Effect of Modified Silica Materials on Polyvinyl Chloride (PVC) Substrates to Obtain Transparent and Hydrophobic Hybrid Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Sol–Gel Silica Materials
2.3. Characterization of the Sol–Gel Silica Materials and of Thin Films
2.3.1. Photopolymerization
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Transmission Electron Microscopy (TEM)
2.3.5. UV/Vis Spectroscopy
2.3.6. Atomic Force Microscopy (AFM)
2.3.7. Spectroscopic Ellipsometry (SE) Measurements
2.3.8. Contact-Angle (CA) Measurements
3. Results and Discussion
3.1. FTIR Spectroscopy
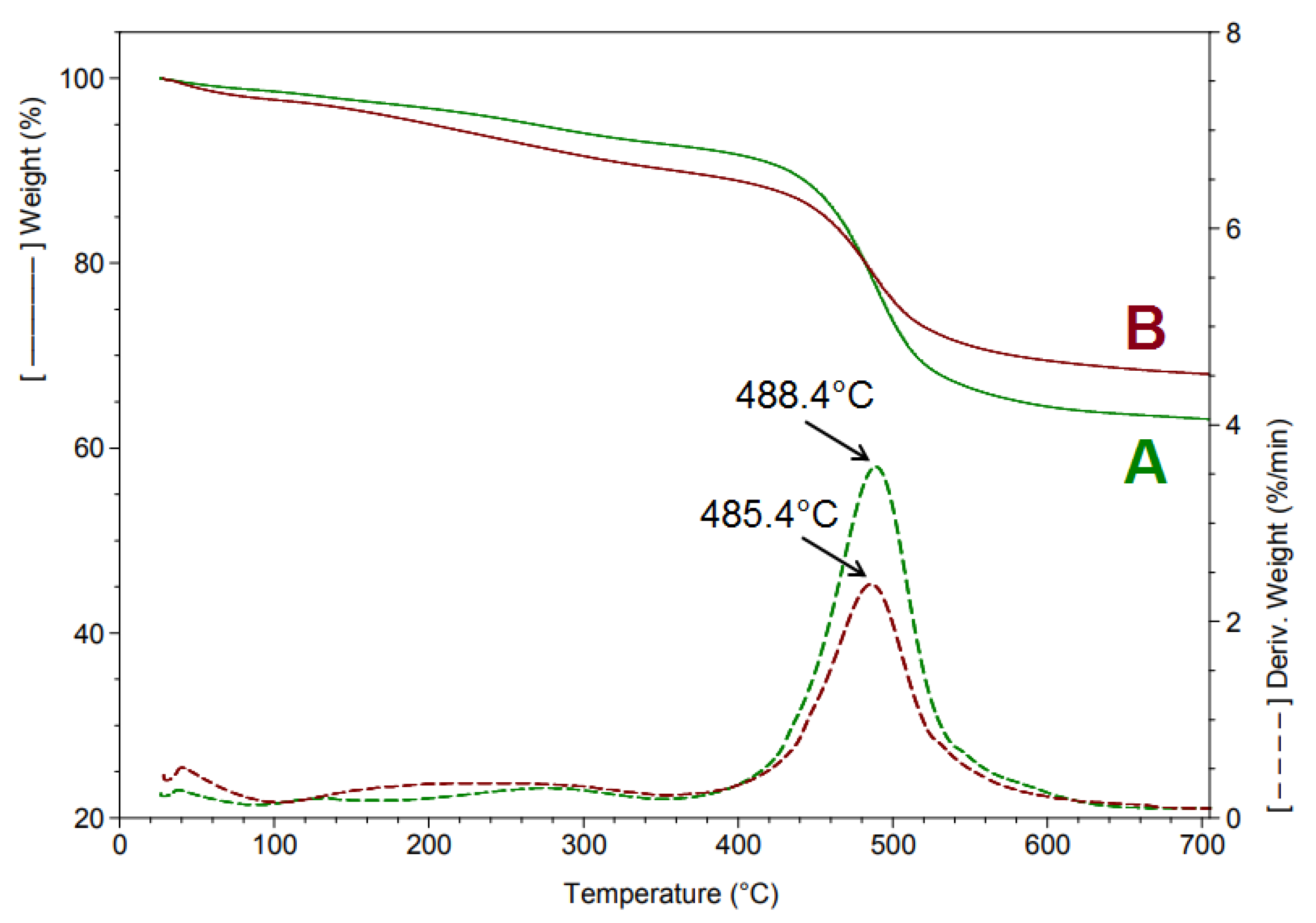
3.2. Thermogravimetric Analysis (TGA)
3.3. TEM Analysis
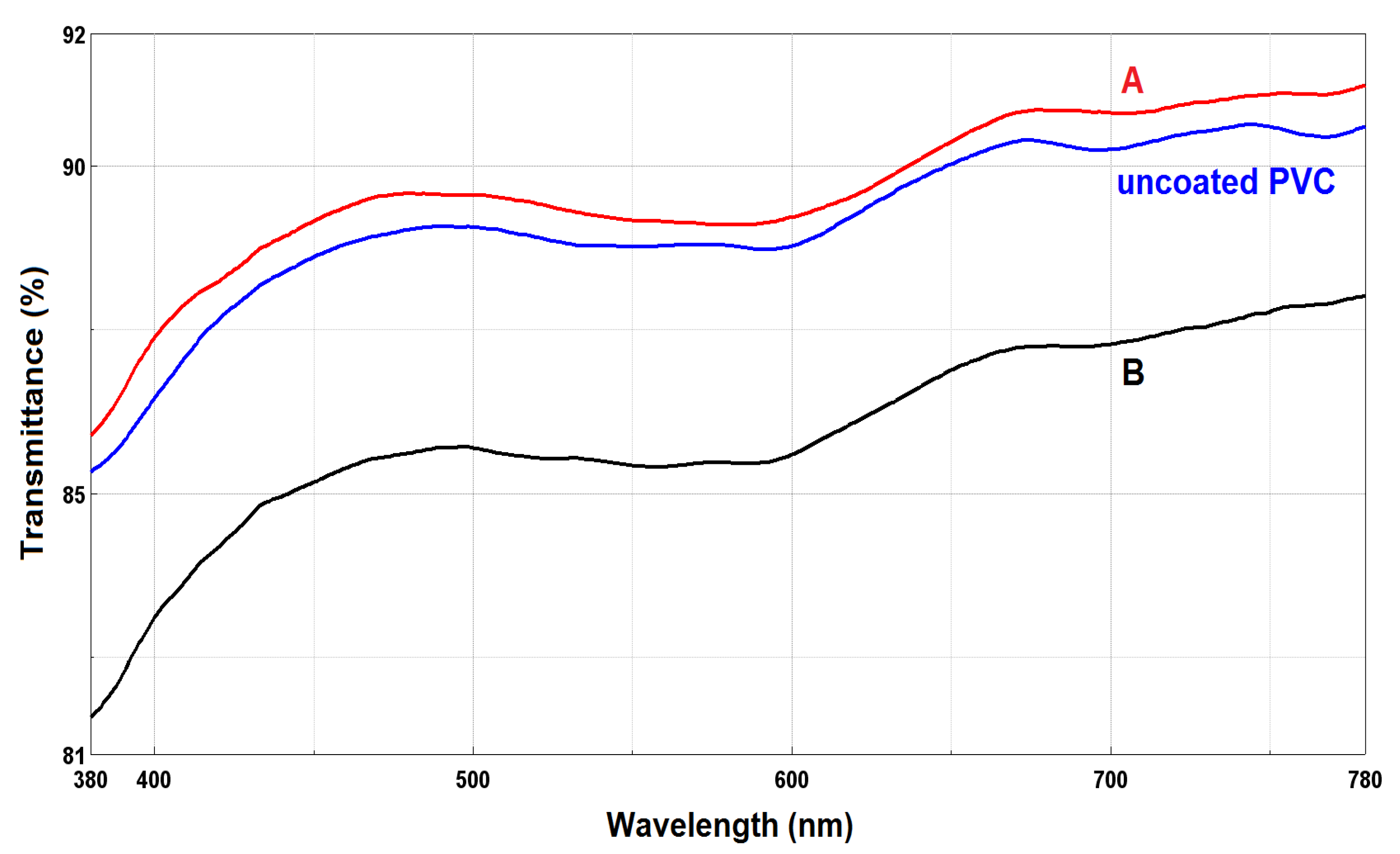
3.4. UV/Vis Spectroscopy
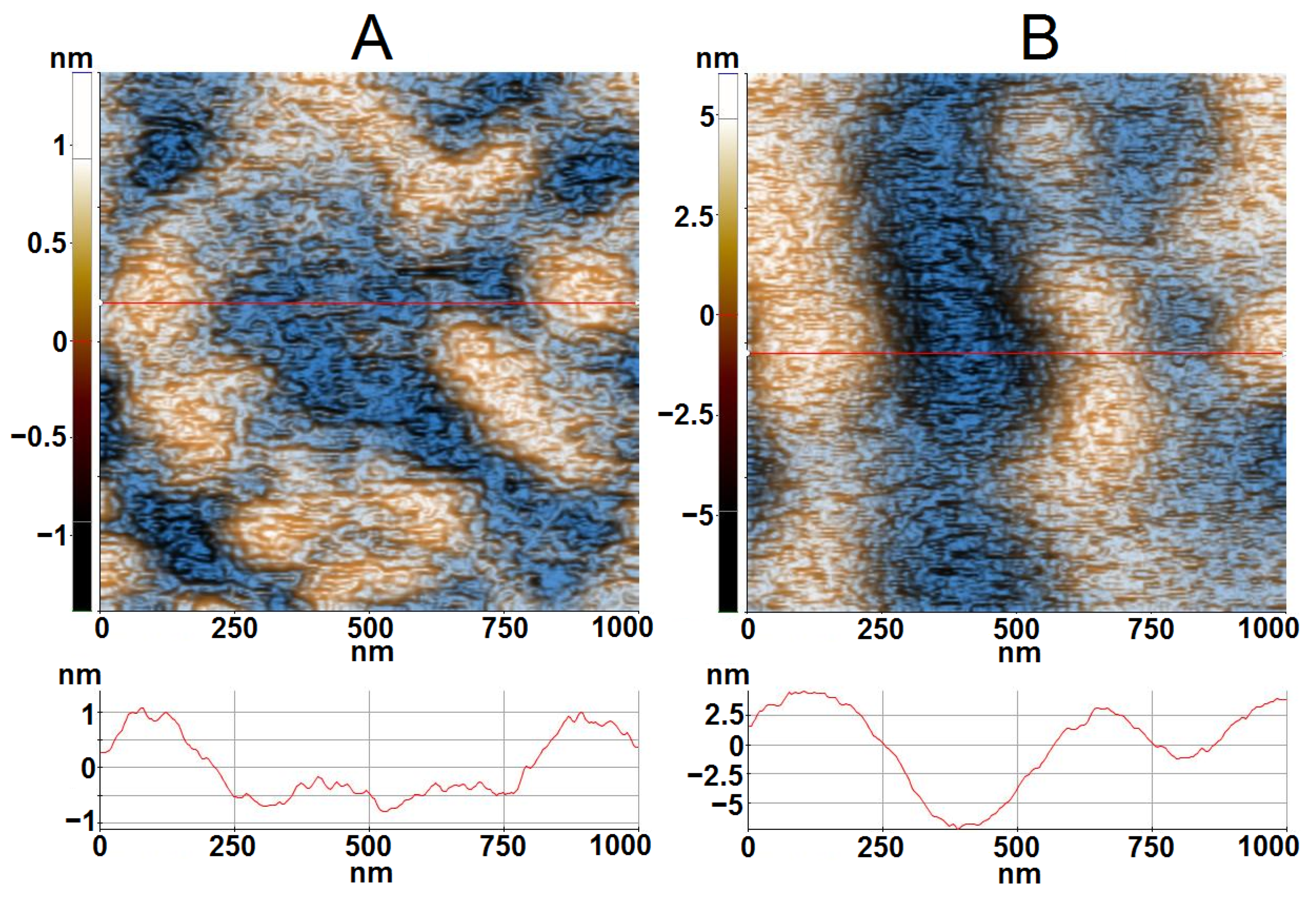
3.5. AFM Analysis and Spectroscopic Ellipsometry (SE) Measurements
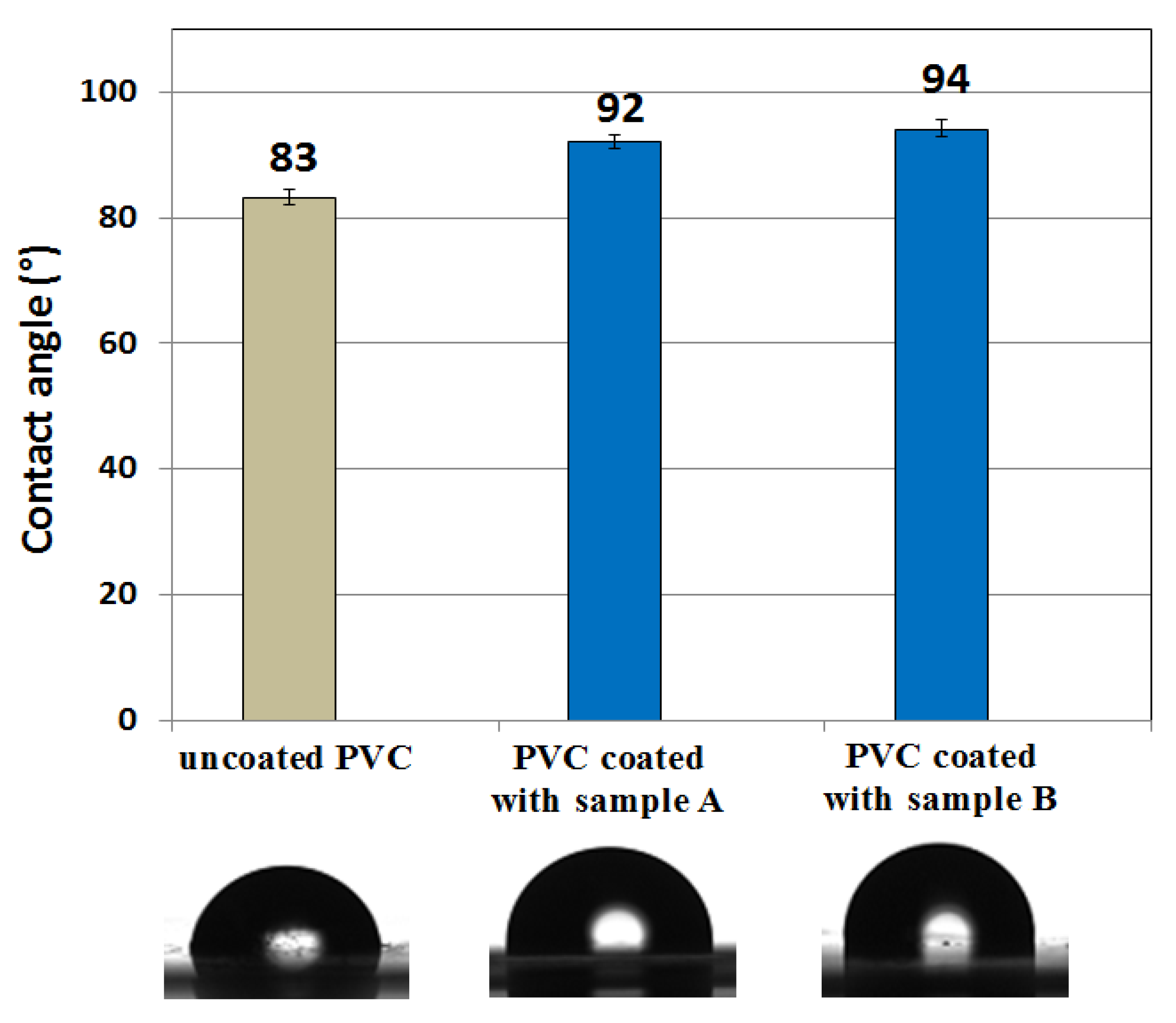
3.6. Contact-Angle Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garlisi, C.; Trepci, E.; Li, X.; Sakkaf, R.A.; Al-Ali, K.; Nogueira, R.P.; Zheng, L.; Azar, E.; Palmisano, G. Multilayer thin film structures for multifunctional glass: Self-cleaning, antireflective and energy-saving properties. Appl. Eng. 2020, 264, 114697–114728. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mourad, I.A.-H.; Pervez, H.; Surkatti, R. Recent developments in multifunctional coatings for solar panel applications: A review. Sol. Energy Mater. Sol. Cells 2019, 189, 75–102. [Google Scholar] [CrossRef]
- Arabatzis, I.; Todorova, N.; Fasaki, I.; Tsesmeli, C.; Peppas, A.; Li, W.X.; Zhao, Z. Photocatalytic, self-cleaning, antireflective coating for photovoltaic panels: Characterization and monitoring in real conditions. Sol. Energy 2018, 159, 251–259. [Google Scholar] [CrossRef]
