Synthesis and Characterization of Biochar-Based Geopolymer Materials
Abstract
:1. Introduction
- -
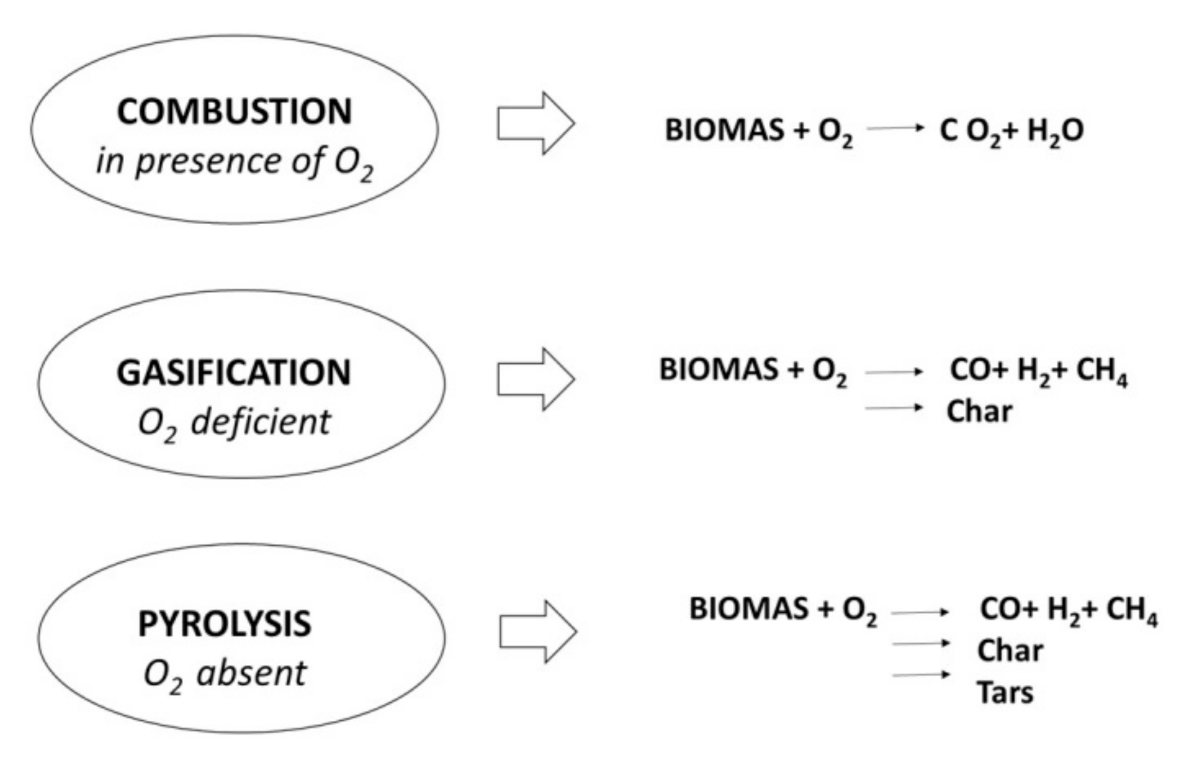
- non-hazardous biochar, which comes from gasification’s process of woody biomasses from river maintenance,
- -
- pre-treated bottom ash from urban incinerators, which acts as a foaming agent,
- -
- metakaolin to optimize the Si/Al ratio in the mixture.
2. Materials and Methods
2.1. Raw Materials
2.1.1. Raw Materials Characterization
2.1.2. Mineralogical Analysis
2.1.3. Physical Characterization
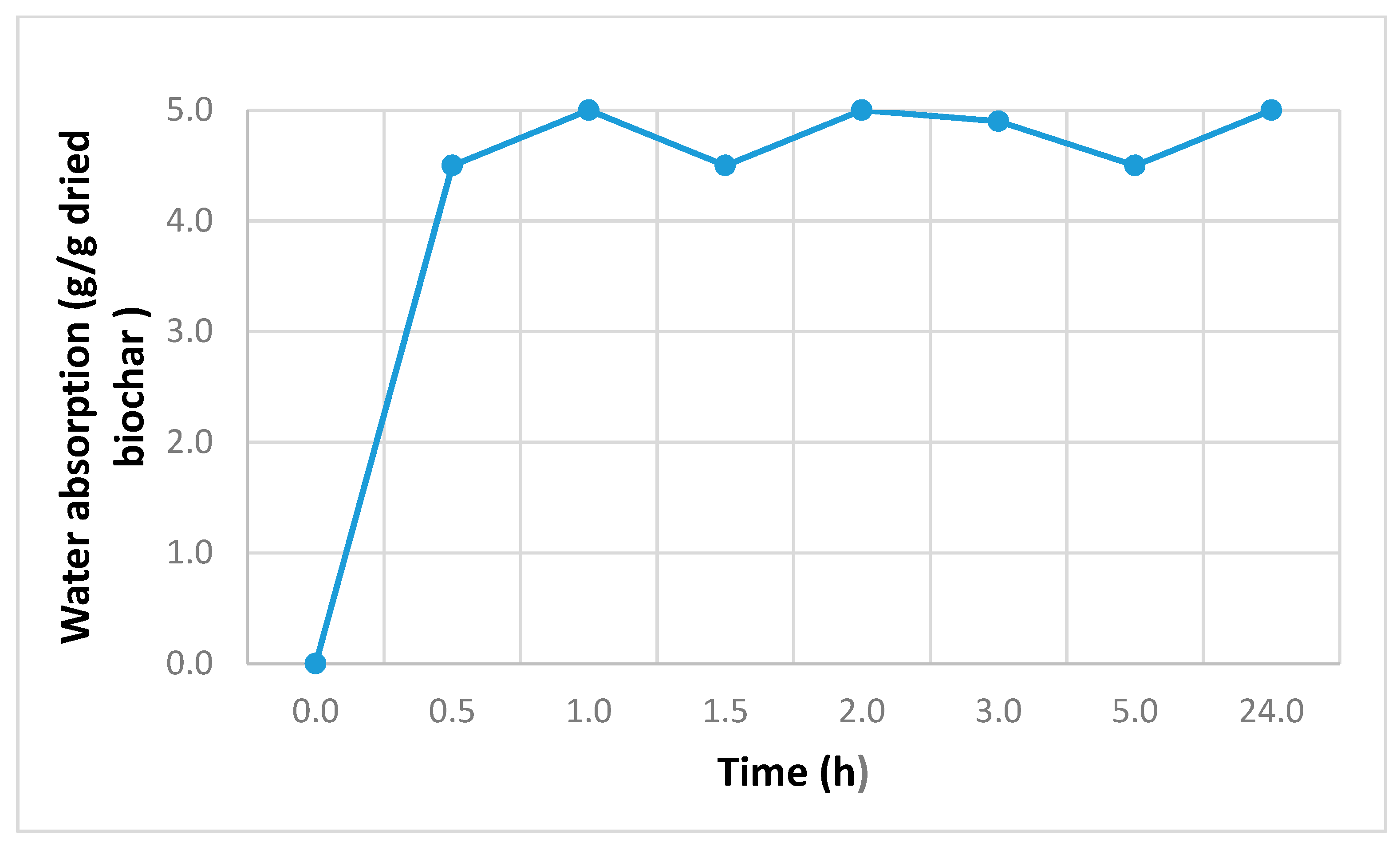
2.1.4. Biochar Absorption
2.2. Geopolymers Preparation
- (1)
- Mixing powders: The first composition was a mix of metakaolin and biochar and the second of metakaolin, IBA and biochar, both shed in a beaker in order to obtain a homogeneous mixture.
