1. Introduction
Radio frequency (RF) alternating electromagnetic fields are extensively used for communication applications, but numerous other industrial applications make use of RF as a dielectric heating technology, e.g., in the paper, lumber, and plastic industry [
1]. In the food industry, RF is already well-established for applications such as drying, post baking, thawing, and tempering [
2,
3,
4,
5,
6]. More recently, there is an increasing research effort towards thermal food pasteurisation and sterilisation applications [
2]. While the RF fields that are relevant for heating applications span from 1 to 300 MHz [
7], only frequencies of 13.56, 27.12, and 40.68 MHz are designated for unlicensed use worldwide in industrial, scientific, and medical (ISM) applications [
8]. When foods are exposed to RF radiation, dielectric heating is accomplished by a combination of (i) the dipolar rotation of free water and (ii) the depolarisation of solvated ions, with the latter being the dominant heating mechanism [
7]. In comparison, microwave (MW) heating (i.e., frequencies of 300–300,000 MHz), which is the most well-known dielectric heating method, is mainly accomplished by the frictional heat generated from the dipolar rotation of free water molecules [
7,
9]. The main advantages of both dielectric heating methods (i.e., RF and MW) compared to conventional heating technologies are fast volumetric heating and high energy efficiencies [
10]. In the food industry, there are three main reasons to select RF heating over MW heating, i.e., (i) larger penetration depths allowing the treatment of larger products, (ii) the possibility to effectively heat low-moisture foods, and (iii) the absence of power intensity interference due to the longer wavelengths [
2,
11]. Even though achieving homogeneous temperature distributions during RF heating processes remains challenging (i.e., especially concerning over-heating at the edge), the larger penetration depths of RF already result in more even heating than for MW processes [
12,
13].
Nowadays, there are two types of commercial RF equipment available for generating and transferring electromagnetic power: (i) the conventional free-running oscillator (FRO) and (ii) the 50 Ω RF amplifier system. The FRO system consists of an RF generator (i.e., where the power is generated) and an RF applicator (i.e., where the material is placed and heated), which are both combined in one device. The applicator controls the power applied by the generator [
14]. One of the main characteristics of this system is the variation of frequency during heating (typically, about 1–2 MHz above the standards). When the temperature rises, the dielectric properties of the product change and so does the capacitance of the oscillator, causing a shift in frequency [
15]. The 50 Ω RF equipment differs from the previous system in that the RF generator is separated from the RF applicator by a high-power coaxial cable [
14]. This system uses a crystal oscillator to stabilise the frequency (e.g., 27.12 MHz) and to produce a weak signal. This signal is amplified and transmitted to the applicator. The generator is fixed at an output impedance of 50 Ω. In order to transfer the power efficiently, the generator is connected to a matching network (built into the applicator) that keeps the impedance fixed at 50 Ω [
2,
14,
16]. The most crucial difference between both systems (FRO and 50 Ω) resides in the higher cost of implementation and complexity of the latter type. The FRO system is commonly used industrially for drying processes (e.g., cookies, crackers), because the RF energy focuses on wet areas more than dry areas, evening out the moisture distribution throughout the process [
2,
17,
18]. The 50 Ω system is more suitable for pasteurisation and sterilisation purposes because of the stability of frequency and power [
2].
The majority of the research currently conducted on RF pasteurisation/sterilisation focuses on the optimisation of the heating uniformity of food products [
13]. In contrast, the amount of fundamental (lab-scale) research conducted towards the bacterial inactivation mechanisms of RF heating is still limited, illustrating the possible research opportunities in this field. A notable example is the research towards the existence of non-thermal effects induced in microorganisms by RF [
19]. However, commercial 50 Ω RF setups are expensive to adapt for small lab-scale samples and therefore are less suitable for more fundamental scientific studies. Numerous RF schemes, recommendations, and lessons on how to build RF generators, amplifiers, and matching circuits can be found online, but all are difficult to implement for non-experts without specific knowledge in the field of RF behaviour and circuit design. In addition, a matching box is needed to transmit the RF energy to the load and to prevent RF reflection from the load to the power supply. In contrast, the free-running oscillator circuit, although not often used for industrial thermal inactivation applications, is suitable to implement at laboratory conditions. The free-running oscillator system is very simple in implementation, requires only a limited number of electronic parts, and can easily be modified with respect to changing research interests in the field of RF radiation [
20]. The design requires preliminary work to be done on winding the coils and calibrating the system. If the frequency of the oscillations is not important for the specific application, a fluorescent or neon lamp is sufficient for the detection of the presence of RF radiation.
