA, Pustovoy VI, Troitskii AV, Shcherbakov IA. Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions. Applied Sciences. 2021; 11(23):11466. https://doi.org/10.3390/app112311466
Chicago/Turabian StyleSarimov, Ruslan M., Alexander V. Simakin, Tatyana A. Matveeva, Sergey V. Gudkov, Gennady A. Lyakhov, Vladimir I. Pustovoy, Alexey V. Troitskii, and Ivan A. Shcherbakov. 2021. "Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions" Applied Sciences 11, no. 23: 11466. https://doi.org/10.3390/app112311466
APA StyleSarimov, R. M., Simakin, A. V., Matveeva, T. A., Gudkov, S. V., Lyakhov, G. A., Pustovoy, V. I., Troitskii, A. V., & Shcherbakov, I. A. (2021). Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions. Applied Sciences, 11(23), 11466. https://doi.org/10.3390/app112311466