- Rosales, A.; Esquivel, K. SiO2@TiO2 Composite Synthesis and Its Hydrophobic Applications: A Review. Catalysts 2020, 10, 171. [Google Scholar] [CrossRef]
- Alarifi, I.M. Advanced selection materials in solar cell efficiency and their properties—A comprehensive review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Chemin, J.-B.; Bulou, S.; Baba, K.; Fontaine, C.; Sindzingre, T.; Boscher, N.D.; Choquet, P. Transparent anti-fogging and self-cleaning TiO2/SiO2 thin flms on polymer substrates using atmospheric plasma. Sci. Rep. 2018, 8, 9603. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Nagappan, S.; Li, X.-H.; Lee, J.-H.; Shi, L.; Yuan, S.; Lee, W.-K.; Ha, C.-S. Highly Transparent, Robust Hydrophobic, and Amphiphilic Organic−Inorganic Hybrid Coatings for Antifogging and Antibacterial Applications. ACS Appl. Mater. Interfaces 2021, 13, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Park, J.J.; Park, S.S.; Ha, C.S. Preparation of superhydrophobic and transparent micro-nano hybrid coatings from polymethylhydroxysiloxane and silica ormosil aerogels. Nano Converg. 2014, 1, 30. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Wang, X.; Xie, A.; Shen, Y.; Duan, W.; Zhang, Y.; Li, J. A simple method for preparation of transparent hydrophobic silica-based coatings on different substrates. Appl. Phys. A 2012, 106, 229–235. [Google Scholar] [CrossRef]
- Du, X.; Xing, Y.; Li, X.Y.; Huang, H.W.; Geng, Z.; He, J.H.; Wen, Y.Q.; Zhang, X.J. Broadband antireflective superhydrophobic self-cleaning coatings based on novel dendritic porous particles. RSC Adv. 2016, 6, 7864–7871. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Yan, L.; Li, Y.; Zhang, S.; He, M.; Li, H.; Yan, H.; Jiang, B. Preparation of silica coatings with continuously adjustable refractive indices and wettability properties via sol–gel method. RCS Adv. 2018, 8, 6091–6098. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Bretler, S.; Cohen, S.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Margel, S. Engineering of superhydrophobic silica microparticles and thin coatings on polymeric films by ultrasound irradiation. Mater. Today Chem. 2021, 21, 100520–100529. [Google Scholar]
- Chang, C.-C.; Oyang, T.-Y.; Hwang, F.-H.; Chen, C.-C.; Cheng, L.-L. Preparation of polymer/silica hybrid hard coatings with enhanced hydrophobicity on plastic substrates. J. Non-Cryst. Solids 2012, 358, 72–76. [Google Scholar] [CrossRef]
- Zhang, X.P.; Lan, P.J.; Lu, Y.H.; Li, J.; Xu, H.; Zhang, J.; Lee, Y.; Rhee, J.Y.; Choy, K.L.; Song, W.J. Multifunctional antireflection coatings based on novel hollow silica-silica nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 1415–1423. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Alssad, A.M.; Ahmad, A.A.; Telfah, A. A novel optical model of the experimental transmission spectra of nanocomposite PVC-PS hybrid thin films doped with silica nanoparticles. Heliyon 2020, 6, e04177. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Baset, T.; Elzayat, M.; Mahrous, S. Characterization and optical and dielectric properties of polyvinyl chloride/silica nanocomposites films. Int. J. Polym. Sci. 2016, 1, 1707018. [Google Scholar] [CrossRef]
- Youn, D.-H.; Lee, K.-S.; Jung, S.-K.; Kang, M. Fabrication of a Simultaneous Highly Transparent and Highly Hydrophobic Fibrous Films. Appl. Sci. 2021, 11, 5565. [Google Scholar] [CrossRef]