The aim of this study was to design and construct a lab-scale low-power free-running RF oscillator operating at a frequency of 27.12 MHz (i.e., the RF frequency most commonly used for food pasteurisation) at a fixed load, i.e., for a fixed sample volume. An FRO setup design was selected over a 50 Ω system, because the FRO type can be constructed without extensive technical knowledge, with the lower cost being an additional advantage. This is especially useful taking into account that the intended users of the setup would be part of microbiologically oriented research groups. In order to be suitable for fundamental scientific studies, the setup was specifically designed for the thermal inactivation of foodborne pathogens in fundamental lab-scale studies. Therefore, the setup was calibrated to treat two commonly used laboratory media for bacterial growth, i.e., Brain–Heart Infusion broth (BHI) and Tryptic Soy Broth (TSB). The stability of the free-running RF oscillator over the course of the inactivation treatments was investigated by means of the temperature, frequency, and power evolution with time.
Listeria monocytogenes (Gram-positive) and
Salmonella Typhimurium (Gram-negative) were used as model microorganisms to assess the thermal inactivation potential of the RF setup. Those bacteria were selected because of their relevance for European food safety, with
Salmonella being the most common causative agent of foodborne outbreaks (i.e., 30.7% out of 5098 total outbreaks in 2018), and illnesses caused by
Listeria monocytogenes exhibiting the highest fatality rate (i.e., 15.6% in 2018) [
21].
2. Materials and Methods
2.1. Radio Frequency Setup
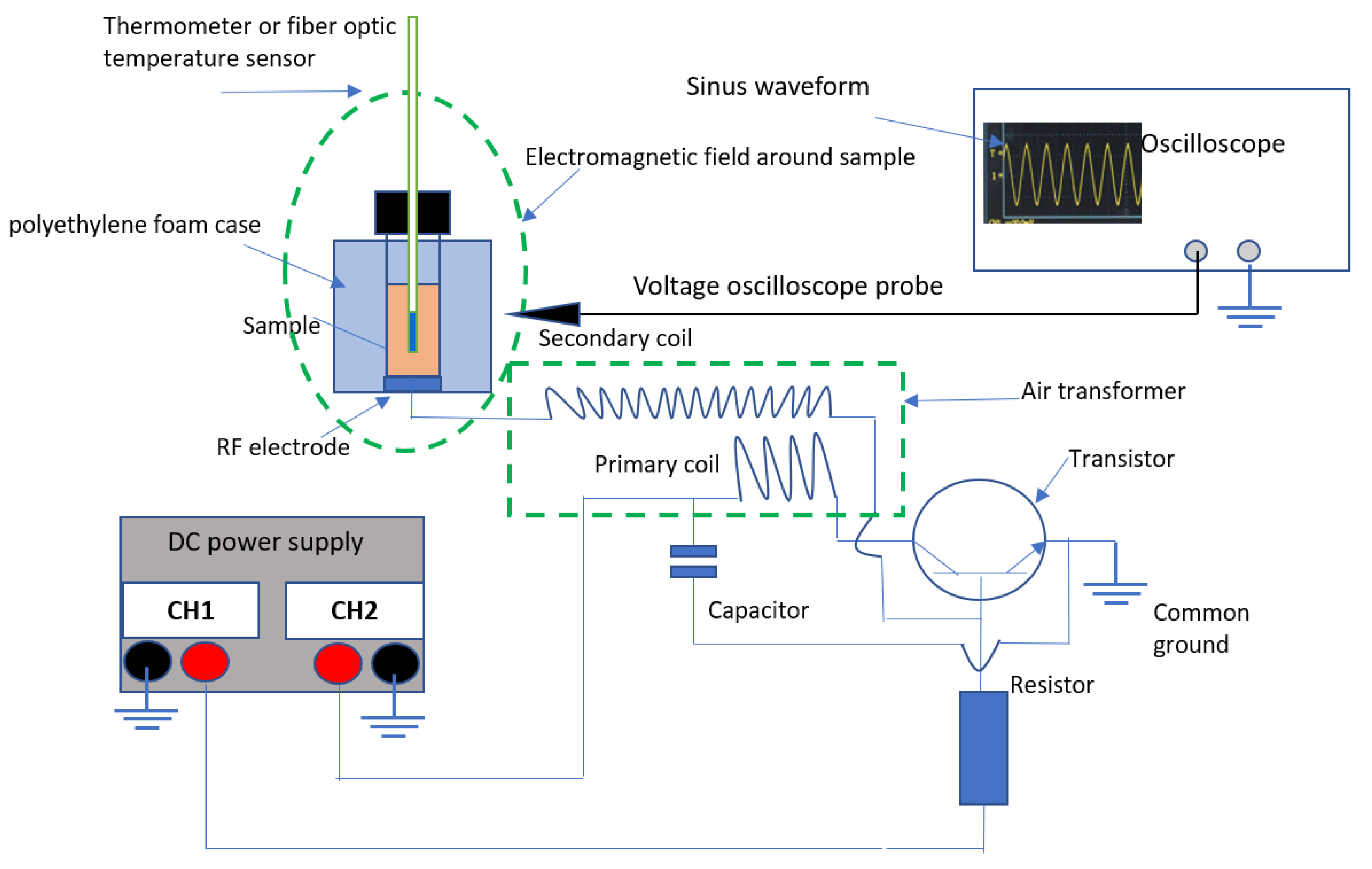
Figure 1 provides a schematic overview of the in-house developed RF setup, while
Figure 2 provides a picture of the setup. RF electromagnetic waves with frequencies in the range of 26.7–29.3 MHz were generated at an input power of 12–14 W. The RF generator was built according to the flyback topology design using an air-core transformer (i.e., Tesla coil). The hot end of the high voltage transformer was connected to the electrode terminal made of a copper coin with a diameter of 16.25 mm. The cold end was connected to the base terminal of an NPN bipolar junction transistor (type 2SC2275A, SavantIC Semiconductor, Irvine, CA, USA). The primary and secondary coil consisted of enamelled copper wire winded around glass tubes, with no pinch between turns. A resistor with a resistance of 887 Ω was used in addition to two power supplies (Aim-TTi EX354RD, 280 W, RS Components, Brussels, Belgium), the first to provide a permanent positive bias at the base-emitter terminal of the transistor, and the second to provide adjustable power. A cooling fan was installed to prevent damage to the secondary coil from over-heating during energy transmission to the load.
