Effect of Soil Washing with Ferric Chloride on Cadmium Removal and Soil Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Batch Experiment
2.3. Measurements
2.4. Statistical Analysis
3. Results and Discussion
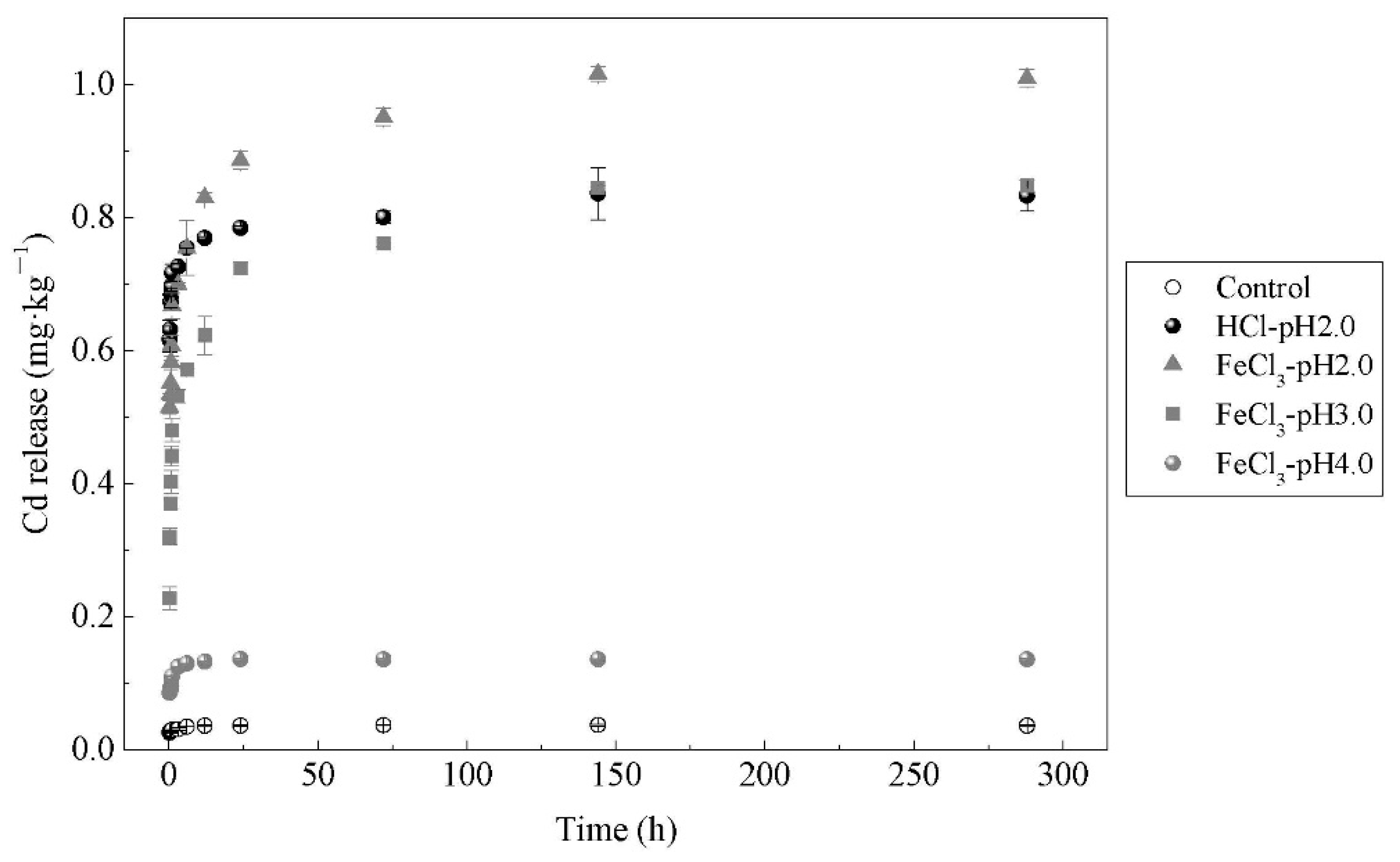
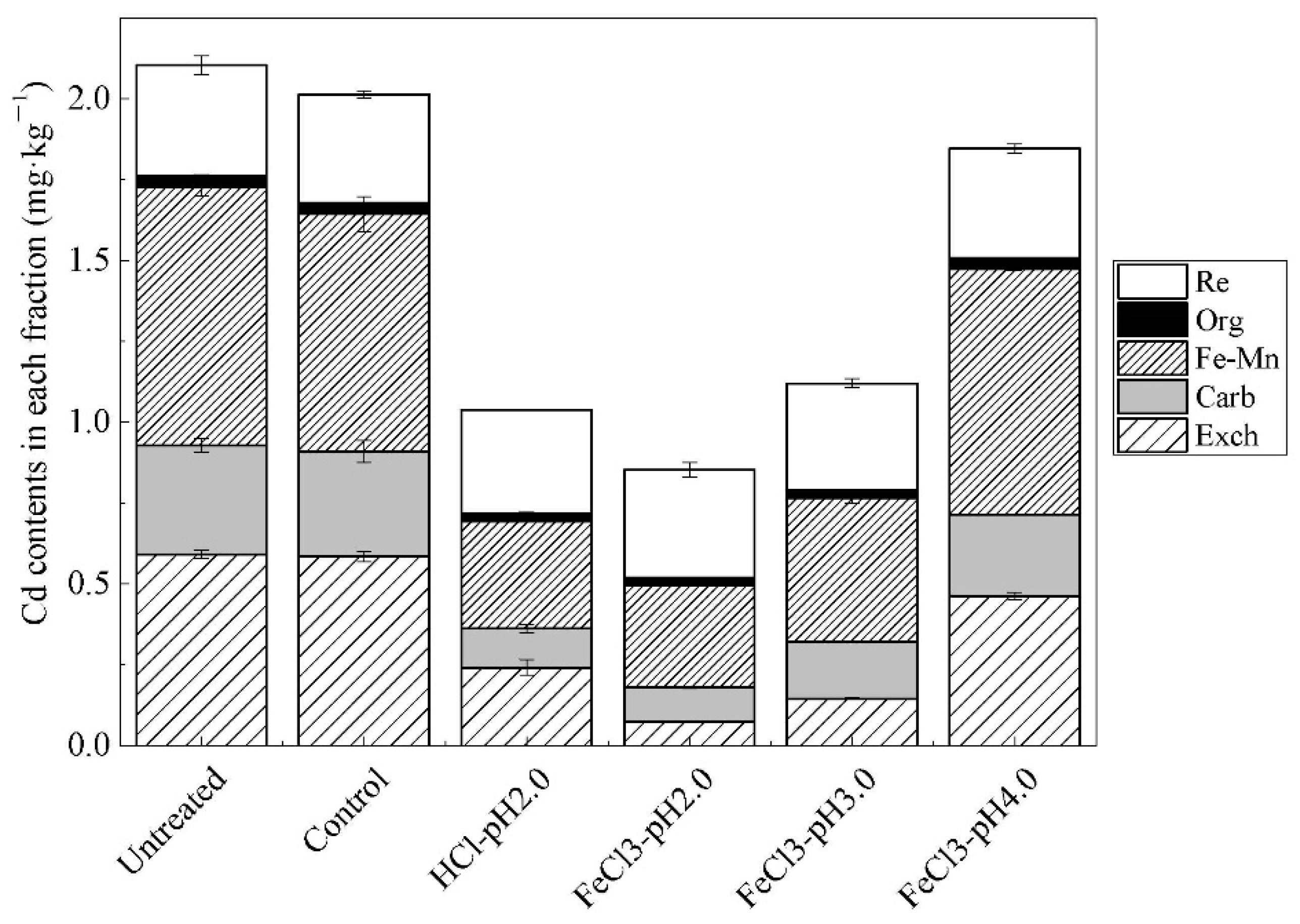
3.1. Effects of Ferric Chloride on Cd Extraction Efficiency
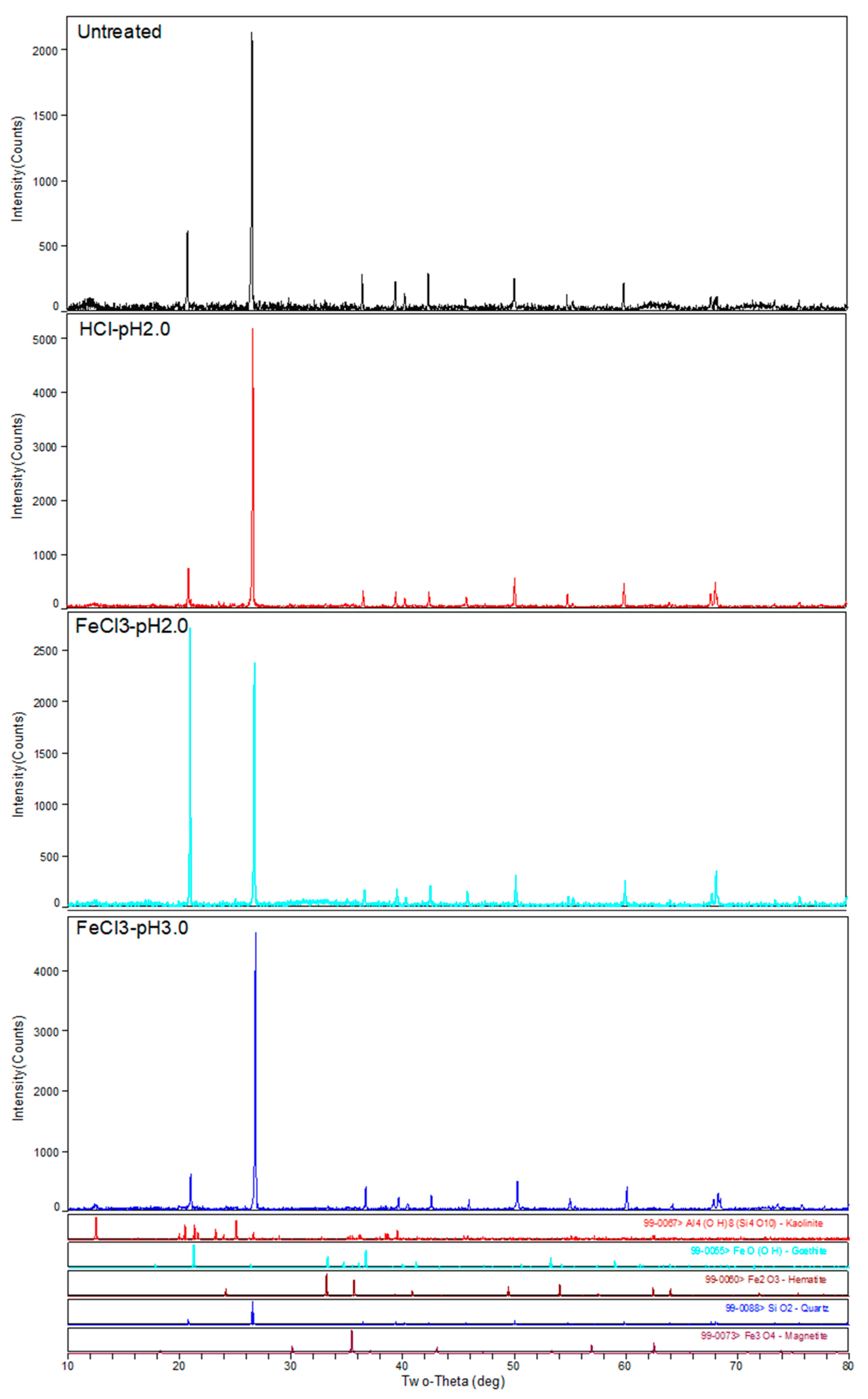
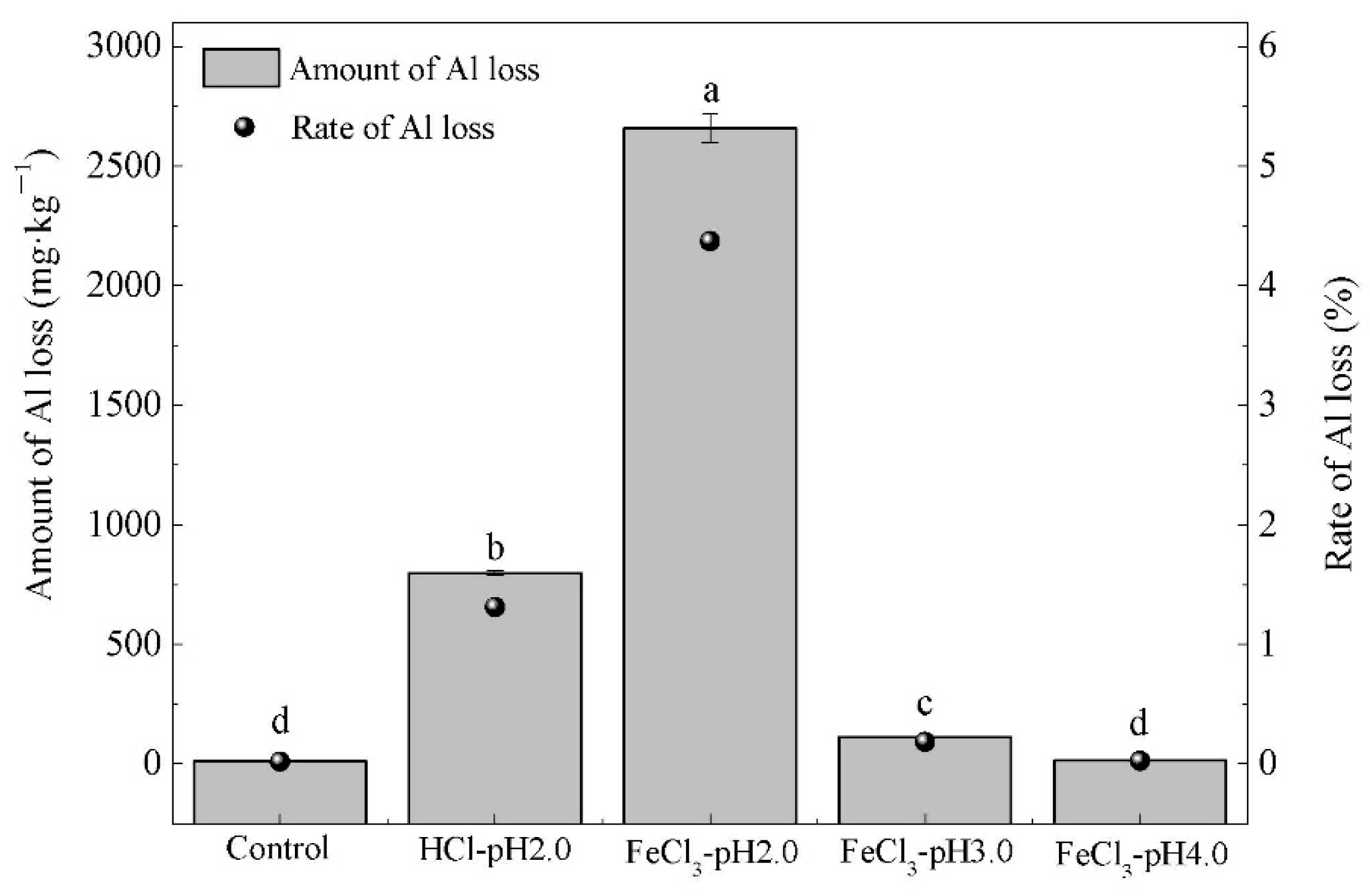
3.2. Changes in Soil Clay Minerals during Extraction
3.3. Changes in Total Organic Carbon during Extraction
3.4. Applications and Suggestions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.J.; Tian, G.J.; Jiang, D.; Zhang, C.; Kong, L.Q. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. 2016, 23, 17941–17952. [Google Scholar] [CrossRef]
- MEP. Environmental Quality Standard for Soils (GB 15618-1995); MEP: Beijing, China, 1995. [Google Scholar]
- MEP. The Ministry of Land and Resources Report on the National Soil Contamination Survey; MEP: Beijing, China, 2014. [Google Scholar]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium-historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Makino, T.; Maejima, Y.; Akahane, I.; Kamiya, T.; Takano, H.; Fujitomi, S.; Ibaraki, T.; Kunhikrishnan, A.; Bolan, N. A practical soil washing method for use in a Cd-contaminated paddy field, with simple on-site wastewater treatment. Geoderma 2016, 270, 3–9. [Google Scholar] [CrossRef]
