Extraction of Pest Insect Characteristics Present in a Mirasol Pepper (Capsicum annuum L.) Crop by Digital Image Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. First Stage: Scale-Invariant Feature Transform (SIFT)
2.1.1. Scale-Space Extrema Detection
2.1.2. Key Point Localization
2.1.3. Orientation Assignment
2.1.4. Key Point Descriptor
2.1.5. Scale-Invariant Coordinates
2.2. Second Stage: Color Transformation and Regions of Interest
2.2.1. Grayscale
G = image (:, :, 2);
B = image (:, :, 3);
2.2.2. CIE*L*A*B
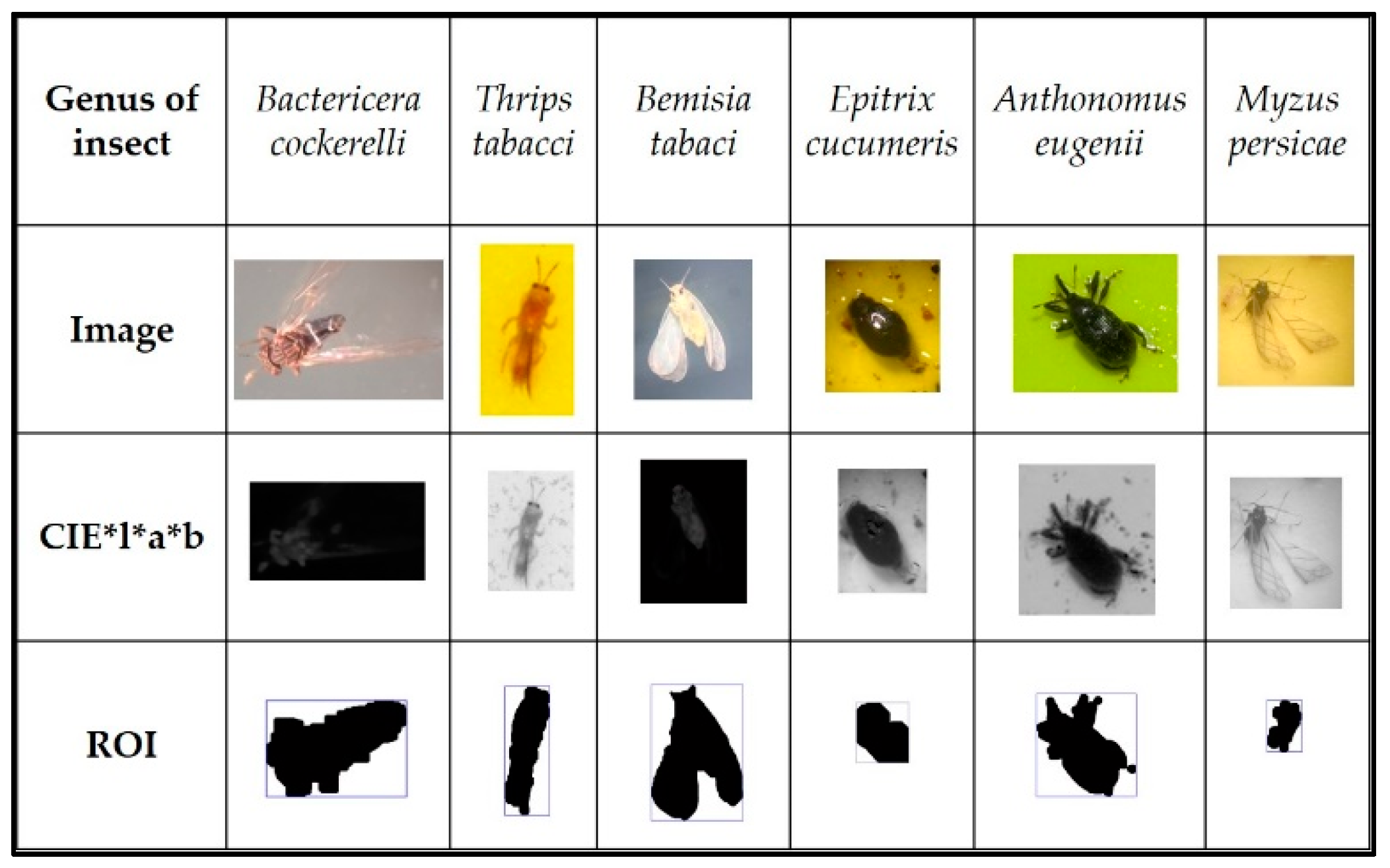
2.2.3. Regions of Interest
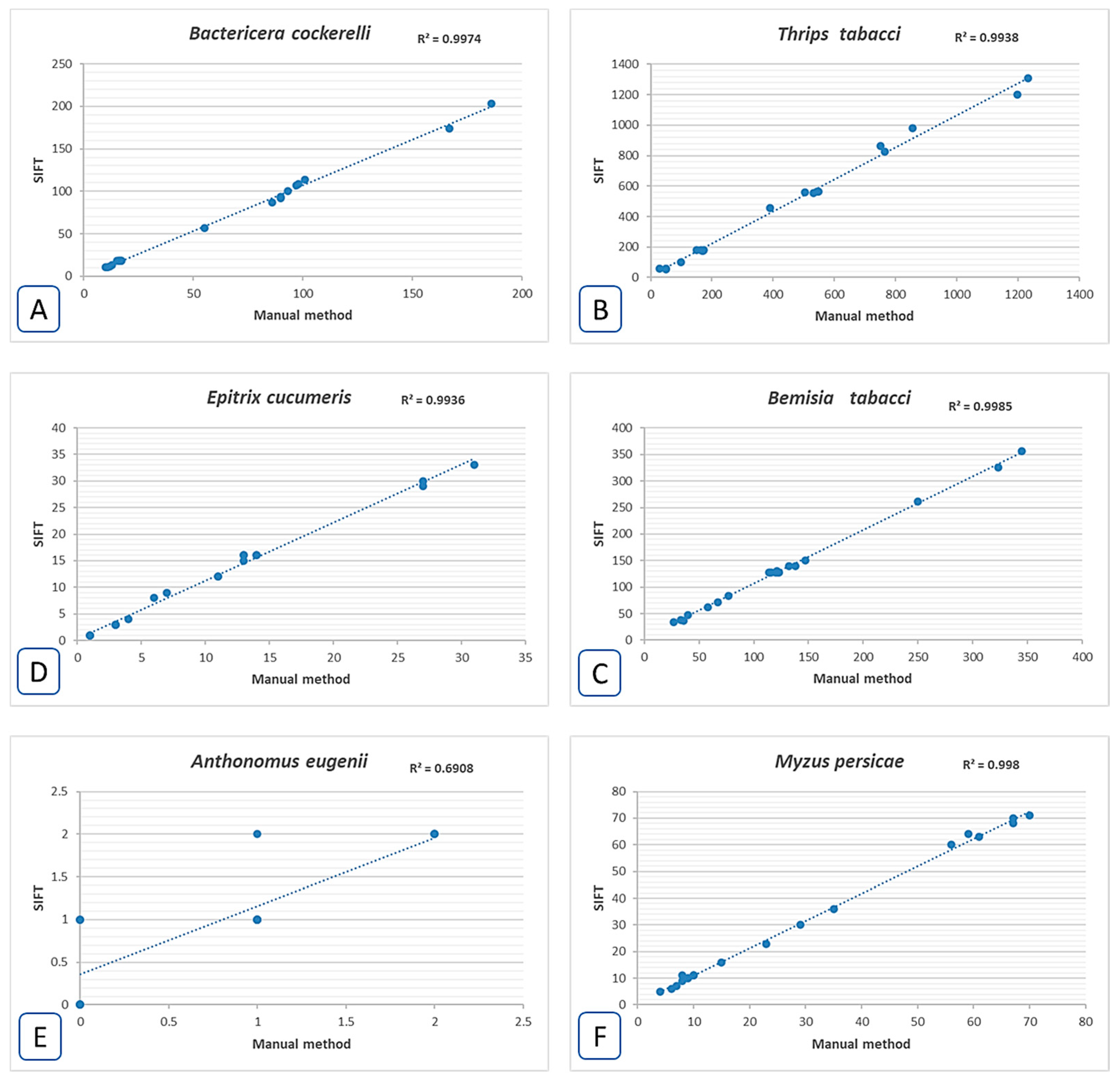
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Da Silveira, F.; Lermen, F.H.; Amaral, F.G. An overview of agriculture 4.0 development: Systematic review of descriptions, technologies, barriers, advantages, and disadvantages. Comput. Electron. Agric. 2021, 189, 106405. [Google Scholar] [CrossRef]
- Eastwood, C.; Klerkx, L.; Nettle, R. Dynamics and distribution of public and private research and extension roles for technological innovation and diffusion: Case studies of the implementation and adaptation of precision farming technologies. J. Rural. Stud. 2017, 49, 1–12. [Google Scholar] [CrossRef]
- Karim, F.; Karim, F.; Frihida, A. Monitoring system using web of things in precision agriculture. Procedia Comput. Sci. 2017, 10, 402–409. [Google Scholar] [CrossRef]
- Latinovic, N.; Pešic, A. Architecting an IoT-enabled platform for precision agriculture and ecological monitoring: A case study. Comput. Electron. Agric. 2017, 140, 255–265. [Google Scholar]
- Agroasemex, S. Las Plagas Producen Pérdidas de Hasta un 40 por Ciento en la Producción Agrícola, Revela Estudio de la FAO. 2019. Available online: https://www.gob.mx/agroasemex/articulos/las-plagas-producen-perdidas-de-hasta-un-40-por-ciento-en-la-produccion-agricola-revela-estudio-de-la-fao?idiom=es (accessed on 19 January 2020).
- Macías Valdez, L.M.; Baltazar Brenes, E.; González Gaona, E.; Gómez Serrano, C.; Galindo Reyes, M.A.; Maciel Pérez, L.H.; Robles Escobedo, F.J. Nueva Tecnología de Manejo Para el Control de la Marchitez del Chile en Aguascalientes; Primera, Ed.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Aguascalientes, México, 2010. [Google Scholar]
