Abstract
Aim: Although the association between left ventricular dilation and mitral annulus dilation is well understood, the potential variation in the size of the mitral annulus during dilation of the left atrium is currently unknown. In order to investigate the link between the two variables, we used multidetector computed tomography (MDCT) and looked at patients who had a dilated left atrium, assessing if the mitral valve also dilates. Materials and Methods: The study included 107 patients with paroxysmal and persistent atrial fibrillation, in whom catheter ablation was performed using pulmonary vein isolation ± atrial substrate modification. Eighty patients were male (74.8%), with a mean age of 55.8 years (±9.87 with a minimum age of 26 years and a maximum age of 79 years), of which 57.1% had paroxysmal AF and the rest had persistent fibrillation. All the patients underwent multiple-detector CT (MDCT) with contrast medium before the ablation. CT images were integrated into the three-dimensional mapping system CARTO 3, after which the diameters of the mitral annulus, area, and circumference were measured. Left atrial size was evaluated by measuring the diameters, area, and volume. Results: The left atrial area was 247 ± 65.7 cm2 and the left atrial volume was 139 ± 56.3 mL. The transverse mitral annulus (MA) was 29.9 ± 5.3 mm and the longitudinal diameter was 41.9 ± 7.6 mm. The MA circumference and area were 15.0 ± 3.5 cm and 14.2 ± 4.6 cm2, respectively. The following statistically significant correlation was identified between the dimensions of the mitral annulus and the diameters of the left atrium: the transverse mitral annulus correlates with the antero-posterior (AP) LA diameter (R = 0.594, p < 0.01) and the longitudinal MA diameter correlates with the latero-lateral (LL) LA diameter (R = 0.576, p < 0.01). Furthermore, the MA area correlates with the LA volume (R = 0.639, p < 0.001). Conclusions: The volume of the left atrium correlates with the area of the mitral annulus. In patients with paroxysmal and persistent AF, an increase in left atrial dimensions is further associated with an increase in mitral valve dimensions.
1. Introduction
Normal dimensions of the mitral annulus are important for maintenance of the valve’s normal function. Enlargement of the mitral annulus (MA) produces abnormal coaptation of the valve leaflets and subsequent mitral regurgitation. Previous research has shown that left ventricular dilation and dysfunction are the most common causes of functional mitral regurgitation [1,2]. In addition, in patients with ischemic or non-ischemic dilated cardiomyopathy, mitral regurgitation is frequent. Nonetheless, there have been no prospective data that could provide evidence of the association between LA enlargement and the severity of MA dilation. Gertz et al. reported annular dilation and severe mitral regurgitation in 6.5% of AF patients who showed a decrease in MR grade after cardioversion to sinus rhythm. Other studies, on the other hand, disputed these findings. According to Otsuji et al. [3] and Zhou et al. [4], isolated AF does not result in annular dilation.
The aim of our study was to investigate the effect of left atrial enlargement on the size of the mitral annulus, measured by multidetector computed tomography angiography (MDCT).
The most common imaging approach for evaluating mitral valve function is echocardiography. However, the precise and comprehensive assessment of the MA given by MDCT makes this imaging method the preferable approach to measure both the MV and the LA with sub-millimeter spatial resolution.
2. Materials and Methods
2.1. Study Subjects and Procedure
A total of 107 patients who underwent MDCT and atrial fibrillation catheter ablation in the Rehabilitation Hospital Cluj-Napoca, Romania, were included in our retrospective study. The research was conducted according to the guidelines of the Declaration of Helsinki. There was no need for informed consent as our study was retrospective. MDCT scans were performed in our radiology department using a 32-detector General Electric system. All images were integrated with the CARTO 3 Biosense Webster system using the image integration tool and 3-dimensional images were obtained for each left atrium. The mitral annulus was automatically traced by the system after manually defining 6 to 8 points of the mitral plane, with circumference and area of the annulus automatically calculated by the software.
The patients we excluded from this study were those with the following: abnormal LV systolic function or dilated LV, organic valvular disease, such as rheumatic mitral stenosis or regurgitation, MV prolapse, moderate or severe calcifications of the mitral valve, and patients who underwent surgery for mitral valvuloplasty or replacement of the mitral valve.