- (2)
- Addition of sodium hydroxide solution (8 M) and sodium silicate solution (Si/Na = 3) (Rm = 3).
- (3)
- Possible addition of few millilitres of water in order to obtain a liquid mixture. Water must be added if strictly necessary, otherwise it could slow down the subsequent hardening.
- (4)
- Intensive shaking until a homogeneous and fluid paste was formed, in order to avoid formation of bubbles.
- (5)
- Casting of the paste into a plastic mould.
- (6)
- Closing of the mould inside a plastic bag for 24 h in order to avoid cracking and breakage.
- (7)
- Maintaining the cast at room temperature.
- (8)
- The curing phase at room temperature lasted 7 days, 30 days and 90 days.
2.3. Geopolymers Characterization
2.3.1. Integrity Test
2.3.2. Mineralogical Analysis
2.3.3. Chemical Stability
2.3.4. Physical Characterization
2.3.5. Adsorption Capability of Alkali-Activated Materials
3. Results
3.1. Raw Materials Characterization
3.1.1. Mineralogical Analysis
3.1.2. Physical Characterization
3.2. Geopolymer Characterization
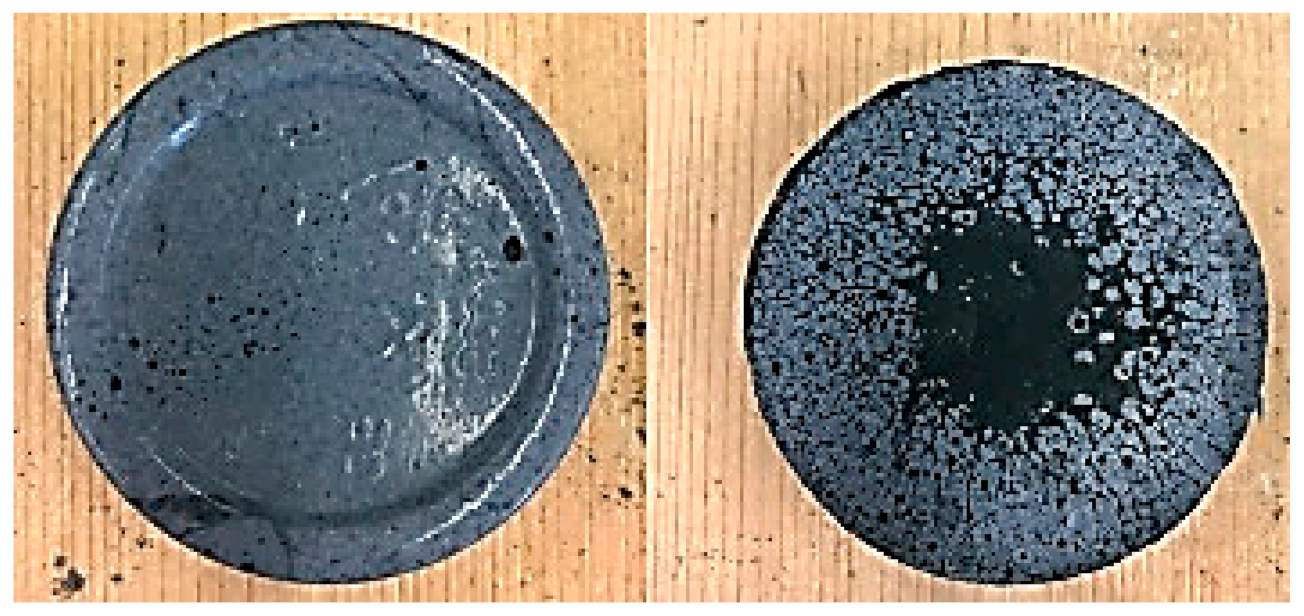
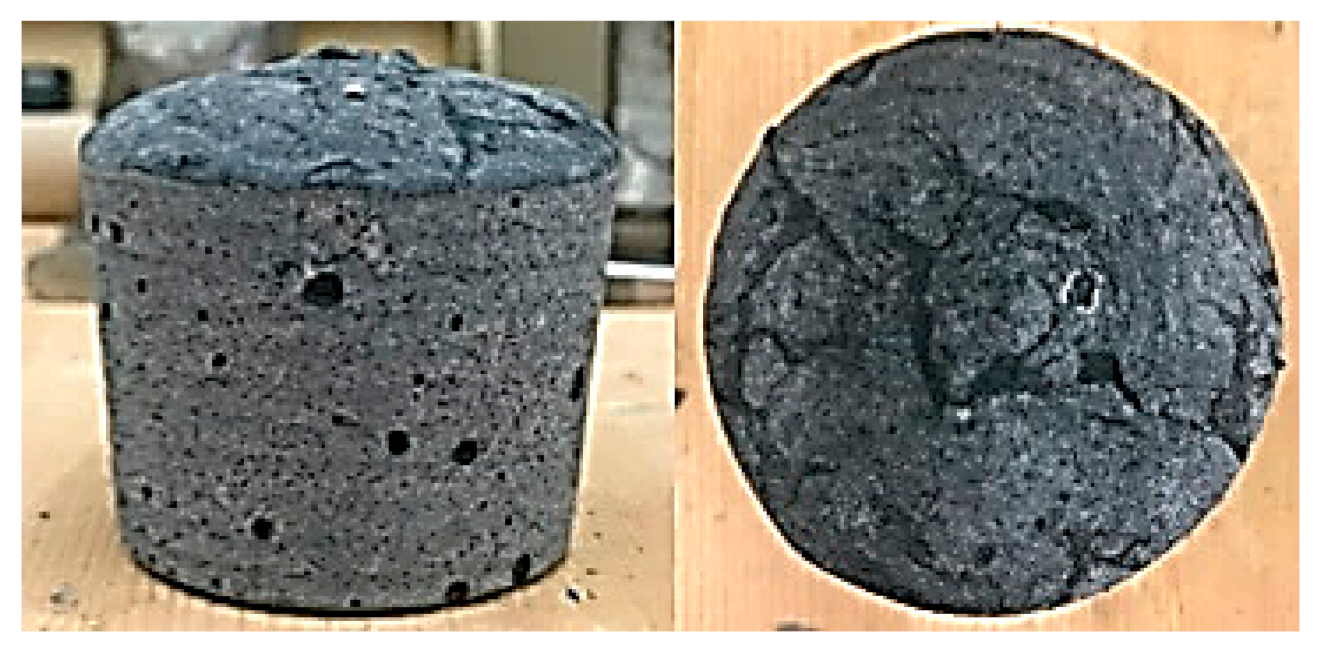
3.2.1. Integrity Test
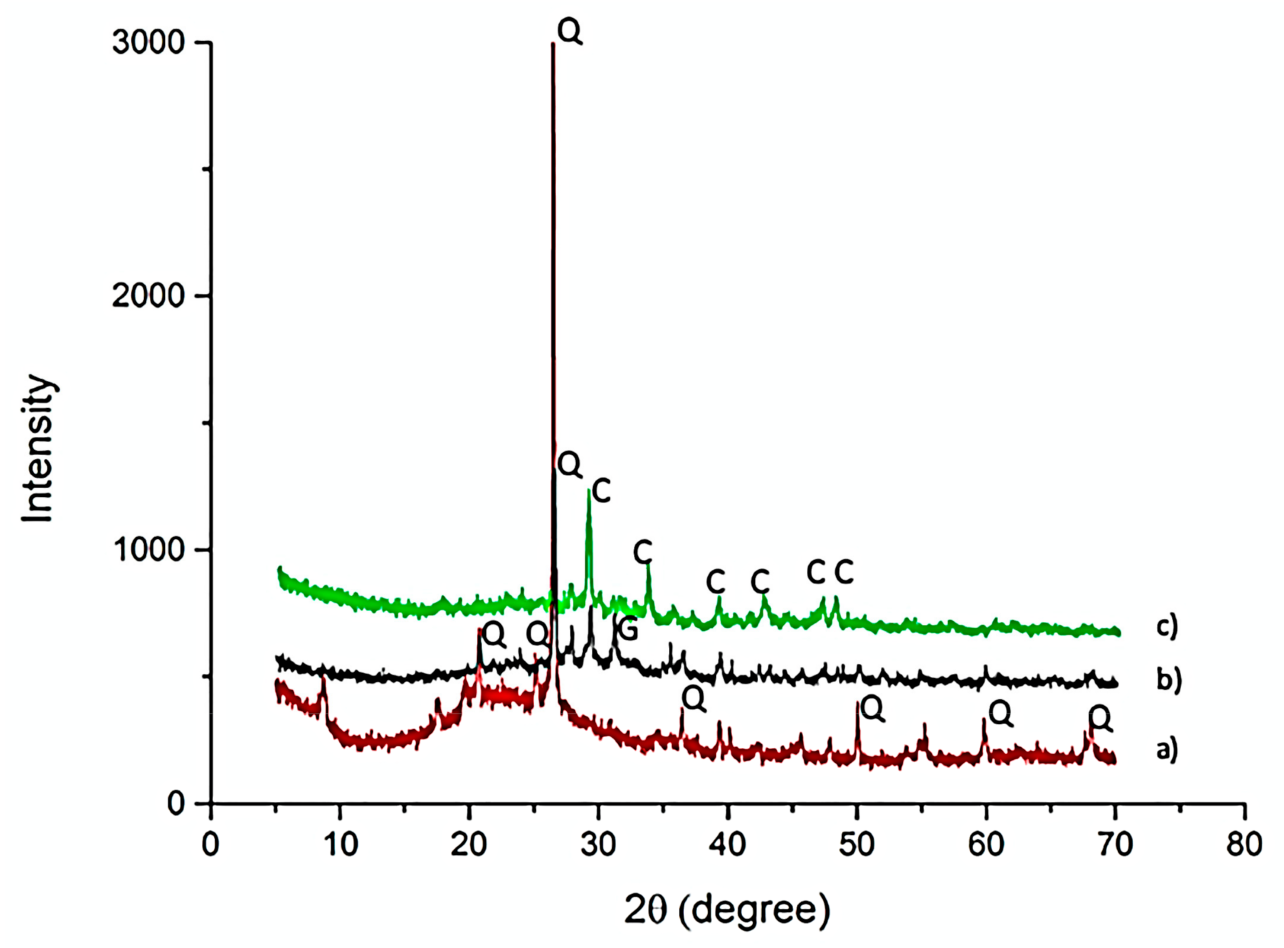
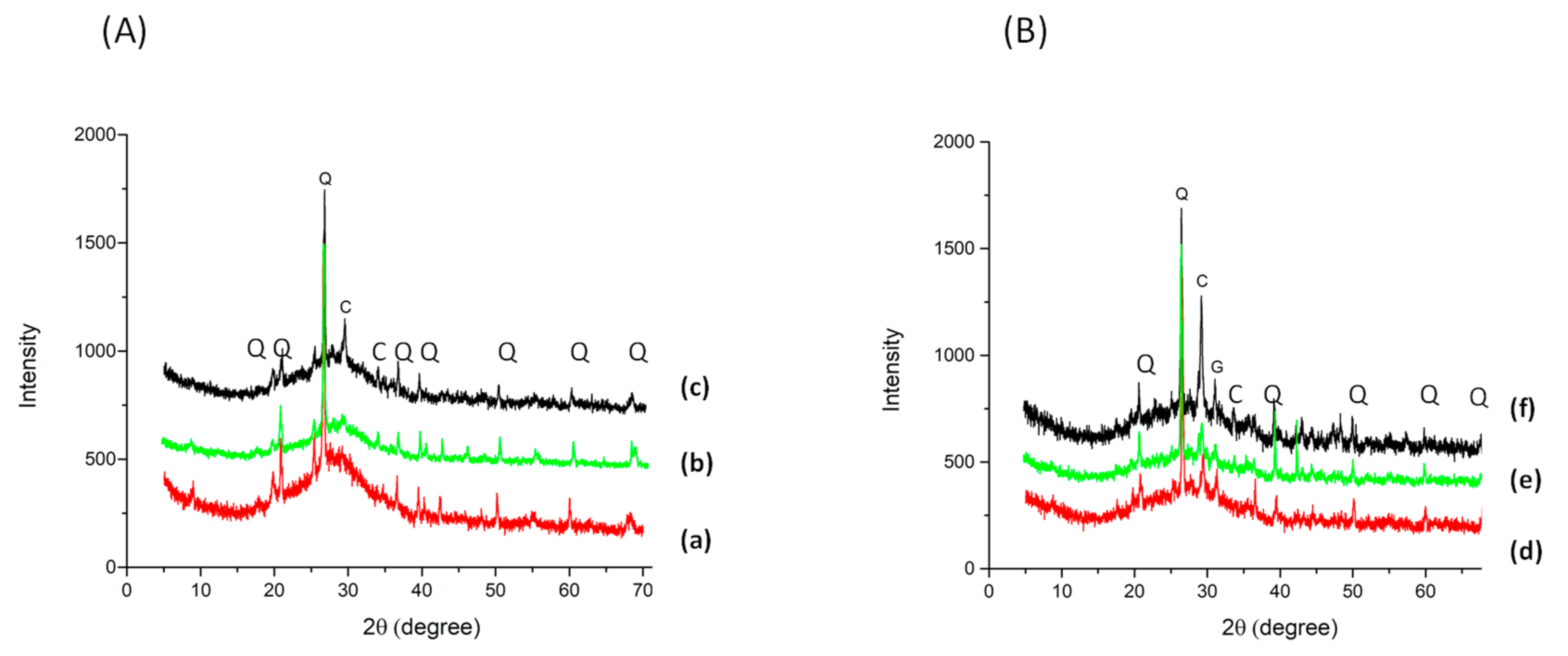