- Boissard, P.; Martin, V.; Moisan, S. A cognitive vision approach to early pest detection in greenhouse crops. Comput. Electron. Agric. 2008, 62, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Lozano, Á.G.B.; González, G.G.; Ramírez, M.D.A. Tecnología de Producción de Chile Seco, 5th ed.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Aguascalientes, México, 2006. [Google Scholar]
- CESAVEG. Manual de Plagas y Enfermedades en Chile; CESAVEG: Guanajuato, México, 2010; p. 28. [Google Scholar]
- Dirección General de Estudios Agropecuarios y Pesqueros. Línea de Acción: Determinación del Nivel Riesgo Fitosanitario Para los Cultivos de Importancia Económica en México; Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: México City, México, 2016; p. 64. [Google Scholar]
- Servin, R. Especies de mosquita blanca en agrosistemas deserticos de Baja Balifornia Sur, México y fenología de sus hospederos. Rev. Biol. 2004, 18, 57–64. [Google Scholar]
- Padilla, M.M.; Casasola, L.E.; Umaña, F.M. SFE Desarrolla Plan de Acción ante la Cercanía de la Paratrioza (Bactericera Cockerelli Sulc); Actualidad Fitosanitaria: San José, Costa Rica, 2010. [Google Scholar]
- Tiznado, J.A.G. Paratrioza (Bactericera) Cockerelli Sulc, Vector de la Bacteria Candidatus Liberibacter Solanacearum- Zebra Chip en Papa; Facultad de Ciencias Químico-Biológicas, Universidad Autónoma de Sinaloa: Culiacán, México, 2010. [Google Scholar]
- Garzón-Tiznado, J.A.; Cárdenas-Valenzuela, O.G.; Bujanos-Muñiz, R. Association of hemiptera: Triozidae with the disease ‘Permanente del tomate’ in México. Agric. Tec. Mex. 2009, 35, 61–72. [Google Scholar]
- Stuart, R.R.; Gao, Y.-L.; Lei, Z.-R. Thrips: Pests of Concern to China and the United States. Agric. Sci. China 2011, 10, 867–892. [Google Scholar] [CrossRef]
- Boonham, N.; Smith, P.; Walsh, K.; Tame, J.; Morris, J.; Spence, N.; Bennison, J.; Barker, I. The detection of Tomato spotted wilt virus (TSWV) in individual thrips using real time fluorescent RT-PCR (TaqMan). J. Virol. Methods 2002, 101, 37–48. [Google Scholar] [CrossRef]