- Tao, C.; Zou, X.; Reddy, K.M.; Zhanga, L.; Jiang, B. A hydrophobic ultralow refractive-index silica coating towards double-layer broadband antireflective coating with exceptionally high vacuum stability and laser-induced damage threshold. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 340–349. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Alzamani, M.; Eghdam, E.; Karimi, M.; Mastali, S. SiO2-TiO2 Nanostructure Films on Windshields Prepared by Sol-Gel Dip-Coating Technique for Self-Cleaning and Photocatalytic Applications. Nanosci. Nanotechnol. 2012, 2, 16–21. [Google Scholar] [CrossRef]
- Pingan, H.; Mengjun, J.; Yanyan, Z.; Ling, H. A silica/PVA adhesive hybrid material with high transparency, thermostability and mechanical strength. RSC Adv. 2017, 7, 2450–2459. [Google Scholar] [CrossRef]
- Hegde, M.; Kavanagh, Y.; Duffy, B.; Tobin, E.F. Multifunctional hybrid sol-gel coatings for Marine Renewable Energy Applications: Synthesis, Characterization and Comparative Analysis with Organically Modified Silicon Precursor Coatings. MRS Adv. 2020, 5, 1757–1764. [Google Scholar] [CrossRef]
- Picolo, N.; Tavares de Moraes, V.; Lebrão, G.W.; Lebrão, S.M.G. Sol-gel processed Superhydrophobic Plastic Surfaces Modified with Perfluorooctyltriethoxysilane (POTS). Mater. Res. 2019, 22, e20190488. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self–cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Sriramulu, D.; Reed, E.L.; Annamalai, M.; Venkatesan, T.V.; Valiyaveettil, S. Synthesis and Characterization of Superhydrophobic, Self-cleaning NIR-reflective Silica Nanoparticles. Sci. Rep. 2016, 6, 35993. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, C.; Zhang, W.; Yu, L.; Wang, Q.; Que, W.; Wang, Y.; Hu, F. Multifunctional TiO2/ormosils organic-inorganic hybrid films derived by a sol-gel process for photonics and UV nanoimprint applications. Opt. Mater. Express 2019, 9, 304–314. [Google Scholar] [CrossRef]