- Liu, L.W.; Li, W.; Song, W.P.; Guo, M.X. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Li, B.; Lai, Y.W.; Zang, L.B.; Tang, X.D. Process design and validation of a new mixed eluent for leaching Cd, Cr, Pb, Cu, Ni, and Zn from heavy metal-polluted soil. Anal. Methods 2021, 13, 1269–1277. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Zhang, W.H.; Lo, I.M.C. Copper extraction effectiveness and soil dissolution issues of EDTA-flushing of contaminated soils. Chemosphere 2007, 68, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; An, G.N.; Zhu, S.S.; Wang, L.; Ma, F. Can Cd translocation be attenuated in Oryza sativa L. by arbuscular mycorrhizal fungi in the presence of EDTA? Environ. Sci. Pollut. Res. 2018, 25, 9380–9390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Huang, H.; Tan, F.F.; Wang, H.; Qiu, R.L. Influence of EDTA washing on the species and mobility of heavy metals residual in soils. J. Hazard. Mater. 2010, 173, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Sigg, L. Adsorption of EDTA and metal–EDTA complexes onto goethite. J. Colloid Interf. Sci. 1996, 177, 106–121. [Google Scholar] [CrossRef]
- Krishnamurti, G.S.R.; Cieslinski, G.; Huang, P.M.; Van Rees, K.C.J. Kinetics of cadmium release from soils as influenced by organic acids: Implication in cadmium availability. J. Environ. Qual. 1997, 26, 271–277. [Google Scholar] [CrossRef]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef]