Transverse diameter of the mitral annulus was defined as the longest septal to lateral distance, perpendicular to the contact line between LVOT and mitral annulus. The longitudinal diameter of the mitral annulus was defined as the longest distance measured between intercommissural points, perpendicular to the transverse diameter (Figure 1 and Figure 2). Supero-inferior diameter of the left atrium was defined as the distance between the point of intersection between longitudinal and transversal diameters of the mitral annulus and the highest left atrial point. Latero-lateral diameter of the left atrium was defined as the longest distance between the lateral and septal walls of the left atrium, parallel to the mitral annulus. AP diameter of the left atrium was defined as the distance between the anterior and posterior walls of the LA, perpendicular to the LL diameter.
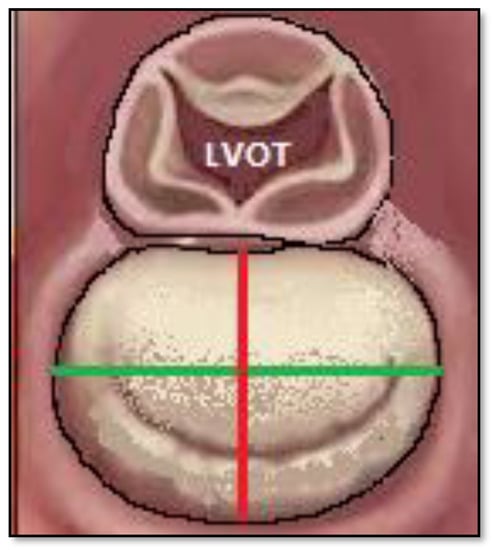
Figure 1.
Diameters of the mitral annulus are depicted in the image. With red color indicating transverse diameter; with green color indicating longitudinal diameter.
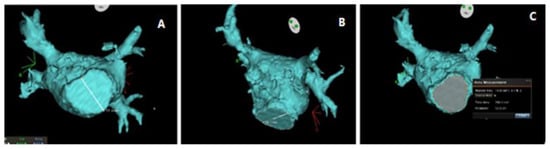
Figure 2.
Assessment of mitral valve dimensions using MDCT. (A) White line shows measurement of the transverse diameter of the mitral annulus. (B) White line shows measurement of the longitudinal diameter of the mitral annulus. (C) Measurement of the mitral valve area and perimeter. The system calculates the perimeter and area based on the points defining the mitral annulus; perimeter = 12.2 cm, area = 10.8 cm2.
All catheter ablation procedures were guided by the 3D CARTO 3 Biosense Webster mapping system. Electrogram mapping at the level of the pulmonary vein (PV)’s ostium guided pulmonary vein isolation. To map the pulmonary veins and the LA, a PentaRay or lasso catheter was used, and RF ablation lesions were performed using an open-irrigated 7 French 3.5 mm ablation catheter (SmartTouch, Biosense Webster). The procedure ended with the dissociation of PV potentials (PVP) or the full disappearance of PVP. In the case of persistent AF, we used lines between the left and right PV, mitral isthmus line, cavo-tricuspid isthmus line, or vein of Marshall ethanol ablation following PV isolation. A second ablation procedure was performed in 3 of the 107 patients.
2.2. Statistical Analysis
Continuous variables (age, diameter, area, circumference, volume) are presented as means ± standard deviation in cases of normal distribution or median + interquartile range in cases of not normal distribution. Categorical variables (sex) are presented as frequencies and percentages. Pearson or Spearman correlation was used to assess association between MV dimensions and LA volume, and linear regression was used to obtain the formula that predicts LA volume based on MV area. Bland–Altman test of agreement was used to establish limits of agreement and the mean bias between measured volume and estimated LA volume using the formula. All tests were performed using SPSS statistics program version 25, and p values < 0.05 were considered statistically significant.
3. Results
A total of 107 patients were included in this study (mean age of 55.8± 9.8 years, 74.8% male). The mean weight of the patients was 80.3 ± 15.5 kg and the mean BMI was 29.6 ± 6.0. The majority of the patients had paroxysmal atrial fibrillation (57.1%) and the rest had persistent AF (42.9%). The mean LV systolic diameter was 52.4 ± 5.8 mm and the mean LV diastolic diameter was 37.6 ± 6.8 mm; the mean ejection fraction of the left ventricle was 54.2 ± 4.2%. All the patients underwent multiple-detector computed tomography angiography (MDCT) of the left atrium and pulmonary veins prior to catheter ablation of atrial fibrillation.