- Bosco, L.; Giacometto, E.; Tavella, L. Colonization and predation of thrips (Thysanoptera: Thripidae) by Orius spp. (Heteroptera: Anthocoridae) in sweet pepper greenhouses in Northwest Italy. Biol. Control 2008, 44, 331–340. [Google Scholar] [CrossRef]
- Brown, J.K.; Bird, J. Whitefly-Transmitted Geminiviruses and Associated Disorders in the Americas and the Caribbean Basin. Am. Phytopathol. Soc. 1992, 76, 220–225. [Google Scholar] [CrossRef]
- Lowe, B.; Boudjelas, S.; De Poorter, M. 100 de las Especies Exóticas Invasoras más dañinas del mundo. Aliens 2000, 12, 12. [Google Scholar]
- Rojas, S.R.; Pedroza, A.S.; Nakagome, T. Manual de Plagas y Enfermedades del Jitomate, Tomate de Cáscara y Cebolla; Secretaria de agricultura, Ganadería y Desarrollo Rural: Morelos, México, 2001. [Google Scholar]
- Ballestín, P.; Barber, A.; Cambra, M.; Aguado, A.; López, A. Epitrix spp. Pulgillas de la Papa; Dirección General de Alimentación y Fomento Agroalimentario: Zaragoza, Spain, 2016. [Google Scholar]
- Jiménez, A.J.G. Conozca el Barrenillo del Chile y su Control; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Aguascalientes, México, 1991. [Google Scholar]
- Niu, J.-Z.; Hull-Sanders, H.; Zhang, Y.-X.; Lin, J.-Z.; Dou, W.; Wang, J.-J. Biological control of arthropod pests in citrus orchards in China. Biol. Control 2014, 68, 15–22. [Google Scholar] [CrossRef]
- Carmo, D.D.G.D.; Lopes, M.C.; de Araújo, T.A.; Ramos, R.S.; Soares, J.R.S.; Paes, J.D.S.; Picanço, M.C. Conventional sampling plan for green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in bell pepper crops. Crop. Prot. 2021, 145, 105645. [Google Scholar] [CrossRef]
- Soares, J.R.S.; Paes, J.D.S.; De Araújo, V.C.R.; Araújo, T.; Ramos, R.S.; Picanço, M.C.; Zanuncio, J.C. Spatiotemporal Dynamics and Natural Mortality Factors of Myzus persicae (Sulzer) (Hemiptera: Aphididae) in Bell Pepper Crops. Neotrop. Entomol. 2020, 49, 445–455. [Google Scholar] [CrossRef]
- Zayas, I.; Flinn, P. Detection of insects in bulk wheat samples with machine vision. Trans. Am. Soc. Agric. Eng. 1998, 41, 883–888. [Google Scholar] [CrossRef]