- Purcar, V.; Răditoiu, V.; Răditoiu, A.; Manea, R.; Raduly, F.M.; Ispas, G.C.; Frone, A.N.; Nicolae, C.A.; Gabor, R.A.; Anastasescu, M.; et al. Preparation and Characterization of Some Sol-Gel Modified Silica Coatings Deposited on Polyvinyl Chloride (PVC) Substrates. Coatings 2021, 11, 11. [Google Scholar] [CrossRef]
- Tao, C.; Yan, H.; Yuan, X.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Sol-gel based antireflective coatings with superhydrophobicity and exceptionally low refractive indices built from trimethylsilanized hollow silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 307–313. [Google Scholar] [CrossRef]
- Capeletti, B.L.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; Mark, T.S., Ed.; IntechOpen: London, UK, 2016; pp. 1–21. [Google Scholar]
- Tuo, J.; Chi, M.; Brisbin, L.; Mu, L.; Robertson, C.G.; Dong, Y.; Zhu, J. Organosilane grafted silica: Quantitative correlation of microscopic surface characters and macroscopic surface properties. Appl. Surf. Sci. 2017, 399, 565–572. [Google Scholar]
- Albers, P.; Maier, M.; Reisinger, M.; Hannebauer, B.; Weinand, R. Physical boundaries within aggregates—Differences between amorphous, para-crystalline, and crystalline Structures. Cryst. Res. Technol. 2015, 50, 846–865. [Google Scholar] [CrossRef]
- Castro, M.; Losch, P.; Farès, C.; Haouas, M.; Taulelle, F.; Breynaert, E.; Kirschhock, C.; Park, W.; Ryoo, R.; Schmidt, W. Self-organization of silicates on different length scales exemplified by amorphous mesoporous silica and mesoporous zeolite beta using multiammonium surfactants. RSC Adv. 2020, 10, 20928–20938. [Google Scholar] [CrossRef]