The mean LA volume was 139 ± 56.3 mL and the total area of the left atrium was 247 ± 65.7 cm2. Only 15 patients (14%) had a left atrial volume of <90 mL, with the rest presenting dilated left atria (Table 1).

Table 1.
Means and standard deviation for mitral annulus and left atrial dimensions.
The dimensions of the mitral annulus (MA) were as follows: Longitudinal diameter = 41.8 ± 8.3 mm; transversal diameter = 29.8 ± 4.9 mm; MA area = 13.0 ± 4.5 cm2; MA circumference = 14.4 ± 3.2 cm (Table 1).
There was a significant difference in terms of age, AP diameter of the LA, and longitudinal diameter of the mitral valve between patients with paroxysmal and persistent atrial fibrillation (Table 2).

Table 2.
Comparison between LA and MV diameters in both paroxysmal and persistent AF.
As expected, a good correlation between the longitudinal and transverse diameters of the mitral valve was noted (R = 0.726, p < 0.0001). Furthermore, there was a statistically significant correlation between the longitudinal LA diameter and the longitudinal MV diameter (R = 0.576, p < 0.0001), as well as a significant correlation between the transverse LA diameter and the transverse MA diameter (R = 0.594, p < 0.0001) (Table 3). Most importantly, we identified a significant correlation between the left atrial volume and the mitral valve area, which could further be expressed by the following formula that estimates LA volume based on MA area: LA volume = 12 × MA area + 50 mL (Figure 3).

Table 3.
Correlations between LA and MA dimensions.
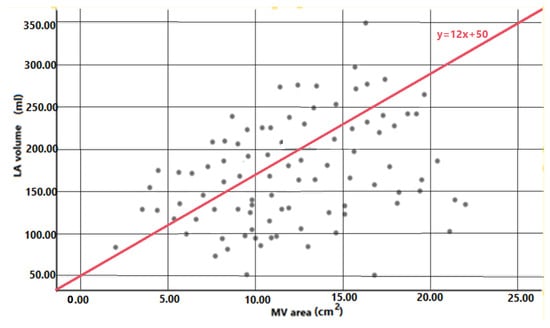
Figure 3.
Simple regression analysis between mitral valve area and left atrial volume in patients with catheter ablation of atrial fibrillation. Reference line from regression equation is depicted in the image (LA volume = 12 × MV area + 50 mL).
In order to validate the formula, the agreement between the measured LA volume (LAVm) and the estimated LA volume (LAVe) was calculated by plotting the differences and the means on a Bland–Altman graph (Figure 4).
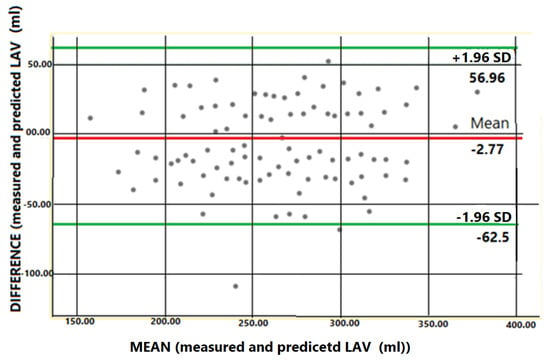
Figure 4.
Bland–Altman test of agreement between measured left atrial volume and predicted left atrial volume using the following formula: 12 × MV area + 50 mL. The mean difference is depicted as a red solid line and 95 confidence intervals (CI) as dashed green lines.
In the multivariate analysis, the transverse diameter of the mitral valve (p = 0.004, 95% CI = 1.8–9.4) was independently associated with the LA volume, after adjusting for confounding factors, such as Sex, age, and type of atrial fibrillation (Figure 5).