- Solis-Sánchez, L.O.; Escalante, J.J.G.; Castañeda-Miranda, R.; Torres-Pacheco, I.; Guevara-González, R. Machine vision algorithm for whiteflies (Bemisia tabaciGenn.) scouting under greenhouse environment. J. Appl. Entomol. 2009, 133, 546–552. [Google Scholar] [CrossRef]
- Solis-Sánchez, L.O.; Castañeda-Miranda, R.; Escalante, J.J.G.; Torres-Pacheco, I.; Guevara-González, R.G.; Castañeda-Miranda, C.L.; Alaniz-Lumbreras, P.D. Scale invariant feature approach for insect monitoring. Comput. Electron. Agric. 2011, 75, 92–99. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Wu, W.; Sun, C.; Guo, W.; Zhu, X. Detection of aphids in wheat fields using a computer vision technique. Biosyst. Eng. 2016, 141, 82–93. [Google Scholar] [CrossRef]
- Maharlooei, M.; Sivarajan, S.; Bajwa, S.G.; Harmon, J.P.; Nowatzki, J. Detection of soybean aphids in a greenhouse using an image processing technique. Comput. Electron. Agric. 2017, 132, 63–70. [Google Scholar] [CrossRef]
- Xia, C.; Chon, T.-S.; Ren, Z.; Lee, J.-M. Automatic identification and counting of small size pests in greenhouse conditions with low computational cost. Ecol. Inform. 2015, 29, 139–146. [Google Scholar] [CrossRef]
- Zevallos, D.M.P.; Vänninen, I. Yellow sticky traps for decision-making in whitefly management: What has been achieved? Crop. Prot. 2013, 47, 74–84. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Cheng, Q.; Zhou, H.; Li, M.; Fan, Y.; Shan, G.; Damerow, L.; Lammers, P.S.; Jones, S.B. A smart-vision algorithm for counting whiteflies and thrips on sticky traps using two-dimensional Fourier transform spectrum. Biosyst. Eng. 2017, 153, 82–88. [Google Scholar] [CrossRef]
- Luo, S.; Naranjo, S.E.; Wu, K. Biological control of cotton pests in China. Biol. Control 2014, 68, 6–14. [Google Scholar] [CrossRef]
- Lowe, D. Object Recognition from Local Scale-Invariant Features. In Proceedings of the Seventh IEEE International Conference on Computer Vision, Corfu, Greece, 20–25 September 1999; IEEE Computer Society: Washington, DC, USA, 1999; Volume 2, pp. 1150–1157. [Google Scholar]
- Moreno-Lucio, M.; Espinoza-García, G.; Solís-Sánchez, L.O.; Olvera-Olvera, C.A.; Covarruvias, J.M.; Guerrero-Osuna, H.A. PDI para obtener descriptores de diferentes géneros de chicharritas (Hemiptera: Cicadellidae) presentes en un cultivo de chile. In Memorias del Congreso Internacional de Investigación, Academia Journals, Chiapas 2019, 2019th ed.; México, 2019; Volume 11, pp. 816–821. [Google Scholar]