The setup was designed to treat samples in small glass vials (4 mL, 45 × 14.7 mm, BGB Analytik Benelux B.V., Harderwijk, the Netherlands) with a polypropylene screw cap lid with a hole in the middle. To reduce heat dissipation through the glass vial during RF heating, vials were placed inside a polyethylene foam case (
Figure 1). In order to record sample temperatures during RF treatments, an ethanol-filled thermometer was inserted into the samples through the hole in the lid, using PTFE tape for fixation. In future studies, researchers could also use optical fibre sensors to measure temperature, in order to minimise interactions with the electromagnetic field. Electromagnetic resonances were quantified by means of an external function generator (Aim-TTi TG5011A, 50 MHz, RS Components, Brussels, Belgium). The RF frequency and waveform were quantified by means of the voltage probe of an oscilloscope (RS PRO IDS1152AU, Bandwidth 150 MHz, RS Components, Brussels, Belgium).
2.2. Calibration of RF Setup
The RF setup was specifically tuned to treat samples with an RF frequency of 27.12 ± 0.5 MHz at the sample load. The calibration to this predetermined frequency was achieved by modifying the following design specifications: (i) position of the primary coil (inductor) on the core of the secondary coil to transfer maximum energy to the load, (ii) the selection of a suitable capacitor to build the LC circuit, (iii) enlarging the surface area of the hot electrode terminal by adding pieces of aluminium foil to the main electrode (copper coin) to achieve the required frequency of oscillation, and (iv) the adjustment of the positive voltage bias at the base-emitter terminal of the NPN transistor to achieve immediate oscillations at the load.
The RF setup was calibrated in a series of trials using vials containing 3 mL of one of two nutrient broths commonly used in microbiological experiments, i.e., Tryptic Soy Broth (TSB, VWR International, Leuven, Belgium) or Brain–Heart Infusion broth (BHI, VWR International, Leuven, Belgium). For each trial, a broth-containing vial was treated for a period of 30 min, during which the evolution of the core temperature of the samples and the RF parameters (i.e., voltage (V), current (A), and frequency (MHz)) were recorded every minute. For each nutrient broth, at least 3 independent replicates were performed. The aim of the calibration was to achieve repeatable frequency stability and temperature profiles among treatments.
2.3. RF Inactivation Trials
2.3.1. Microorganisms and Preculture Conditions
L. monocytogenes strain LMG 26484 and S. Typhimurium strain SL 1344 were acquired from the BCCM/LMG bacterial collection of Ghent University in Belgium. The storage and culturing of the strains were performed using the broths BHI and TSB for L. monocytogenes and S. Typhimurium, respectively. The strains were stored at −80 °C in a mixture of 80% (v/v) of the relevant broth and 20% (v/v) glycerol (Acros Organics, Geel, Belgium).
Prior to each experiment, a purity plate was prepared by thawing a frozen culture of the target microorganism, streaking a loopful of the culture over an agar plate (broth supplemented with 15 g/L bacteriological agar (VWR International, Leuven, Belgium)) and incubating the plate for 24 h. One colony from each purity plate was transferred into 20 mL of nutrient broth in separate 50 mL Erlenmeyers. After incubating for 24 h under static conditions, 20 μL of each culture was inoculated into 20 mL of fresh broth and incubated for 24 h, resulting in stationary-phase cultures with approximate cell densities of 9 log10 CFU/mL. Purity plates and precultures were incubated at temperatures of 30 and 37 °C for L. monocytogenes and S. Typhimurium, respectively.