3.2.2. Mineralogical Analysis
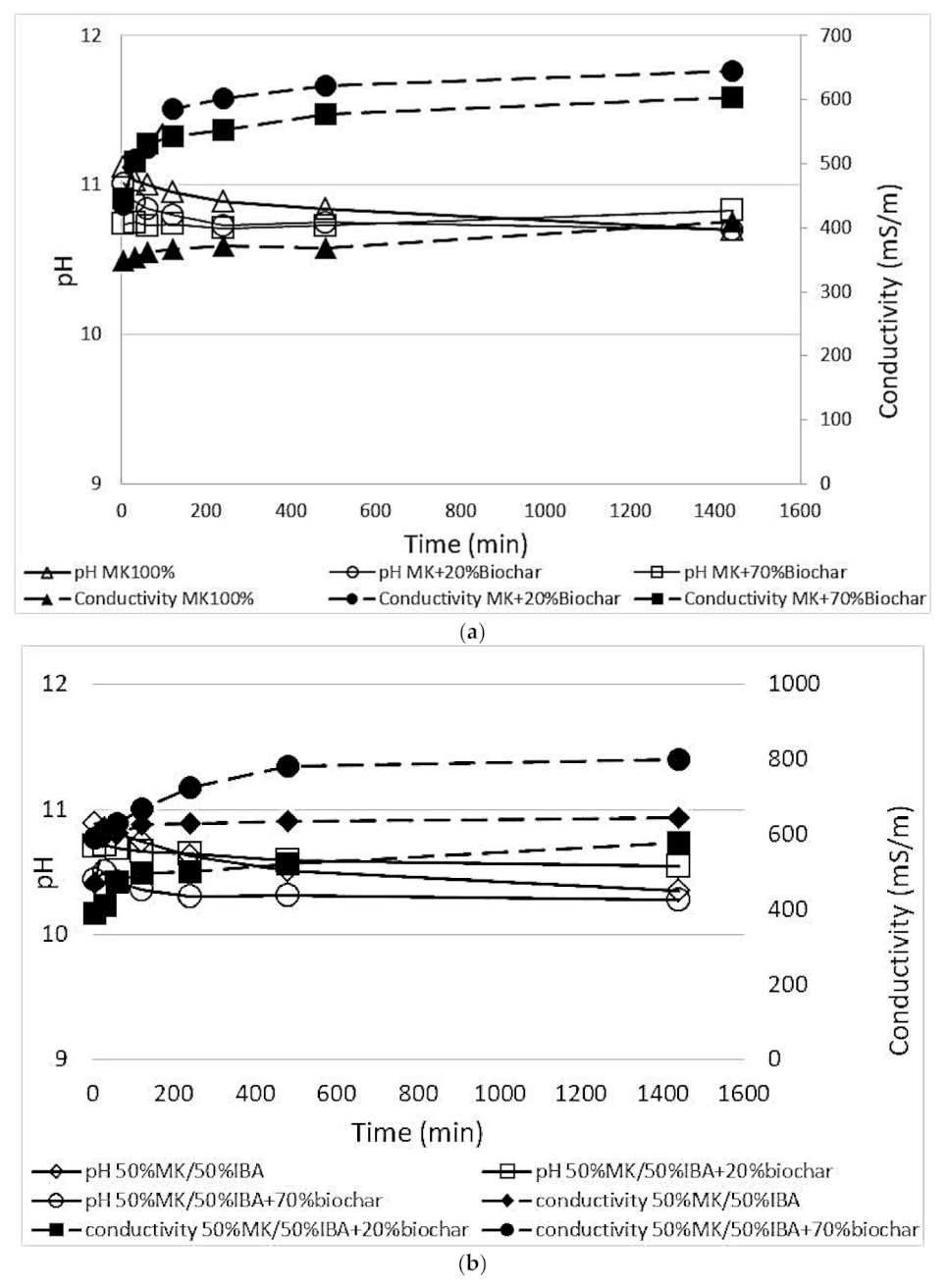
3.2.3. Chemical Stability
3.2.4. Physical Analysis
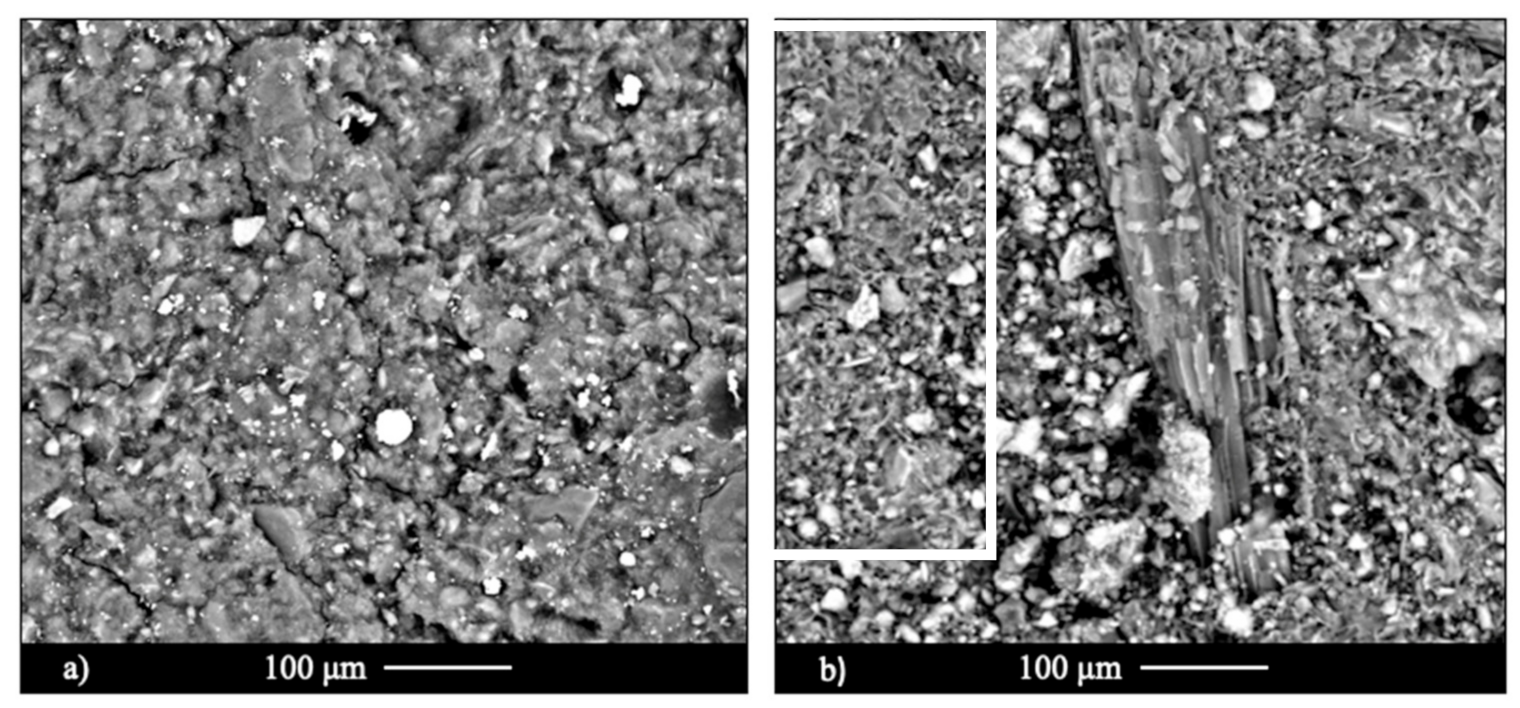
3.2.5. Microstructural Analysis
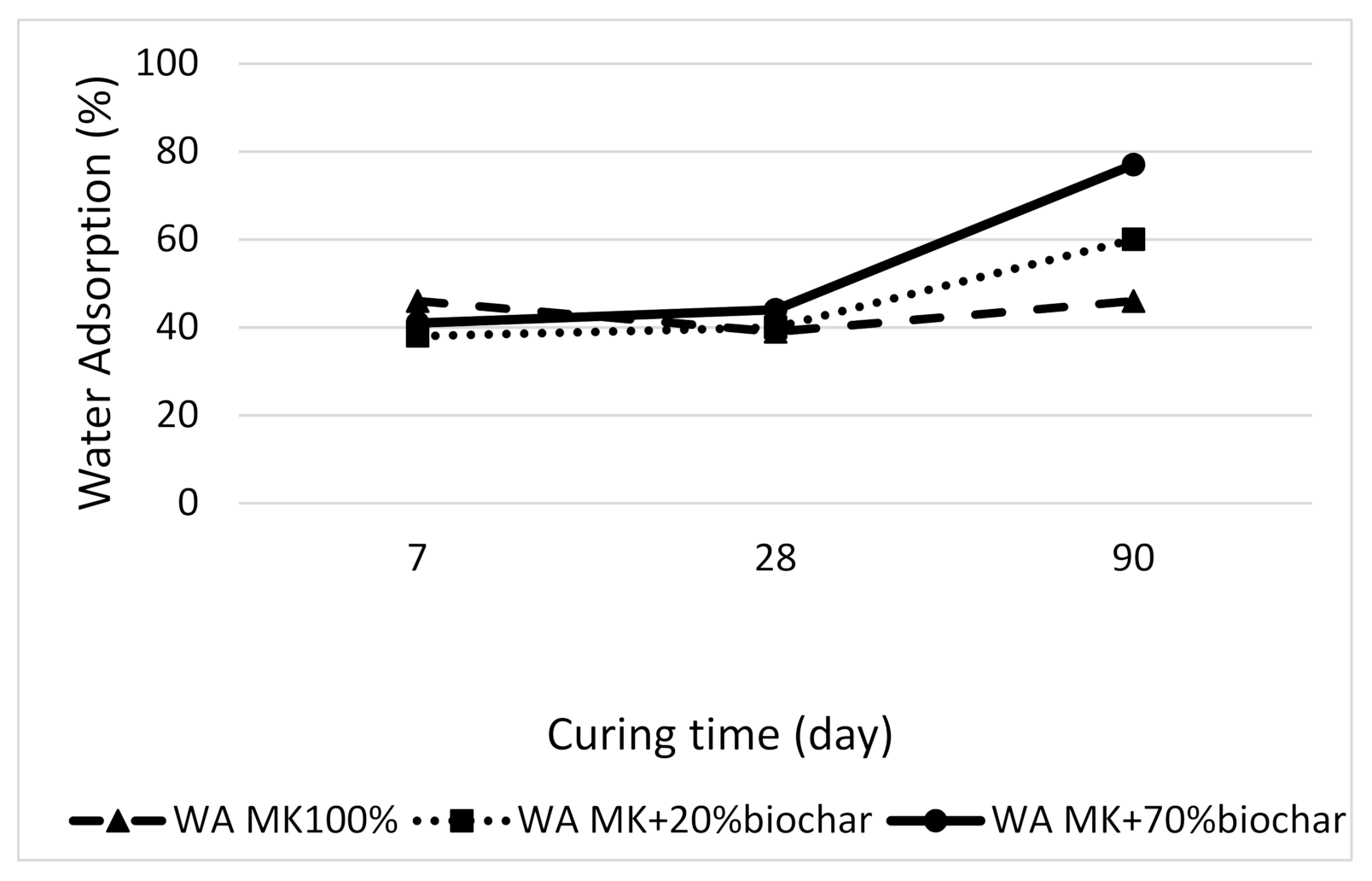
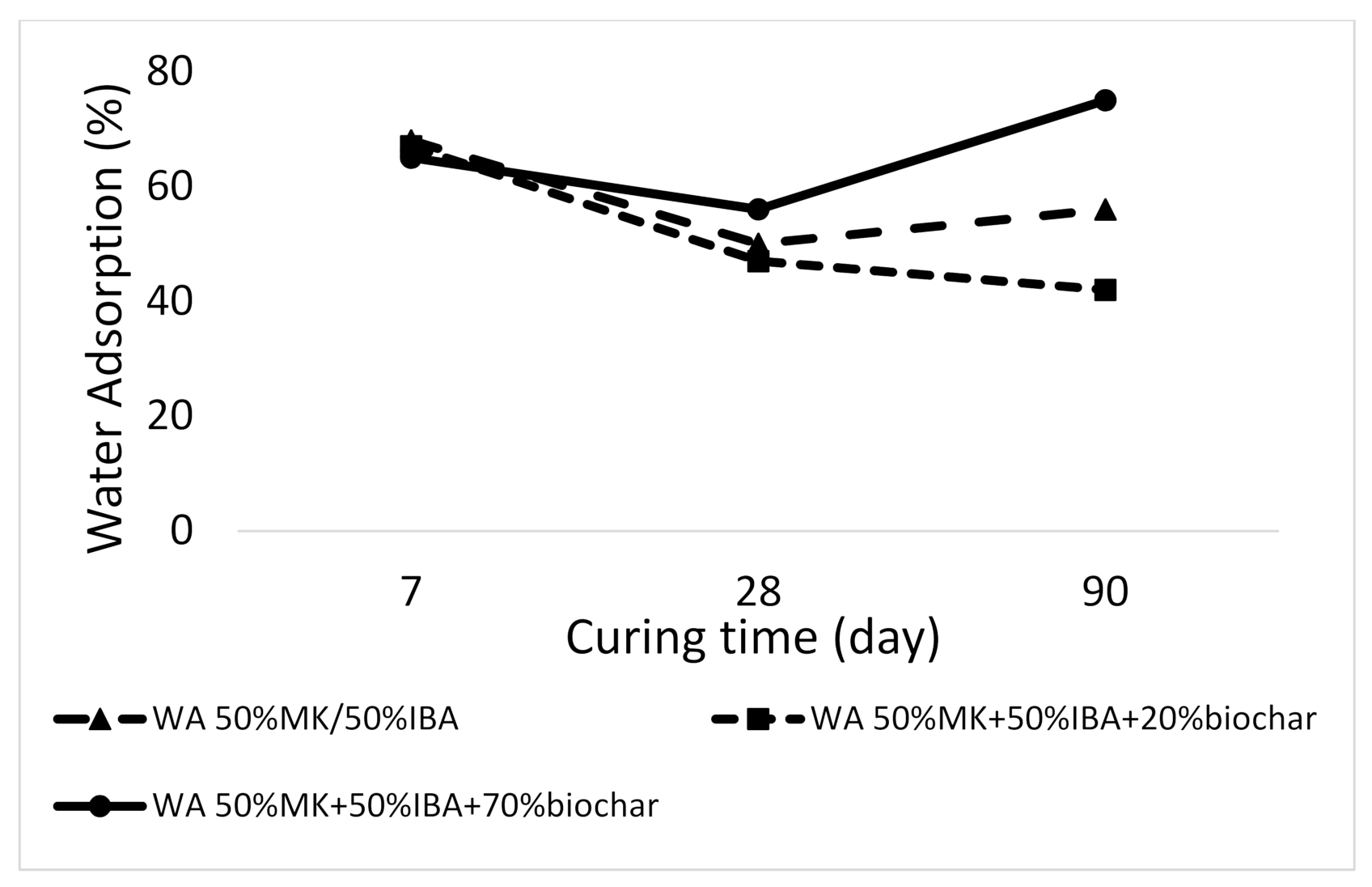
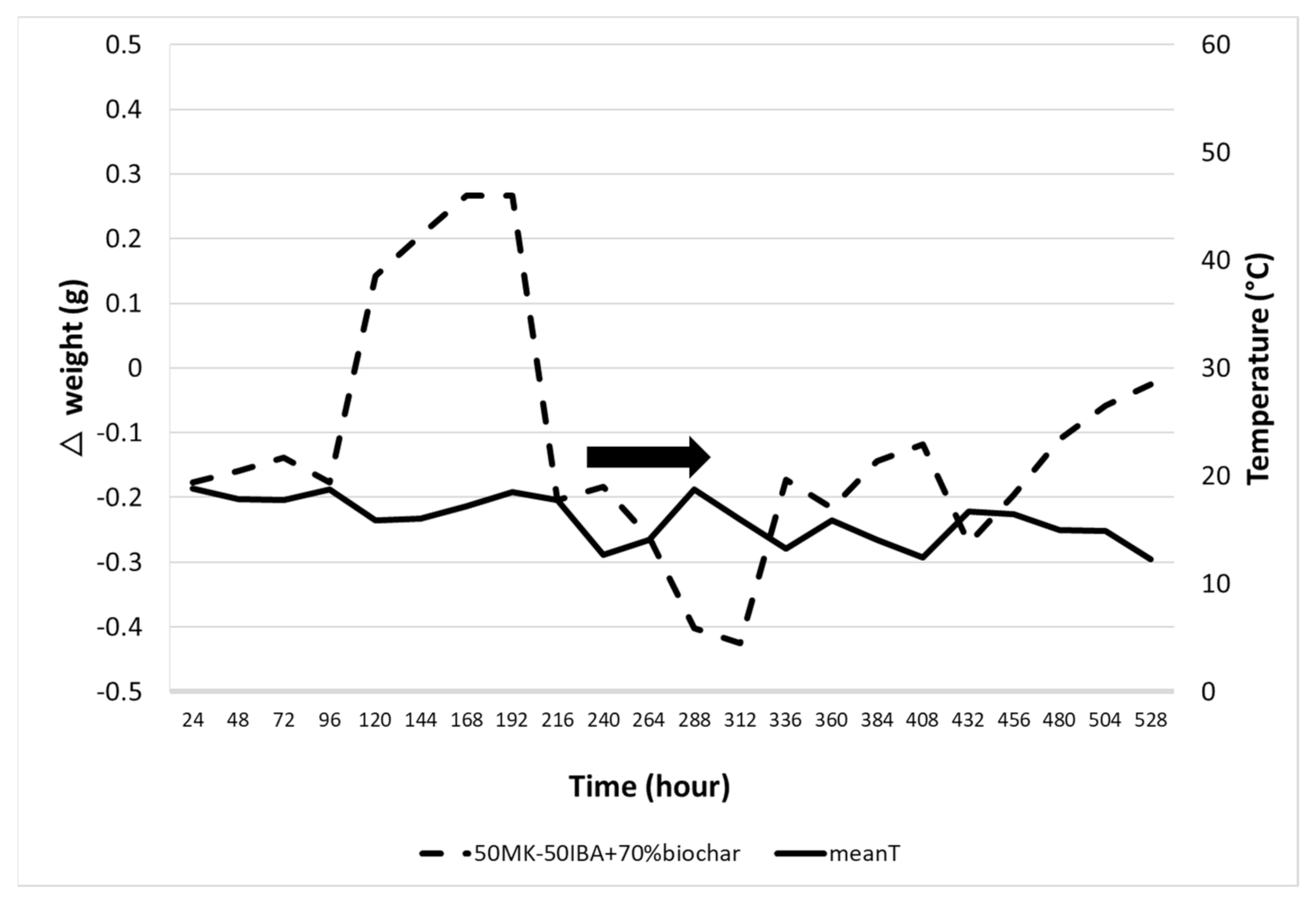
3.2.6. Absorption of Alkali-Activated Materials Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Zwieten, L.; Kimber, S.; Sinclair, K.; Chan, K.Y.; Downie, A. Biochar: Potential for Climate Change Mitigation, Improved Yield and Soil Health. In Proceedings of the 23rd Annual Conference of the Grassland Society of NSW 152, Tamworth, UK, 21–23 July 2008; Available online: http://grasslandnsw.com.au/news/wp-content/uploads/2009/07/Van-Zweiten-Kimber-Sinclair-Chan-Downie-2008.pdf (accessed on 10 November 2021).