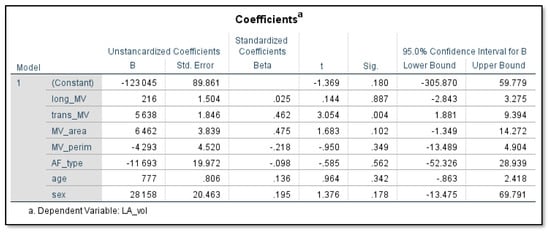
Figure 5.
In multivariate analysis only the transverse diameter of the mitral valve (p = 0.004, 95% CI = 1.8–9.4) was independently associated with LA volume.
4. Discussion
The present study sought to investigate the variation in size of the mitral annulus during dilation of the left atrium. The following are the most important findings of the current paper: (1) the longitudinal diameter of the mitral annulus correlates with the latero-lateral LA diameter; (2) the transverse diameter of the mitral annulus correlates with the antero-posterior LA diameter; (3) the MA area correlates with the LA volume; (4) the LA volume can be estimated from the MA area using the following formula: LAV = 12 × MA area + 50 mL. This is, to the best of our knowledge, the first study using MDCT that demonstrates an association between mitral annulus dimensions and left atrial dimensions.
Our hereby proposed formula can be used to predict LA volume (LAV) based on MA area. The Bland–Altman difference plot [5] was used to test the agreement between the measured LAV and predicted LAV based on the formula. Plotting the differences against means showed that there was good agreement between the predicted and the measured LA volumes. Furthermore, we have calculated the 95 percent limits of agreement for each comparison, showing the difference between the measured and estimated LAV for the majority of patients. We have also calculated the confidence intervals for the 95% limits of agreement. Because the discrepancies within the means are not clinically significant, the two techniques for estimating the LAV can be employed interchangeably, and a simple mitral valve area measurement can thus predict the dilation of the left atrium. This applies exclusively to patients without LV dysfunction, organic valvular disease, or replacement of the mitral valve.
This study used MDCT to estimate the size of the left atrium and the mitral annulus, since it offers a better soft-tissue resolution due to the use of intravenous contrast and better delineation of cardiac structures, compared to transthoracic and transesophageal ultrasonography. Several computed tomography studies within the literature attempted to find the normal reference values for the left atrial volume. In the study conducted by Stojanovska et al. [6], which included 74 subjects, values of the left atrial volume were determined and normalized by age, sex, and body surface area. A statistically significant difference was noted for different ages; the mean left atrial volume varied from 63 mL (30–39 patient age interval) to 68 mL (40–49 years), 83 mL (50–59 years), and 80 mL (60–70 years) [6].
We defined a normal mitral annulus and normal left atrium based on the work of Stojanovska et al. [7], Grover et al. [8], and Naoum et al. [9]. Stojanovska’s study measured the normal left atrium in MDCT for both men and women, and obtained the following results: 74 mL for women, 86 mL for men, and 80 mL for all subjects. According to Grover et al. and Naoum et al., normal mitral valve dimensions differ between male and female subjects in the following way: area = 8.4 ± 1.2 for female, 9.3 ± 1.6 for male, and 8.9 ± 1.5 cm2 overall; perimeter = 107.0 ± 7.0 mm for female, 113 ± 10 mm for male, and 110 ± 9.0 mm overall; longitudinal diameter = 36.1 ± 2.9 for female, 38.8 ± 3.9 for male, and 37.6 ± 3.7 mm overall; transversal diameter = 27.1 ± 2.3 for female, 27.8 ± 3.0 for male, and 27.5 ± 2.7 mm overall.
The volumes of the left atrium found in our study (139 ± 56.3 mL) are higher than those found in the ROMICAT study (97.4 ± 27.3 mL) [10]. All our patients displayed atrial fibrillation, 42.9% of which presented persistent AF. This can explain the increased volume of the left atrium, in contrast to patients from the ROMICAT trial, which comprised individuals with risk factors for coronary artery disease. Our data complete the results of Hirasawa et al. [8], who measured the MA area with MDCT and found values in the range of 9.9 ± 3.0 cm2; Hirasawa et al. [11] also found values, regarding the MA circumference and longitudinal diameter, of 115 ± 18 mm for the MA circumference and 35 ± 5 cm for the longitudinal diameter.