2.3.2. RF Inactivation Treatments
Sterile glass vials containing 2700 μL of broth (i.e., BHI and TSB for
L. monocytogenes and
S. Typhimurium, respectively) were inoculated with 300 μL of the second preculture of the microorganisms, resulting in an initial cell population of approximately 8 log
10 CFU/mL. Based on preliminary experiments, maximum treatment times of 20 min were selected for both microorganisms. After RF treatment, samples were stored in ice water to stop microbial inactivation prior to further processing. Serial decimal dilutions were directly prepared from an aliquot of the samples using 0.85% (
w/
v) saline solution (NaCl, Sigma Aldrich, Diegem, Belgium), and plated on Brain–Heart Infusion agar (BHIA) and Tryptic Soy Agar (TSA) using the drop technique [
22] for
L. monocytogenes and
S.
Typhimurium, respectively. Prior to enumeration, plates were incubated for at least 24 h at 30 or 37 °C, for
L. monocytogenes and
S.
Typhimurium, respectively.
2.4. Statistical Analysis
Significant differences between the measured system characteristics (e.g., temperature, frequency, power) were determined using an analysis of variance (ANOVA, single variance) test at a 95.0% confidence level (α = 0.05). Fisher’s Least Significant Difference test was used to distinguish which means were significantly different from others. Standardised skewness and standardised kurtosis were used to assess if datasets came from normal distributions. The analyses were performed using MATLAB version R2020b (TheMathworks Inc., Natick, MA, USA). Test statistics were regarded as significant when p ≤ 0.05.
3. Results and Discussion
The experimental design of this study consisted of four parts. First, the RF setup was calibrated to achieve operation within the required frequency range of 27.12 ± 0.5 MHz, using TSB and BHI as sample loads, and to achieve reproducible temperature profiles. Secondly, the evolution of the temperature, frequency, and power consumption was monitored over the course of the treatment of the two microbial media using the acquired calibrated operational parameters. Thirdly, these experiments were conducted for media containing bacterial cells (i.e., S. Typhimurium and L. monocytogenes, for TSB and BHI, respectively), to assess the effect of the cell presence on the RF parameters. Finally, the (thermal) inactivation potential of the RF setup against the two microorganisms was investigated.
3.1. Calibration of the RF Setup
The main objective of this study was to achieve RF treatments with repeatable temperature evolutions and stable oscillations around the target frequency of 27.12 MHz. For this purpose, the differences in temperature profiles and RF frequency between repetitions were aimed not to exceed ±5 °C and ±0.5 MHz, respectively. A sample volume of 3 mL was selected based on preliminary experiments to (i) allow fundamental microbiological research in small sample volumes, (ii) provide enough sample volume for a variety of analytical techniques, and (iii) acquire stable electromagnetic oscillations.
The initial parameters of the RF setup were calibrated based on the load of TSB and BHI broths without bacteria during a number of trials (data not shown). An overview of the resulting initial parameter values (i.e., when no sample is present in the setup) is provided in
Table 1. It should be noted that the calibration parameters can differ significantly among different laboratory media and/or food products, meaning that these parameters should be revised when using the RF setup for other types of samples.
3.2. RF Treatment of Nutrient Broths without Bacteria