- Flores, P.; Braun, J. Algoritmo SIFT: Fundamento Teórico; Instituto de electrónica, Universidad de la república: Montevideo, Uruguay, 2011. [Google Scholar]
- Lowe, D.G. SIFT—The Scale Invariant Feature Transform. Int. J. 2004, 2, 91–110. [Google Scholar]
- Lowe, D.G. Distinctive Image Features from Scale-Invariant Keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar] [CrossRef]
- Hunter Associates Laboratory Inc. Hunter Lab vs. CIE Lab; Hunter Associates Laboratory Inc.: Reston, VA, USA, 2012; p. 4. [Google Scholar]
- Robertson, A.R.; Lozano, R.D.; Alman, D.H.; Orchard, S.E. CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. COLOR Res. Appl. 1977, 2, 5–6. [Google Scholar]
- Ortiz-Rodriguez, J.M.; Guerrero-Mendez, C.; Martinez-Blanco, M.D.R.; Castro-Tapia, S.; Lucio, M.M.; Jaramillo-Martinez, R.; Solis-Sanchez, L.O.; Martinez-Fierro, M.D.L.L.; Garza-Veloz, I.; Galvan, J.C.M.; et al. Breast Cancer Detection by Means of Artificial Neural Networks. In Advanced Applications for Artificial Neural Networks; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Gonzalez, R.C.; Woods, R.E.; Masters, B.R. Digital Image Processing, Third Edition. J. Biomed. Opt. 2009, 14, 029901. [Google Scholar] [CrossRef]
- Hejduk, A.; Czajka, S.; Lulek, J. Impact of co-processed excipient particles solidity and circularity on critical quality attributes of orodispersible minitablets. Powder Technol. 2021, 387, 494–508. [Google Scholar] [CrossRef]
- Solà, M.; Lundgren, J.G.; Agusti, N.; Riudavets, J. Detection and quantification of the insect pest Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) in rice by qPCR. J. Stored Prod. Res. 2017, 71, 106–111. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, J.; Li, R.; Li, J.; Hong, P.; Xia, J.; Chen, P. Automatic classification for field crop insects via multiple-task sparse representation and multiple-kernel learning. Comput. Electron. Agric. 2015, 119, 123–132. [Google Scholar] [CrossRef]

| Time in Weeks | Number of Specimens | ||||||