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Italian Law DM 22/06/2015 Updated by D.L 75/2010 (all.2,6,7) relative to the reorganization and revision of regulations on fertilizers published in GU 12/08/2015.
- Hasegawa, T.; Iwasaki, S.; Shibutani, Y.; Abe, I. Preparation of superior humidity-control materials from kenaf. J. Porous Mater 2009, 16, 129–134. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, J.; Ma, Q.; Dai, X.; Chen, Z. Experimental study on humidity control performance of diatomite-based building materials. Appl. Therm. Eng. 2017, 114, 450–456. [Google Scholar] [CrossRef]
- Ding, Y.; Meng, J.; Liang, J.; Li, G.; Liang, X. Preparation and their property of porous mineral ecomaterials for ambient humidity self-controlling. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2004, 33 (Suppl. S2), 114–118. [Google Scholar]
- Park, J.H.; Kim, Y.U.; Jeon, J.; Yun, B.Y.; Kang, Y.; Kim, S. Analysis of biochar-mortar composite as a humidity control material to improve the building energy and hygrothermal performance. Sci. Total Environ. 2021, 775, 145552. [Google Scholar] [CrossRef]
- Tan, T.H.; Mo, K.H.; Ling, T.C.; Lai, S.H. Current development of geopolymer as alternative adsorbent for heavy metal removal. Environ. Technol. Innov. 2020, 18, 100684. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M. Preparation, Characterization, and Application of Metakaolin-Based Geopolymer for Removal of Methylene Blue from Aqueous Solution. J. Chem. 2019, 2019, 4212901. [Google Scholar] [CrossRef]
- El Alouani, M.; Alehyen, S.; El Achouri, M.; Taibi, M. Comparative study of the adsorption of micropollutant contained in aqueous phase using coal fly ash and activated coal fly ash: Kinetic and isotherm studi. Chem. Data Collect. 2019, 23, 100265. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Zhang, J.; Zhang, Y.; Ge, Y.; Cui, X. In-situ synchronous carbonation and self-activation of biochar/geopolymer composite membrane: Enhanced catalyst for oxidative degradation of tetracycline. Chem. Eng. 2020, 397, 125528. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B.; Duan, H. Improvement effect of pyrolyzed agro-food biochar on the properties of magnesium phosphate cement. Sci. Total Environ. 2020, 718, 137422. [Google Scholar] [CrossRef] [PubMed]
- Sirico, A.; Bernardi, P.; Belletti, B.; Malcevschi, A.; Dalcanale, E.; Domenichelli, I.; Fornoni, P.; Moretti, E. Mechanical characterization of cement-based materials containing biochar from gasification. Constr. Build. Mater. 2020, 246, 118490. [Google Scholar] [CrossRef]
- Yang, S.; Wi, S.; Lee, J.; Lee, H.; Kim, S. Biochar-red clay composites for energy efficiency as eco-friendly building materials: Thermal and mechanical performance. J. Haz. Mater. 2019, 373, 844–855. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Guo, B.; Yang, J.; Shen, Z.; Hou, D.; Ok, Y.S.; Poon, C.S. Biochar as green additives in cement-based composites with carbon dioxide curing. J. Clean. Prod. 2020, 258, 120678. [Google Scholar] [CrossRef]
- Gupta, S.; Krishnan, P.; Kashani, A.; Kua, H.W. Application of biochar from coconut and wood waste to reduce shrinkage and improve physical properties of silica fume-cement mortar. Constr. Buid. Mater. 2020, 262, 120688. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Dai Pang, S.; Kua, H.W. Waste Valorisation using biochar for cement replacement and internal curing in ultra-high performance concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Gupta, S.; Palansooriya, K.N.; Dissanayake, P.D.; Ok, Y.S.; Kua, H.W. Carbonaceous inserts from lignocellulosic and non-lignocellulosic sources in cement mortar: Preparation conditions and its effect on hydration kinetics and physical properties. Constr. Build. Mater. 2020, 264, 120214. [Google Scholar] [CrossRef]
- Gupta, S.; Wei, K.H.; Dai, P.S. Effect of biochar on mechanical and permeability properties of concrete exposed to elevated temperature. Constr. Build. Mater. 2020, 234, 11733. [Google Scholar] [CrossRef]
- Vezzali, V.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Pozzi, P.; Allesina, G.; Pedrazzi, S.; Tartarini, P. Gasification of biomass from river maintenance and char application in building materials production. Env. Eng. Manag. J. 2018, 17, 2485–2496. [Google Scholar] [CrossRef]
- Joseph, D. Properties of geopolymer cements. In Proceedings of the 1st International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 24–26 June 1994; pp. 131–149. [Google Scholar]
- Blengini, G.A.; Busto, M.; Fantoni, M.; Fino, D. Eco-efficient waste glass recycling: Integrated waste management and green product development through LCA. Waste Manag. 2012, 32, 1000–1008. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, X.; Rajabipour, F.; Hendrickson, C.T. Comparative life cycle assessment of conventional, glass powder, and alkali-activated slag concrete and mortar. J. Infrastruct. Syst. 2014, 20, 04014020. [Google Scholar] [CrossRef]
- Ferraris, M.; Salvo, M.; Ventrella, A.; Buzzi, L.; Veglia, M. Use of vitrified MSWI bottom ashes for concrete production. Waste Manag. 2009, 29, 1041–1047. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Barbieri, L.; Leonelli, C. Alkali activation process for incinerator residue management. Waste Manag. 2013, 33, 1740–1749. [Google Scholar] [CrossRef]
- Lancellotti, I.; Ponzoni, C.; Bignozzi, M.C.; Barbieri, L.; Leonelli, C. Incinerator bottom ash and ladle slag for geopolymers preparation. Waste Biomass Valorization 2014, 5, 393–401. [Google Scholar] [CrossRef]
- Rambaldi, E.; Esposito, L.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Vassura, I. The recycling of MSWI bottom ash in silicate based ceramic. Ceram. Int. 2010, 36, 2469–2476. [Google Scholar] [CrossRef]