At this stage, the calibrated setup was used to treat the two nutrient broths without any bacteria. The most important system characteristics were monitored as a function of time, i.e., temperature, frequency, voltage, and current. The goal was to study the evolution of these characteristics and to evaluate their dependence on the treated medium.
3.2.1. Temperature
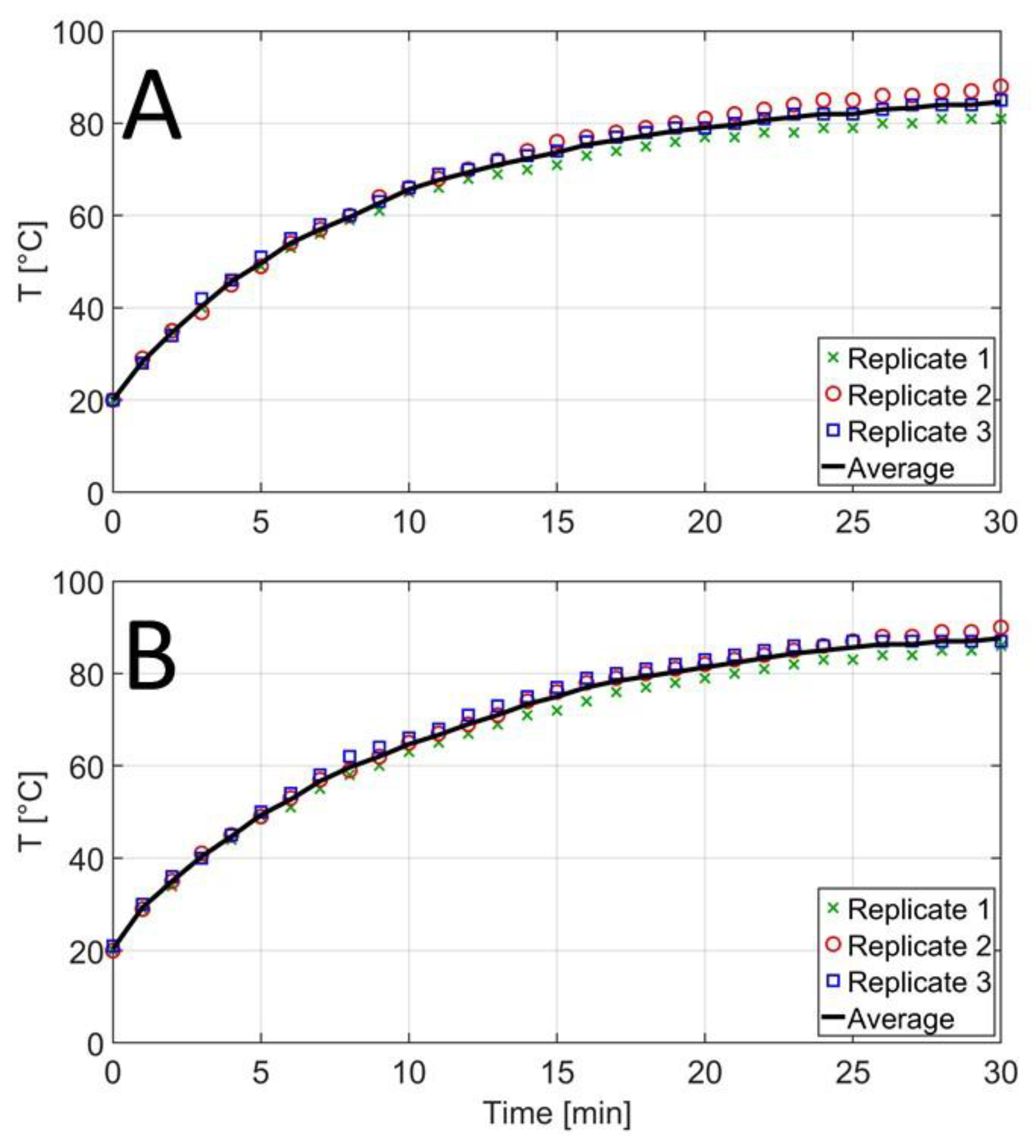
Concerning temperature, the objective was to develop an RF setup capable of providing treatments with a maximum temperature difference of 5 °C between different replicates for each nutrient broth, i.e., TSB and BHI.
Figure 3 shows the temperature profiles during the high-frequency treatment of TSB and BHI media for various repetitions. After 15 min, temperatures of 71–76 and 71–75 °C were reached in TSB and BHI, respectively, hence satisfying the prerequisite of a maximum 5 °C temperature variation over the different replicates. However, the observed temperature variations would exert a significant influence on microbial inactivation rates during thermal inactivation treatments. If temperature variations cannot be further reduced, temperatures should be monitored in future studies during each inactivation trial (i.e., rather than relying on an assumed average temperature profile) in order to allow correct data interpretation.
The temperatures of 71–76 °C reached after 15 min are sufficiently severe to acquire the thermal inactivation of both
L. monocytogenes and
S. Typhimurium in liquid food products such as milk [
23], and the relatively slow heating rate enables frequent sampling to study the inactivation kinetics. In future studies, the input voltage could also be controlled once a certain target temperature is reached in order not to expose bacteria to excessive temperatures. After 30 min, temperatures of 81–88 °C and 86–90 °C were reached in TSB and BHI, respectively. Consequently, the prerequisite of a maximum temperature variation of 5 °C was not always satisfied for the relatively high temperatures reached during the 30 min treatment. Since such high temperatures are generally not required for the inactivation of common vegetative foodborne pathogens, this limitation is not a major disadvantage for the developed RF setup. In addition, the high temperatures reached during the 30 min treatments indicate that microorganisms that are more heat resistant than
L. monocytogenes and
S. Typhimurium can potentially also be inactivated with this RF setup, barring the further improvement of the repeatability of the obtained temperature profiles.
It should be noted that temperature measurements were in this study only conducted at one location in the sample. Therefore, the measured temperature is not representative for the entire sample volume, as non-uniform heating and the resulting heterogeneous temperature distributions that lead to varying effects on bacterial cells are a well-known challenge for RF treatments [
24]. However, due to the small sample sizes used (i.e., 3 mL) and the relatively fast convection in liquid samples, temperature heterogeneities in the samples are limited compared to commercial RF setups. In future studies, it is possible to measure the temperature at different locations in the sample, using infrared temperature sensors or optical fibre temperature sensors, which do not interact with the electromagnetic field. Theoretically, it is also possible to use computer simulations to model the temperature distribution in the samples during the RF treatment, which is based on measured temperatures and sample properties (e.g., dielectric properties, rheological properties, density, geometrical parameters) [
25,
26].