- Guo, X.F.; Wei, Z.B.; Wu, Q.T.; Li, C.P.; Qian, T.W.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.C.; Park, S.M.; Yoon, G.S.; Tsang, D.C.W.; Baek, K. Effects of lead mineralogy on soil washing enhanced by ferric salts as extracting and oxidizing agents. Chemosphere 2017, 185, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.L.; Zhu, P.F.; Guo, G.G.; Hu, H.Q.; Zhu, J.; Fu, Q.L. Efficiency of several leaching reagents on removal of Cu, Pb, Cd, and Zn from highly contaminated paddy soil. Environ. Sci. Pollut. Res. 2016, 23, 23271–23280. [Google Scholar] [CrossRef] [PubMed]
- Miroslava, S.; Dusan, I.; Elena, K.; Miriam, J. Soil Particle Size Analysis by Laser Diffractometry: Result Comparison with Pipette Method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 245, p. 072025. [Google Scholar]
- Yakimovich, P.V.; Alekseev, A.V. Analysis of Wastewater by ICP-MS. Metallurgist 2018, 62, 1–7. [Google Scholar] [CrossRef]
- Tesfaldet, Z.O.; van Staden, J.F.; Stefan, R.I. Sequential injection spectrophotometric determination of iron as Fe(II) in multi-vitamin preparations using 1,10-phenanthroline as complexing agent. Talanta 2004, 64, 1189–1195. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Laffely, A.E. Root Biomass and Other Soil Properties Affecting the CO2 Flush from Laboratory Dried and Rewetted Soils; The University of Maine: Orono, ME, USA, 2019. [Google Scholar]
- Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Gericke, G.; Sithole, S.P. Cyclodextrin nanosponges in the removal of organic matter for ultrapure water in power generation. J. Water Supply Res. Technol.-Aqua 2009, 58, 299–304. [Google Scholar] [CrossRef]