Interestingly, in the case of our patients who presented with AF, the measurements obtained are also comparable to the values recorded in three-dimensional transesophageal echocardiography (3D-TEE) studies, such as the Cong et al. study [12], which found an MA area of 8.86 ± 1.03 cm2 in the control group without AF, and 12.6± 0.85 cm2 in the AF + significant mitral regurgitation group; an MA circumference of 10.8 ± 0.9 cm in the control group and 13.4 ± 1.3 cm in the AF + significant MR group; a transversal diameter of 30.6 ± 2.4 mm in the control group and 32.9 ± 20.4 mm in the MR+ group; a longitudinal diameter of 36.3 ± 2.3 mm in the control group and 41.0 ± 3.8 mm in the MR + AF group. In the patients with no-to-mild mitral regurgitation, compared to moderate-to-severe mitral regurgitation, the mitral annulus dimensions were lower, as follows: a transversal diameter of 32.0 vs. 32.9 mm; a longitudinal diameter of 38.3 vs. 41.0 mm; an MV area of 9.58 vs. 12.66 cm2; a circumference of 11.5 vs. 13.4 cm [12]. The following similar results were obtained by Mihaila Baldea et al. [13], using three-dimensional transthoracic echocardiography: The MA transverse diameter was 34 mm for patients with functional mitral regurgitation and 28 mm in control subjects. The longitudinal diameter was 44 mm in patients with functional MR and 39 mm in control subjects, whilst the circumference was 12.9 mm and 11.2 mm, respectively. The MA area was 12.2 cm2 and 8.9 cm2, respectively.
Our retrospective analysis shows that the mitral annulus is enlarged in patients with left atrial dilation. While the association between left ventricular dilation and mitral annulus dilation is well understood, the same cannot be said for the mitral annulus alterations caused by left atrial dilation. In 1996, Tanimoto et al. [14] showed that left atrial enlargement increased the size of the mitral annulus. However, due to the lack of technical advancements, the mitral annular area was computed assuming elliptical geometry of the valve and using two diameters from two different echographic views. The mitral valve area was found to be 6.9 cm2 in patients with an LA diameter <45 mm and 9.2 cm2 in patients with an LA diameter >45 mm. Furthermore, the longitudinal diameter was 31 mm in the LAD <45 mm group and 35 mm in the LAD >45 mm group. The discrepancies between the results of Tanimoto and our results are most likely due to the fact that only 40.3% of Tanimoto’s patients had atrial fibrillation. It is unclear if this was paroxysmal or persistent, but the authors state that the LA diameter was 40 mm in one half of the group and 53 mm in the other half.
In addition, numerous studies have shown that left atrial enlargement itself can contribute to mitral annulus dilation. Gertz et al. [15] compared 53 patients with moderate-to-severe type I functional MR and a normal LV ejection fraction during the first AF ablation with a matched AF group with trivial/mild MR. Despite having equal LV size and function, the patients with MR exhibited significantly larger LA and MA dimensions. Persistent AF, age, and isolated MA dilatation were associated with the significance of MR. In the non-recuring AF subgroup, there was a substantial reduction in LA size (LA volume index of 28.2 cm3/m2 vs. 23.9 cm3/m2) and MA dimensions (3.41 cm vs. 3.24 cm). As a result, rather than being a result of MR, AF may be considered as a promoter of functional MR, conveying its effect through LA and MA dilatation. Another study showed that mitral annulus dilation, due to atrial remodeling in atrial fibrillation, can influence, by itself, the level of mitral regurgitation in time, in patients without any left ventricular pathology. The study included 86 patients with quantified mitral regurgitation evaluated through 3D ultrasound. A total of 53 patients had nonvalvular persistent AF without LV dysfunction or dilation, and 33 were normal controls [16]. The relationship between LA volume and MA size is likely bidirectional, as mitral valve dysfunction causes atrial stretch, with cellular and tissue alterations that can lead to fibrosis [17]. Atrial stretch leads to conduction slowing across the pulmonary veins–left atrial junction, predisposing to local reentry [18]. The effective refractory period of the left atrium is affected by heterogeneous tissue changes, which further increase the risk of atrial fibrillation. Subsequently, AF increases the LA size and mitral annulus diameter, and leads to MV dysfunction and regurgitation.