3.2.2. Frequency
One of the characteristics of free-running oscillation is the reduction of the initial frequency (i.e., without sample load) caused by the absorption of RF energy by the load. Apart from the frequency, the absorption of the RF energy by the sample is mainly affected by the temperature-dependent dielectric properties of that sample, i.e., the dielectric constant ε’ and the dielectric loss factor ε” [
20]. Therefore, it was important to validate that the RF frequency remained within the predefined frequency range limits of 27.12 ± 0.50 MHz during the entire treatment of the nutrient broths for which the setup was calibrated.
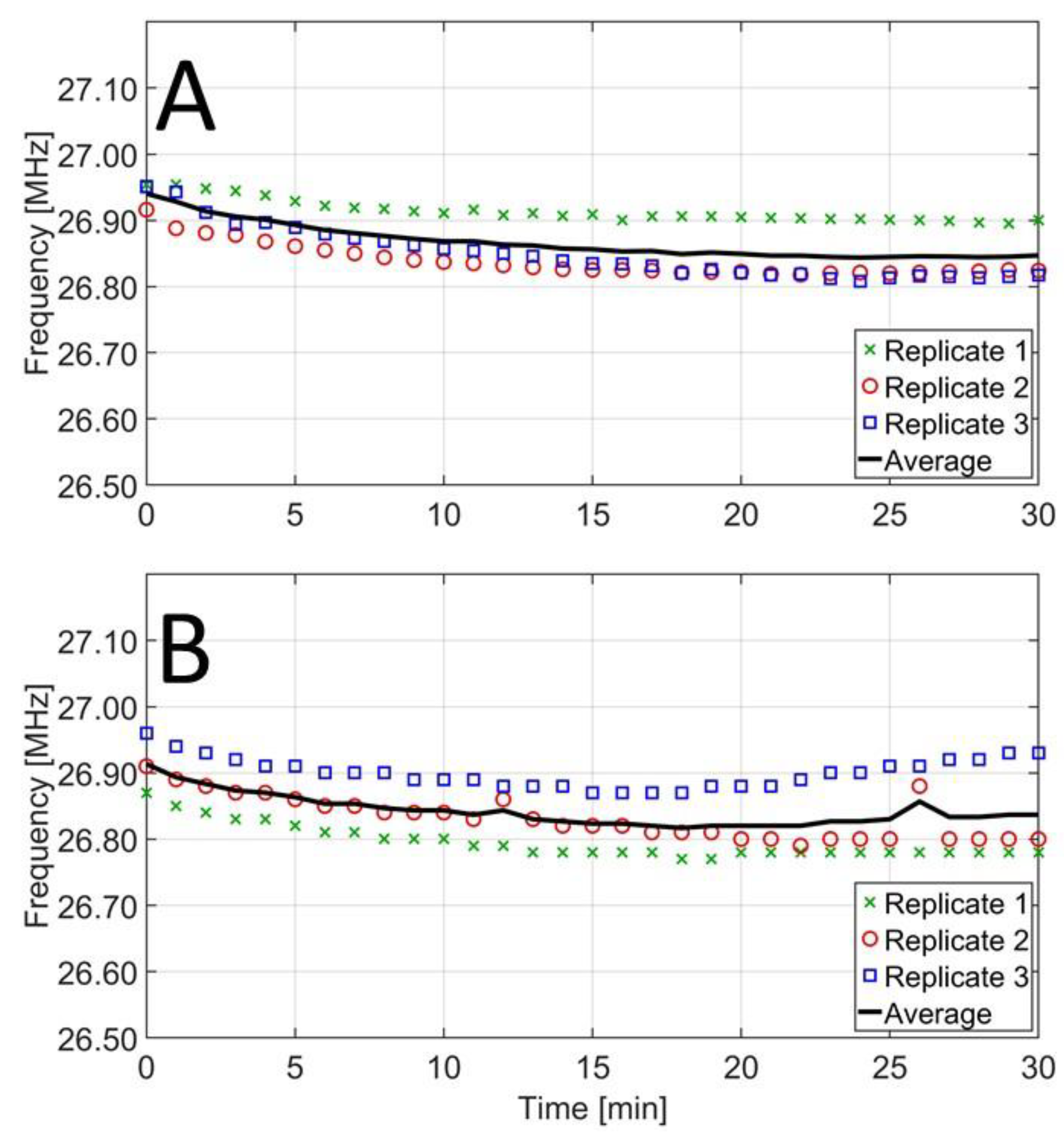
Figure 4 shows the RF frequency evolution in TSB and BHI. For both media, it was observed that the initial RF frequency varied only slightly among different replicates, ranging from 26.92 to 26.95 MHz and from 26.87 to 26.96 MHz, for TSB and BHI, respectively. As such, initial frequencies ranged in a bandwidth of just 0.09 MHz. As the treatments progressed, the frequency decreased with increasing sample temperature, approximately reaching a constant value after 15 min. As can be seen in
Figure 3, this treatment time corresponded to temperatures of 71–76 °C, indicating that the influence of temperature on the dielectric properties started to become less important at these temperatures. The constant frequency was on average higher in TSB (26.86 ± 0.05 MHz) than in BHI (26.82 ± 0.05 MHz), but differences were not significant. Overall, the RF frequency remained within the predefined range of 27.12 ± 0.50 MHz during the entire treatment for both broths. Nevertheless, more precise fine-tuning is necessary in future studies to achieve the more narrow frequency range of 27.12 ± 0.16 MHz designated for ISM applications. This fine-tuning is expected to be feasible, since the entire set of treatments ranged in a bandwidth of 0.19 MHz, which is smaller than the ISM band of 0.32 MHz. Therefore, the initial calibration needs to improve with the specific media or food product and sample size in mind. Parameters to adapt in order to fine-tune the frequency include the voltage, the current, and the position of the secondary coil.