- Singh, V.; Agrawal, M.H. Qualitative soil mineral analysis by EDXRF, XRD and AAS probes. Radiat. Phys. Chem. 2012, 81, 1796–1803. [Google Scholar] [CrossRef]
- Yoo, J.C.; Shin, Y.J.; Kim, E.J.; Yang, J.S.; Baek, K. Extraction mechanism of lead from shooting range soil by ferric salts. Process. Saf. Environ. 2016, 103, 174–182. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. Pb, Zn and Cd mobility, availability and fractionation in aged soil remediated by EDTA leaching. Chemosphere 2009, 74, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.C.; Lee, C.D.; Yang, J.S.; Baek, K. Extraction characteristics of heavy metals from marine sediments. Chem. Eng. J. 2013, 228, 688–699. [Google Scholar] [CrossRef]
- Yoo, J.C.; Jeon, P.Y.; Tsang, D.C.W.; Eilhann, E.K.; Baek, K. Ferric-enhanced chemical remediation of dredged marine sediment contaminated by metals and petroleum hydrocarbons. Environ. Pollut. 2018, 243, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.Q.; Li, Z.W.; Huang, B.; Luo, N.L.; Huang, M.; Zhang, Q.; Zeng, G.M. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total. Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef]
- Cutler, W.G.; El-Kadi, A.; Hue, N.V.; Peard, J.; Scheckel, K.; Ray, C. Iron amendments to reduce bioaccessible arsenic. J. Hazard. Mater. 2014, 279, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.N.; Kan, J.J.; Inamdar, S.; Chen, C.; Sparks, D. Dissimilatory microbial iron reduction release DOC (dissolved organic carbon) from carbon-ferrihydrite association. Soil Biol. Biochem. 2016, 103, 232–240. [Google Scholar] [CrossRef]