- Protsak, I.; Pakhlov, E.; Tertykh, V.; Le, Z.-C.; Dong, W. A New Route for Preparation of Hydrophobic Silica Nanoparticles Using a Mixture of Poly(dimethylsiloxane) and Diethyl Carbonate. Polymers 2018, 10, 116. [Google Scholar] [CrossRef]
- Twej, W.A.A.; Alattar, A.M.; Drexler, M.; Alamgir, F.M. Tuned optical transmittance in single-step-derived silica aerogels through pH-controlled microstructure. Int. Nano Lett. 2017, 7, 257–265. [Google Scholar] [CrossRef]
- Manea, R.; Purcar, V.; Rădițoiu, V.; Rădițoiu, A.; Raduly, M.F.; Ispas, G.C.; Frone, A.; Nicolae, C.A.; Wagner, L.E. Preparation of Transparent and Antireflective Silica Thin Coatings on Plastic Substrates by Sol-Gel Process. Proceedings 2020, 57, 6. [Google Scholar] [CrossRef]
- Alam, K.; Ali, S.; Saboor, A.; Salman, M.; Maoz; Humayun, M.; Sadiq, M.; Arif, M. Antireflection, Superhydrophilic Nano-Porous SiO2 Coating based on Aerosol Impact Spray Deposition Technique for Solar PV Module. Coatings 2019, 9, 497. [Google Scholar] [CrossRef]
- Xing, Y.; Du, X.; Li, X.Y.; Huang, H.W.; Li, J.Q.; Wen, Y.Q.; Zhang, X.J. Tunable dendrimer-like porous silica nanospheres: Effects of structures and stacking manners on surface wettability. J. Alloys Compd. 2018, 732, 70–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhao, S.; Dong, W. Hydrophobic and optical properties of silica antireflective coating prepared via sol-gel method. Mater. Res. Express 2021, 8, 046403. [Google Scholar] [CrossRef]
- Castellano, M.; Marsano, E.; Turturro, A.; Conzatti, L.; Busca, G. Dependence of surface properties of silylated silica on the length of silane arms. Adsorption 2012, 18, 307–320. [Google Scholar] [CrossRef]