All things considered, the main mechanism of mitral annular dilation remains to be left ventricular dilation. The mechanism of mitral annulus dilation, due to left atrial dilation, is primarily biomechanical; the mitral annulus is a narrow fibrous tissue that connects the atrioventricular junction to the fibrous trigone anteromedially. Because it lacks its own contractile fibers, it is possible that it will alter its size in response to the numerous dilator and constrictor forces operating on it from both the atrium and the ventricle [19]. The constrictor forces include contraction of the supra-annular portion of the left atrium during atrial contraction, contraction of the base of the left ventricle during ventricular contraction, and papillary muscle forces acting centripetally when the mitral valve is closed, through chordae tendineae and mitral leaflets. The posterior annulus is vulnerable to traction. Enlargement of the left atrium exerts tension on the posterior mitral leaflet by displacing the posterior left atrial wall, altering the mitral annular planarity and interfering with normal mitral leaflet coaptation [20,21]. This finding is supported by a study conducted by Otsuji et al., which suggests that isolated atrial fibrillation is generally not an etiology for mitral regurgitation, despite a few cases of such an association. There is a subset of patients with AF who develop MR without LV dysfunction; in this case, the pathology is termed atrial functional mitral regurgitation. The etiologies are considered to be left atrial and mitral annular dilation associated with reduced leaflet coaptation in the context of abnormal leaflet remodeling, derangement of the MA saddle shape, or atriogenic tethering of the posterior mitral leaflet [22,23,24,25].
5. Limitations
There are, of course, certain limitations to our research. First and foremost, because all of our values are projected dimensions, there may be some sizing bias. The mitral annulus is a three-dimensional structure that cannot be defined by a single annular diameter [26]. However, Abdelghani et al. [17] established that the projected dimensions of the MA are strongly correlated with the effective annular diameter, circumference, and area. Furthermore, the differences between the 2D MA area and 3D MA area are not significant, as shown by the following: 12.2 ± 3 cm2 vs. 12.4 ± 3.1 cm2 in functional mitral regurgitation and 8.9 ± 2.1 cm2 vs. 9.2 ±2.2 cm2 [27]. Furthermore, projected values are also used to predict the size of prosthetics in minimally invasive mitral valve replacement [28]. Secondly, because the annular points were manually traced, the analysis process was tedious. The mitral annulus was automatically traced by the system, after manually defining six to eight points of the mitral plane. The software also computed the circumference and area of the MA. Last, but not least, this is a retrospective single-site study with a relatively small number of patients included; therefore, it is possible that significant correlations between the mitral valve and the size of the left atrium may not have been discovered. Furthermore, we need to corroborate our findings with a larger number of patients from multiple centers.
6. Conclusions
To summarize, there is an important correlation between mitral annulus diameters and left atrial diameters, and between left atrial volume and mitral annulus area. A regression formula can estimate the left atrial volume based on the mitral valve area. In patients with atrial fibrillation, an increase in left atrial dimensions is further associated with an increase in mitral annulus dimensions.
Author Contributions
Conceptualization, G.C. and A.T.; methodology, I.V., M.C.Z., R.R., M.P., G.G., L.M., R.T., I.-A.M. and C.M.F.; software, G.C., A.T., A.C., M.C.Z. and I.V.; validation, R.R., D.P. and D.Z.; formal analysis, G.C., A.T., A.C., M.C.Z. and I.V.; investigation, G.C., A.T., M.C.Z. and I.V.; resources, R.R., G.G., D.P. and D.Z.; data curation, R.R., G.C., G.G., I.-A.M. and C.M.F.; writing—original draft preparation, I.V. and M.C.Z.; writing—review and editing, G.C., A.T., I.V., M.C.Z. and R.T.; visualization, G.C., A.T., A.C., I.V., M.C.Z., G.G., I.-A.M., C.M.F. and D.Z.; supervision, D.Z. and D.P.; project administration, G.C.; funding acquisition, R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Rehabilitation Hospital Cluj-Napoca.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be found, upon request in a google drive file in SPSS format.