3.2.3. Power Consumption
The maximum power consumption of the samples was calculated using the recorded voltage and current (P = UI). However, it should be taken into account that not all emitted power was absorbed by the sample, as the environment and the electrical system also consume a certain amount of the electromagnetic energy.
The evolution of the power consumption during the RF treatments of TSB and BHI is illustrated in
Figure 5. For both media, the power consumption decreased during the first minutes of the treatments, remaining at an approximately constant value afterwards, i.e., 12.14–12.25 W and 12.67–12.85 W, for TSB and BHI, respectively. Over the course of the entire treatment, the power consumption in TSB was in general also significantly lower than in BHI. The maximum power consumption in samples exposed to RF energy can be described by Equation (1) [
27].
where
P (W/m³) is the power consumption caused by the conversion from electromagnetic to thermal energy; f (Hz) is the frequency;
ε0 is the permittivity of vacuum (8.854 × 10
−12 F/m);
ε″ is the dielectric loss factor; and
(V/m) is the electric field intensity in the sample. The change in power consumption over the course of the treatments occurs according to Equation (1). The most probable cause for the decrease in power absorption observed in
Figure 5 is a change in the electrical capacitance of the media with increasing temperature, which is possibly due to a decrease in the dielectric loss factor. In addition, the decrease in power consumption during the first treatment minutes for both media is a possible cause for the relatively fast decrease in frequency observed during that time period, as seen in
Figure 4. In future studies, the quantification of the temperature-dependent dielectric properties of TSB and BHI could elucidate the underlying physical mechanisms causing the observed frequency and power consumption profiles.
3.3. Effect of the Presence of Bacteria
Based on the complete dataset collected for the RF treatments of TSB and BHI using the calibrated setup, all parameters (i.e., temperature, frequency, power consumption) were accepted for further use. To assess whether the presence of bacterial cells in the two media affected the different parameters, the experiments from the previous section were repeated in the presence of bacteria. TSB was inoculated with S. Typhimurium, while BHI was inoculated with L. monocytogenes, both to a cell density of approximately 8 log10 CFU/mL. Experiments were only conducted for 20 min, since preliminary experiments indicated that the complete bacterial population was already inactivated after this treatment time due to the high temperatures.
Figure 6 shows the comparison of the temperature evolution in TSB and BHI both for the case with and without bacterial cells. Both in TSB and BHI, temperatures were generally higher in the presence of bacterial cells, but differences were rarely statistically significant (results not shown). The increased heating rate in the presence of bacterial cells that sometimes occurred was probably caused by the influence of the cells on the dielectric properties of the samples. The effect of the presence of cellular material on the dielectric properties of solutions is well-known and has even led to the development of applications for the real-time estimation of microbial biomass during growth experiments [
28,
29,
30]. The exact influencing mechanisms of this phenomenon are quite complex, and hence, a detailed explanation falls outside the scope of this study. In brief, bacterial cell membranes are characterised by a low conductance, exerting a significant influence on the total dielectric properties of cells suspensions, especially at the high cell densities that are applied in this study [
31]. The principal mechanism for the dielectric polarisation at frequencies in the MHz range is the accumulation of charges from extra- and intracellular fluids (i.e., the Maxwell–Wagner effect) at the membrane [
32,
33].
The effect of bacterial cell presence on the frequency inside both media is illustrated in
Figure 7. Both for TSB and BHI, the statistical analysis (results not shown) showed no significant differences between the situations with and without cells. While the frequency in BHI was consistently higher in the presence of
L. monocytogenes, the maximum difference was rather small, i.e., 0.02–0.04 MHz. Therefore, the presence of the bacterial cells did not seem to influence the frequency of the RF waves.