- Xia, B.; Luo, J.; Li, Y.; Yang, B.; Zhang, S.; Jiang, B. Preparation of sponge-like porous SiO2 antireflective coatings with excellent environment-resistance by an acid-catalysed sol–gel method. RSC Adv. 2017, 7, 26834–26838. [Google Scholar] [CrossRef]
- Yildirim, A.; Khudiyev, T.; Daglar, B.; Budunoglu, H.; Okyay, A.K.; Bayindir, M. Superhydrophobic and omnidirectional antireflective surfaces from nanostructured ormosil colloids. ACS Appl. Mater. Interfaces 2013, 5, 853–860. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Wang, P.; Ye, L.; Luo, J.; Jiang, B. A convenient sol-gel approach to the preparation of nano-porous silica coatings with very low refractive indices. Chem. Commun. 2014, 50, 13813–13816. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, Y.; Zhang, H.; Yan, H.; Lv, H.; Jiang, B. Sol-gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef] [PubMed]


| Alkoxysilane | Structure | Molecular Formula | Mw (g·mol−1) |
|---|---|---|---|
| TEOS | 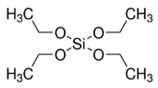 | C8H20O4Si | 208.33 |
| DMVES | 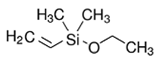 | C6H14OSi | 130.26 |
| OTES | 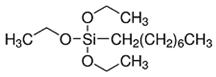 | C14H32O3Si | 276.48 |
| HDTMES | 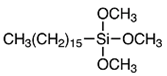 | C19H42O3Si | 346.63 |
| Sample | TEOS (mL) | DMVES (mL) | OTES (mL) | HDTMES (mL) |
|---|---|---|---|---|
| A | 4.2 | 1.52 | 2.9 | - |
| B | 4.2 | 1.52 | - | 0.7 |
| Sample | 30–105 °C | 105–350 °C | 350–700 °C | Residue at 700 °C | ||
|---|---|---|---|---|---|---|
| wt. loss % | wt. loss % | Tmax 1 °C | wt. loss % | Tmax °C | N2% | |
| A | 1.50 | 5.58 | 272.8 | 29.75 | 488.4 | 63.17 |