Acknowledgments
A.T. was supported by the Project PNCDI III 2015–2020 entitled “Increasing the performance of scientific research and technology transfer in translational medicine through the formation of a new generation of young researchers”—ECHITAS, no. 29PFE/18.10.2018.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dwivedi, A.; Vainrib, A.; Saric, M. Functional mitral regurgitation in patients with heart failure and depressed ejection fraction. Curr. Opin. Cardiol. 2016, 31, 483–492. [Google Scholar] [CrossRef]
- Schmitto, J.D.; Lee, L.S.; Mokashi, S.A.; Bolman, R.M., 3rd; Cohn, L.H.; Chen, F.Y. Functional mitral regurgitation. Cardiol. Rev. 2010, 18, 285–291. [Google Scholar] [CrossRef]
- Otsuji, Y.; Kumanohoso, T.; Yoshifuku, S.; Matsukida, K.; Koriyama, C.; Kisanuki, A.; Minagoe, S.; Levine, R.A.; Tei, C. Isolated annular dilation does not usually cause important functional mitral regurgitation: Comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 1651–1656. [Google Scholar] [CrossRef]
- Zhou, X.; Otsuji, Y.; Yoshifuku, S.; Yuasa, T.; Zhang, H.; Takasaki, K.; Matsukida, K.; Kisanuki, A.; Minagoe, S.; Tei, C. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ. J. Off. J. Jpn. Circ. Soc. 2002, 66, 913–916. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. J. R. Stat. Soc. Ser. D 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Stojanovska, J.; Cronin, P.; Patel, S.; Gross, B.H.; Oral, H.; Chughtai, K.; Kazerooni, E.A. Reference normal absolute and indexed values from ECG-gated MDCT: Left atrial volume, function, and diameter. AJR Am. J. Roentgenol. 2011, 197, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, J.; Cronin, P.; Gross, B.H.; Kazerooni, E.A.; Tsodikov, A.; Frank, L.; Oral, H. Left atrial function and maximum volume as determined by MDCT are independently associated with atrial fibrillation. Acad. Radiol. 2014, 21, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Grover, R.; Ohana, M.; Arepalli, C.D.; Sellers, S.L.; Mooney, J.; Kueh, S.H.; Kim, U.; Blanke, P.; Leipsic, J.A. Role of MDCT Imaging in Planning Mitral Valve Intervention. Curr. Cardiol. Rep. 2018, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Naoum, C.; Leipsic, J.; Cheung, A.; Ye, J.; Bilbey, N.; Mak, G.; Berger, A.; Dvir, D.; Arepalli, C.; Grewal, J.; et al. Mitral Annular Dimensions and Geometry in Patients With Functional Mitral Regurgitation and Mitral Valve Prolapse: Implications for Transcatheter Mitral Valve Implantation. JACC Cardiovasc. Imaging 2016, 9, 269–280. [Google Scholar] [CrossRef]
- Hoffmann, U.; Bamberg, F.; Chae, C.U.; Nichols, J.H.; Rogers, I.S.; Seneviratne, S.K.; Truong, Q.A.; Cury, R.C.; Abbara, S.; Shapiro, M.D.; et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: The ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J. Am. Coll. Cardiol. 2009, 53, 1642–1650. [Google Scholar] [CrossRef]
- Hirasawa, K.; Vo, N.M.; Gegenava, T.; Pio, S.M.; van Wijngaarden, S.E.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Mitral Valve Annulus Dimensions Assessment with Three-Dimensional Echocardiography Versus Computed Tomography: Implications for Transcatheter Interventions. J. Clin. Med. 2021, 10, 649. [Google Scholar] [CrossRef]
- Cong, T.; Gu, J.; Lee, A.P.; Shang, Z.; Sun, Y.; Sun, Q.; Wei, H.; Chen, N.; Sun, S.; Fu, T. Quantitative analysis of mitral valve morphology in atrial functional mitral regurgitation using real-time 3-dimensional echocardiography atrial functional mitral regurgitation. Cardiovasc. Ultrasound 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Mihaila Baldea, S.; Muraru, D.; Miglioranza, M.H.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Relation of Mitral Annulus and Left Atrial Dysfunction to the Severity of Functional Mitral Regurgitation in Patients with Dilated Cardiomyopathy. Cardiol. Res. Pract. 2020, 2020, 3261714. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Pai, R.G. Effect of isolated left atrial enlargement on mitral annular size and valve competence. Am. J. Cardiol. 1996, 77, 769–774. [Google Scholar] [CrossRef]