|---|---|---|---|---|---|---|---|
| Temperature °C | Bactericera cockerelli | Thrips tabacci | Bemisia tabaci | Epitrix cucumeris | Anthonomus eugenii | Myzus persicae | |
| 1 | 21.87 | 12 | 56 | 36 | 1 | 0 | 7 |
| 2 | 23.69 | 13 | 58 | 38 | 1 | 1 | 9 |
| 3 | 21.27 | 11 | 54 | 34 | 1 | 0 | 5 |
| 4 | 17.91 | 94 | 455 | 48 | 8 | 2 | 36 |
| 5 | 17.4 | 203 | 983 | 62 | 12 | 1 | 71 |
| 6 | 17.06 | 174 | 865 | 83 | 12 | 2 | 70 |
| 7 | 17.83 | 114 | 565 | 150 | 3 | 1 | 68 |
| 8 | 17.59 | 100 | 553 | 130 | 3 | 1 | 60 |
| 9 | 19.83 | 109 | 565 | 140 | 4 | 2 | 64 |
| 10 | 19.83 | 107 | 560 | 140 | 3 | 1 | 63 |
| 11 | 18.59 | 92 | 1203 | 326 | 30 | 0 | 30 |
| 12 | 17.4 | 87 | 1309 | 357 | 33 | 0 | 23 |
| 13 | 19.87 | 57 | 826 | 261 | 29 | 2 | 16 |
| 14 | 19.41 | 18 | 181 | 128 | 15 | 1 | 11 |
| 15 | 18.73 | 18 | 178 | 128 | 16 | 1 | 10 |
| 16 | 17.9 | 18 | 178 | 128 | 16 | 1 | 11 |
| 17 | 17.67 | 18 | 175 | 128 | 16 | 1 | 10 |
| 18 | 18.03 | 18 | 175 | 128 | 16 | 1 | 11 |
| 19 | 17.18 | 11 | 100 | 72 | 9 | 0 | 6 |
| Genus | Number of Insects Manual Method | Number of Insects Detected whit SIFT | Accuracy Rating |
|---|---|---|---|
| Bactericera cockerelli | 12 | 12 | 100% |
| Thrips tabacci | 56 | 28 | 50% |
| Bemisia tabaci | 36 | 36 | 100% |
| Epitrix cucumeris | 1 | 1 | 100% |
| Myzus persicae | 7 | 7 | 100% |
| Genus | Number of Insects Manual Method | Number of Insects Detected via SIFT | Accuracy Rating |
|---|---|---|---|
| Bactericera cockerelli | 3 | 2 | 67% |
| Thrips tabacci | 3 | 2 | 67% |
| Bemisia tabaci | 3 | 2 | 67% |
| Epitrix cucumeris | 3 | 3 | 100% |
| Myzus persicae | 3 | 3 | 100% |
| Feature | Genus of Pest | ||||||
|---|---|---|---|---|---|---|---|
| Bactericera cockerelli | Thrips tabacci | Bemisia tabaci | Epitrix cucumeris | Anthonomus eugenii | Myzus persicae | ||
| Solidity | 8.60 × 10−1 | 8.84 × 10−1 | 8.09 × 10−1 | 9.66 × 10−1 | 7.59 × 10−1 | 8.82 × 10−1 | |
| Eccentricity | 8.78 × 10−1 | 9.71 × 10−1 | 7.22 × 10−1 | 7.62 × 10−1 | 7.99 × 10−1 | 8.52 × 10−1 | |
| Pixel area | 1.41 × 104 | 6.34 × 103 | 3.61 × 104 | 4.02 × 104 | 2.78 × 104 | 4.94 × 103 | |
| Centroid | X | 1.02 × 102 | 1.18 × 102 | 1.33 × 102 | 1.03 × 102 | 1.62 × 102 | 1.11 × 102 |