- Filep, T.; Rékási, M. Factors controlling dissolved organic carbon (DOC), dissolved organic nitrogen (DON) and DOC/DON ratio in arable soils based on a dataset from Hungary. Geoderma 2011, 162, 312–318. [Google Scholar] [CrossRef]
- Li, M.F.; Wang, J.; Guo, D.; Yang, R.R.; Fu, H. Effect of land management practices on the concentration of dissolved organic matter in soil: A meta-analysis. Geoderma 2019, 344, 74–81. [Google Scholar] [CrossRef]
- Shi, J.Y.; Pang, J.L.; Liu, Q.L.; Luo, Y.T.; Ye, J.; Xu, Q.; Long, B.B.; Ye, B.H.; Yuan, X.F. Simultaneous removal of multiple heavy metals from soil by washing with citric acid and ferric chloride. RSC Adv. 2020, 10, 7432–7442. [Google Scholar] [CrossRef]
- Liang, F.; Guo, Z.H.; Men, S.H.; Xiao, X.Y.; Peng, C.; Wu, L.H.; Christie, P. Extraction of Cd and Pb from contaminated-paddy soil with EDTA, DTPA, citric acid and FeCl3 and effects on soil fertility. J. Cent. South Univ. 2019, 26, 2987–2997. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, M. The Improvement Effects of Different Treatment Methods of Soil Wastewater Washing on Environmental Pollution. Water 2020, 12, 2329. [Google Scholar] [CrossRef]
| Treatments | TOC (g·kg−1) | DOC (mg·L−1) | Fe (II) (mg·L−1) |
|---|---|---|---|
| Control | 22.3 ± 0.24 a | 6.63 ± 0.30 c | 0.08 ± 0.00 c |