Finally,
Figure 8 shows the power consumption evolution in both media for the cases with and without bacterial cells. For TSB, the power consumption was always higher when cells were present in the broths, even though statistical differences were only observed between 1 and 7 min of treatment (results not shown). For BHI, the power consumption was lower when cells were present after approximately 10 min, although differences were only significant after 19 and 20 min. The different behaviour of the two medium-microorganism combinations was probably caused by the combined action of two effects. Firstly, as mentioned, the power consumption without bacterial cells was higher in BHI than in TSB. Secondly, the influence of the presence of bacterial cells on the dielectric properties is dependent on the type of microorganisms, with the effect being considerably larger in Gram-positive bacteria than in their Gram-negative counterparts due to the different cell envelope organisation [
34,
35].
3.4. Thermal Inactivation of Model Foodborne Pathogens
In the previous sections, it was demonstrated that the developed RF setup is capable of providing thermal treatments with the desired parameter values in TSB and BHI, both with and without bacterial cells. In a final step, the RF treatments were assessed for their bacterial inactivation potential.
Figure 9 depicts the inactivation of
S. Typhimurium and
L. monocytogenes over the course of the RF treatments. For
S. Typhimurium, inactivation started after 4–5 min, corresponding to a temperature of 47–51 °C. For
L. monocytogenes, inactivation started after 5–6 min, corresponding to a temperature of 49–54 °C. These inactivation temperatures correspond to the minimal required temperatures for the inactivation of both microorganisms as reported in literature, i.e., approximately 50 °C for both
S. Typhimurium and
L. monocytogenes [
36,
37]. Bacterial counts were below the detection limit (DL) for all replicates after 8 min (T = 63 ± 2 °C) and 10 min (T = 66 ± 1 °C), for
S. Typhimurium and
L. monocytogenes, respectively. For
S.
Typhimurium, bacterial counts were below the DL for samples taken after 8, 9, 10, 15, and 20 min, while for
L. monocytogenes, bacterial counts were below the DL for samples taken after 10, 15, and 20 min. These treatment times of 8-10 min to achieve a 6 log reduction of bacteria in small samples of 3 mL are similar to those achieved in (isothermal) water baths [
38]. Consequently, the developed RF setup is suitable for thermal inactivation experiments on both
S. Typhimurium and
L. monocytogenes. If a slower or faster inactivation rate would be required for certain types of research, this can be achieved by controlling the input voltage of the setup. Different types of temperature profiles can also be achieved by using the voltage as a control parameter, e.g., heating up to a constant temperature at a high input voltage followed by a constant temperature phase at a lower input voltage. However, in this case, the effect of the voltage change on the frequency should be assessed to see whether further calibration is necessary. If researchers require this kind of control over the temperature profile, the voltage change could also be achieved with the power as an adjustable parameter. While the power-control of the setup is at this stage not possible in a direct way (i.e., without specific calculations), this could be implemented in order to acquire a more easily adjustable setup.
In future research, it should also be taken into account that the resistance of bacteria to thermal treatments can be affected by the food model system [
38]. Therefore, depending on their specific research interests, researchers could opt to wash and resuspend the cells in a fresh buffer medium when they want to discard food microstructural and/or compositional influences. Moreover, when studying the microbial inactivation in detail, it will be beneficial to obtain information on potential temperature heterogeneities in the sample. Even though the setup has been designed to minimise temperature heterogeneities compared to commercial systems, small temperature differences could still be worth considering when interpreting microbial inactivation data.
4. Conclusions
Fundamental studies on the (thermal) inactivation mechanisms of RF require the use of lab-scale setups that are more easily controllable than the various (expensive) commercially available RF setups. Therefore, in this study, a lab-scale low-power free-running RF oscillator was developed specifically with this goal in mind. The setup was calibrated to treat two typical microbiological growth media (i.e., TSB and BHI) within a predefined frequency range of 27.12 ± 0.50 MHz. Using fairly low power levels of 12–13 W and small volumes of 3 mL, temperatures above 60 °C were reached within treatments times relevant for microbiological inactivation experiments (approximately 10 min). Thermal treatments were repeatable among different replicates, and the influence of the presence of bacterial cells (i.e., S. Typhimurium and L. monocytogenes, for TSB and BHI, respectively) on the operating parameters was insignificant. The microbial inactivation potential of the setup was validated, illustrating its potential use in more fundamental microbiological studies on RF inactivation in the future. In such studies, the treatment parameters can be further calibrated to comply with specific research goals by, e.g., constructing more complex temperature profiles as a function of the input voltage and/or narrowing the target frequency range. While only two medium-microorganism combinations were tested in this study, the developed setup can also be used to treat other liquid products containing different microorganisms, although a recalibration may be required for each product to obtain the desired frequency. Barring a more elaborate recalibration, the setup can in future studies also be adopted to treat more complexly structured products of different origins (e.g., dairy, meat, fish, vegetables), including semi-solid and solid products and powders.