| Y | 1.30 × 102 | 1.96 × 102 | 2.26 × 102 | 1.30 × 102 | 1.96 × 102 | 1.38 × 102 | |
| Perimeter | 5.77 × 102 | 4.12 × 102 | 9.96 × 102 | 7.96 × 102 | 9.47 × 102 | 3.17 × 102 | |
| Axis length | Major | 2.04 × 102 | 1.88 × 102 | 2.88 × 102 | 2.84 × 102 | 2.57 × 102 | 1.13 × 102 |
| Minor | 9.75 × 101 | 4.54 × 101 | 1.99 × 102 | 1.84 × 102 | 1.54 × 102 | 5.94 × 101 | |
| Size in mm2 | 4.78 × 10−1 | 2.26 × 10−1 | 9.12 × 10−1 | 2.51 | 7.12 × 10−1 | 1.03 × 10−1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Lucio, M.; Castañeda-Miranda, C.L.; Espinoza-García, G.; Olvera-Olvera, C.A.; Luque-Vega, L.F.; Santiago, A.D.R.-D.; Guerrero-Osuna, H.A.; Martínez-Blanco, M.d.R.; Solís-Sánchez, L.O. Extraction of Pest Insect Characteristics Present in a Mirasol Pepper (Capsicum annuum L.) Crop by Digital Image Processing. Appl. Sci. 2021, 11, 11166. https://doi.org/10.3390/app112311166
Moreno-Lucio M, Castañeda-Miranda CL, Espinoza-García G, Olvera-Olvera CA, Luque-Vega LF, Santiago ADR-D, Guerrero-Osuna HA, Martínez-Blanco MdR, Solís-Sánchez LO. Extraction of Pest Insect Characteristics Present in a Mirasol Pepper (Capsicum annuum L.) Crop by Digital Image Processing. Applied Sciences. 2021; 11(23):11166. https://doi.org/10.3390/app112311166
Chicago/Turabian StyleMoreno-Lucio, Mireya, Celina Lizeth Castañeda-Miranda, Gustavo Espinoza-García, Carlos Alberto Olvera-Olvera, Luis F. Luque-Vega, Antonio Del Rio-De Santiago, Héctor A. Guerrero-Osuna, Ma. del Rosario Martínez-Blanco, and Luis Octavio Solís-Sánchez. 2021. "Extraction of Pest Insect Characteristics Present in a Mirasol Pepper (Capsicum annuum L.) Crop by Digital Image Processing" Applied Sciences 11, no. 23: 11166. https://doi.org/10.3390/app112311166
APA StyleMoreno-Lucio, M., Castañeda-Miranda, C. L., Espinoza-García, G., Olvera-Olvera, C. A., Luque-Vega, L. F., Santiago, A. D. R.-D., Guerrero-Osuna, H. A., Martínez-Blanco, M. d. R., & Solís-Sánchez, L. O. (2021). Extraction of Pest Insect Characteristics Present in a Mirasol Pepper (Capsicum annuum L.) Crop by Digital Image Processing. Applied Sciences, 11(23), 11166. https://doi.org/10.3390/app112311166