| HCl, pH 2.0 | 19.0 ± 0.06 b | 9.01 ± 0.19 b | 0.37 ± 0.08 b |
| FeCl3, pH 2.0 | 16.4 ± 2.15 c | 19.0 ± 1.62 a | 28.9 ± 0.75 a |
| FeCl3, pH 3.0 | 18.5 ± 0.08 bc | 7.64 ± 0.08 c | 0.12 ± 0.04 c |
| FeCl3, pH 4.0 | 21.9 ± 0.45 a | 7.07 ± 0.14 c | 0.08 ± 0.00 c |
| Treatments | Initial pH | Final pH |
|---|---|---|
| Control | 5.06 ± 0.01 | 5.25 ± 0.04 |
| HCl, pH 2.0 | 2.00 ± 0.00 | 2.77 ± 0.12 |
| FeCl3, pH 2.0 | 2.00 ± 0.00 | 2.12 ± 0.05 |
| FeCl3, pH 3.0 | 3.00 ± 0.00 | 3.94 ± 0.05 |
| FeCl3, pH 4.0 | 4.00 ± 0.00 | 4.88 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Sun, Q.; Zhang, X.; Zhou, Y.; Xia, L.; Yuan, J. Effect of Soil Washing with Ferric Chloride on Cadmium Removal and Soil Structure. Appl. Sci. 2021, 11, 10956. https://doi.org/10.3390/app112210956
Dong J, Sun Q, Zhang X, Zhou Y, Xia L, Yuan J. Effect of Soil Washing with Ferric Chloride on Cadmium Removal and Soil Structure. Applied Sciences. 2021; 11(22):10956. https://doi.org/10.3390/app112210956
Chicago/Turabian StyleDong, Jing, Qi Sun, Xue Zhang, Yuan Zhou, Longchao Xia, and Jin Yuan. 2021. "Effect of Soil Washing with Ferric Chloride on Cadmium Removal and Soil Structure" Applied Sciences 11, no. 22: 10956. https://doi.org/10.3390/app112210956
APA StyleDong, J., Sun, Q., Zhang, X., Zhou, Y., Xia, L., & Yuan, J. (2021). Effect of Soil Washing with Ferric Chloride on Cadmium Removal and Soil Structure. Applied Sciences, 11(22), 10956. https://doi.org/10.3390/app112210956