| B | 2.42 | 7.37 | 239.5 | 22.18 | 485.4 | 68.04 |
| Sample | Film Thickness (nm) | Roughness (nm) | MSE |
|---|---|---|---|
| A | 426.96 | 2.90 | 0.89 |
| B | 513.91 | 6.63 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcar, V.; Rădițoiu, V.; Rădițoiu, A.; Raduly, F.M.; Ispas, G.C.; Căprărescu, S.; Frone, A.N.; Trică, B.; Nicolae, C.-A.; Anastasescu, M.; et al. Effect of Modified Silica Materials on Polyvinyl Chloride (PVC) Substrates to Obtain Transparent and Hydrophobic Hybrid Coatings. Appl. Sci. 2021, 11, 11044. https://doi.org/10.3390/app112211044
Purcar V, Rădițoiu V, Rădițoiu A, Raduly FM, Ispas GC, Căprărescu S, Frone AN, Trică B, Nicolae C-A, Anastasescu M, et al. Effect of Modified Silica Materials on Polyvinyl Chloride (PVC) Substrates to Obtain Transparent and Hydrophobic Hybrid Coatings. Applied Sciences. 2021; 11(22):11044. https://doi.org/10.3390/app112211044
Chicago/Turabian StylePurcar, Violeta, Valentin Rădițoiu, Alina Rădițoiu, Florentina Monica Raduly, Georgiana Cornelia Ispas, Simona Căprărescu, Adriana Nicoleta Frone, Bogdan Trică, Cristian-Andi Nicolae, Mihai Anastasescu, and et al. 2021. "Effect of Modified Silica Materials on Polyvinyl Chloride (PVC) Substrates to Obtain Transparent and Hydrophobic Hybrid Coatings" Applied Sciences 11, no. 22: 11044. https://doi.org/10.3390/app112211044
APA StylePurcar, V., Rădițoiu, V., Rădițoiu, A., Raduly, F. M., Ispas, G. C., Căprărescu, S., Frone, A. N., Trică, B., Nicolae, C.-A., Anastasescu, M., & Stroescu, H. (2021). Effect of Modified Silica Materials on Polyvinyl Chloride (PVC) Substrates to Obtain Transparent and Hydrophobic Hybrid Coatings. Applied Sciences, 11(22), 11044. https://doi.org/10.3390/app112211044