- Gertz, Z.M.; Raina, A.; Saghy, L.; Zado, E.S.; Callans, D.J.; Marchlinski, F.E.; Keane, M.G.; Silvestry, F.E. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: Reversal with arrhythmia control. J. Am. Coll. Cardiol. 2011, 58, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Heo, R.; Handschumacher, M.D.; Lee, S.; Choi, Y.S.; Kim, K.R.; Shin, Y.; Park, H.K.; Bischoff, J.; Aikawa, E.; et al. Mitral Valve Adaptation to Isolated Annular Dilation: Insights Into the Mechanism of Atrial Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 665–677. [Google Scholar] [CrossRef]
- Quinn, T.A.; Kohl, P. Cardiac Mechano-Electric Coupling: Acute Effects of Mechanical Stimulation on Heart Rate and Rhythm. Physiol. Rev. 2021, 101, 37–92. [Google Scholar] [CrossRef]
- Walters, T.E.; Lee, G.; Spence, S.; Larobina, M.; Atkinson, V.; Antippa, P.; Goldblatt, J.; O’Keefe, M.; Sanders, P.; Kistler, P.M.; et al. Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: Insights into the mechanism of pulmonary vein arrhythmogenesis. Circ. Arrhythm. Electrophysiol. 2014, 7, 1189–1197. [Google Scholar] [CrossRef]
- Mihaila, S.; Muraru, D.; Miglioranza, M.H.; Piasentini, E.; Aruta, P.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Relationship between mitral annulus function and mitral regurgitation severity and left atrial remodelling in patients with primary mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 918–929. [Google Scholar] [CrossRef][Green Version]
- Levy, M.J.; Edwards, J.E. Anatomy of mitral insufficiency. Prog. Cardiovasc. Dis. 1962, 5, 119–144. [Google Scholar] [CrossRef]
- Perloff, J.K.; Roberts, W.C. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation 1972, 46, 227–239. [Google Scholar] [CrossRef]
- Abdelghani, M.; Spitzer, E.; Soliman, O.I.I.; Beitzke, D.; Laggner, R.; Cavalcante, R.; Tateishi, H.; Campos, C.M.; Verstraeten, L.; Sotomi, Y.; et al. A simplified and reproducible method to size the mitral annulus: Implications for transcatheter mitral valve replacement. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Takahashi, Y.; Shibata, T. Functional mitral regurgitation, updated: Ventricular or atrial? J. Echocardiogr. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Kagiyama, N.; Hayashida, A.; Toki, M.; Fukuda, S.; Ohara, M.; Hirohata, A.; Yamamoto, K.; Isobe, M.; Yoshida, K. Insufficient Leaflet Remodeling in Patients With Atrial Fibrillation: Association With the Severity of Mitral Regurgitation. Circ. Cardiovasc. Imaging 2017, 10, e005451. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, H.O.; Jouni, H.; Oguz, D.; Thaden, J.J.; Nkomo, V.T.; Pislaru, C.; Foley, T.A.; Muraru, D.; Pellikka, P.A.; Pislaru, S.V. Large, Unpredictable Beat-To-Beat Variability of Mitral Annulus Size in Atrial Fibrillation. JACC Cardiovasc. Interv. 2020, 13, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.R.; Bashein, G.; Sheehan, F.H.; Legget, M.E.; Munt, B.; Li, X.N.; Sivarajan, M.; Bolson, E.L.; Zeppa, M.; Arch, M.Z.; et al. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am. Heart J. 2000, 139, 378–387. [Google Scholar] [CrossRef]
- Mihaila, S.; Muraru, D.; Piasentini, E.; Miglioranza, M.H.; Peluso, D.; Cucchini, U.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Quantitative analysis of mitral annular geometry and function in healthy volunteers using transthoracic three-dimensional echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2014, 27, 846–857. [Google Scholar] [CrossRef]
- Vo, A.T.; Nguyen, N.T.H.; Le, K.M.; Vuong, N.L.; Nguyen, T.T.T.; Vu, T.T.; Hoang, S.V.; Nguyen, D.H. Mitral prosthetic size predictor in minimally invasive mitral valve replacement. J. Cardiothorac. Surg. 2020, 15, 147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).