- Schabbach, L.M.; Andreola, F.; Lancellotti, I.; Barbieri, L. Minimization of Pb content in a ceramic glaze by reformulation the composition with secondary raw materials. Ceram. Int. 2011, 37, 1367–1375. [Google Scholar] [CrossRef]
- Schabbach, L.M.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Karamanova, E.; Ranguelov, B.; Karamanov, A. Post-treated incinerator bottom ash as alternative raw material for ceramic manufacturing. J. Eur. Ceram. Soc. 2012, 32, 2843–2852. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zhu, W.; Yang, E. Incinerator bottom ash (IBA) aerated geopolymer. Constr. Build. Mater. 2016, 1121, 1025–1031. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 1481, 49–59. [Google Scholar] [CrossRef]
- Zhu, W.; Teoh, P.J.; Liu, Y.; Chen, Z.; Yang, E. Strategic utilization of municipal solid waste incineration bottom ash for the synthesis of lightweight aerated alkali-activated materials. J. Clean. Prod. 2019, 23520, 603–612. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Utilization of blended fluidized bed combustion (FBC) ash and pulverized coal combustion (PCC) fly ash in geopolymer. Waste Manag. 2010, 30, 667–672. [Google Scholar] [CrossRef]
- Topcu, I.B.; Toprak, M.U. Properties of geopolymer from circulating fluidized bed combustion coal bottom ash. Mater. Sci. Eng. A 2011, 528, 1472–1477. [Google Scholar] [CrossRef]
- Xu, H.; Li, Q.; Shen, L.; Wang, W.; Zhai, J. Synthesis of thermostable geopolymer from circulating fluidized bed combustion (CFBC) bottom ash. J. Hazard. Mater. 2010, 175, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Noor-ul-Amin, S.; Faisal, M.; Muhammad, K.; Gul, S. Synthesis and characterization of geopolymer from bagasse bottom ash, waste of sugar industries and naturally available china clay. J. Clean. Prod. 2016, 12915, 491–495. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- Nkuna, C.N.; Oboirien, B.O.; Sadiku, E.R.; Lekitima, J. A comparative study of geopolymers synthesized from OXY-combustion and chemical looping combustion bottom ashes. Constr. Build. Mater. 2017, 1361, 246–255. [Google Scholar] [CrossRef]
- Khamlue, P.; Lertcumfu, N.; Jaita, P.; Manotham, S.; Tunkasiri, T.; Malasri, P.; Rujijanagul, G. The Effects of Biochar Additive on the Properties of Geopolymer Materials. Key Eng. Mater. 2019, 798, 273–278. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Farges, R.; Gharzouni, A.; Ravier, B.; Jeulin, P.; Rossignol, S. Insulating Foams and Dense Geopolymers from Biochar By-Products. J. Ceram. Sci. Technol. 2018, 9, 193–200. [Google Scholar]
- Devi, P.; Saroha, A.K. Risk assessment and technical feasibility of usage of paper mill sludge biochar-based exhausted adsorbent for geopolymeric brick formation. Environ. Sci. Pollut. Res. 2016, 23, 21641–21651. [Google Scholar] [CrossRef]
- Farzanian, K.; Teixeira, K.P.; Perdigao Rocha, I.; De Sa Carneiro, L.; Ghahremaninezhad, A. The mechanical strength, degree of hydration and electrical resistivity of cement pastes modified with superabsorbent polymers. Constr. Build. Mater. 2016, 109, 156–165. [Google Scholar] [CrossRef]
- Schrofl, C.; Mechtcherine, V.; Gorges, M. Relation between the molecular structure and the efficiency of superabsorbent polymers (SAP) as concrete admixture to mitigate autogenous shrinkage. Cem. Concr. Res. 2012, 42, 865–873. [Google Scholar] [CrossRef]
- Wang, A.; Sun, D.; Cao, G.; Wang, H.; Ren, N.; Wu, W.; Logan, B.E. Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour. Technol. 2011, 102, 4137–4143. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Effect of Temperature on Biochar Properties during Paper Mill Sludge Pyrolysis. Int. J. Chem. Tech. Res. 2013, 5, 682–687. Available online: https://www.researchgate.net/profile/Parmila_Devi/publication/259449899_Effect_Of_Temperature_On_Biochar_Properties_During_Paper_Mill_Sludge_Pyrolysis/links/0deec53637ec17aae7000000.pdf (accessed on 10 November 2021).
- Bedussi, F. Evaluation of the Potential of Biochar as a Component of Cultivation Substrates. Ph.D. Thesis, Department of Agricultural and Environmental Sciences, University of Milan, Milan, Italy, 2015. [Google Scholar]
- Brewer, C.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Kiventera, J.; Lancellotti, I.; Catauro, M.; Dal Poggetto, F.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Webb, P.; Orr, C. Analytical Methods in Fine Particle Technology; Micromeritics Instrument Corp.: Norcross, GA, USA, 1997. [Google Scholar]


| SAMPLE | MK (g) | IBA (g) | BIOCHAR (vol%) | NaOH (mL) | Na2SiO3 (mL) | H2O |
|---|---|---|---|---|---|---|
| MK100 | 50 | \ | \ | 24 | 34 | \ |
| MK + 20%biochar | 50 | \ | (20%) | 24 | 34 | \ |
| MK + 30%biochar | 50 | \ | (30%) | 24 | 34 | \ |
| MK + 40%biochar | 50 | \ | (40%) | 24 | 34 | \ |
| MK + 50%biochar | 50 | \ | (50%) | 24 | 34 | \ |
| MK + 70%biochar | 50 | \ | (70%) | 24 | 34 | 1 |
| 50MK-50IBA | 25 | 25 | \ | 8 | 20 | \ |
| 50MK-50IBA + 20%biochar | 25 | 25 | (20%) | 8 | 20 | 2 |
| 50MK-50IBA + 30%biochar | 25 | 25 | (30%) | 8 | 20 | 5 |
| 50MK-50IBA + 40%biochar | 25 | 25 | (40%) | 8 | 20 | 7 |
| 50MK-50IBA + 50%biochar | 25 | 25 | (50%) | 8 | 20 | 9 |
| 50MK-50IBA + 70%biochar | 25 | 25 | (70%) | 8 | 20 | 11 |
| 50MK-50BC | 35.4 | \ | 14.60 g | 24 | 34 | \ |
| OXIDES (wt%) | MK | IBA | Biochar |
|---|---|---|---|
| SiO2 | 58.97 | 53.90 | 26.10 |
| Al2O3 | 34.70 | 5.67 | 2.42 |
| Fe2O3 | 1.40 | 2.67 | 1.27 |
| CaO | 0.10 | 22.55 | 10.35 |
| MgO | 0.10 | 3.40 | 0.90 |
| Na2O | 0.10 | 4.03 | 0.54 |
| K2O | 0.70 | 0.94 | 2.57 |
| TiO2 | 1.30 | 1.55 | 0.02 |
| LOI * | 2.63 | 5.30 | 55.83 |
| Unity | Biochar | |
|---|---|---|
| BET Surface Area | m2 g−1 | 210.50 ± 5.94 |
| Apparent Density | g cm−3 | 0.16 |
| Absolute density | g cm−3 | 2.23 ± 0.0004 |
| Total Porosity | % | 92.8 |
| SAMPLE | BET (m2 g−1) |
|---|---|
| MK100 | 23.81 ± 0.1848 |
| MK + 50%Biochar | 39.21 ± 0.3538 |
| MK + 70%Biochar | 46.45 ± 0.7282 |
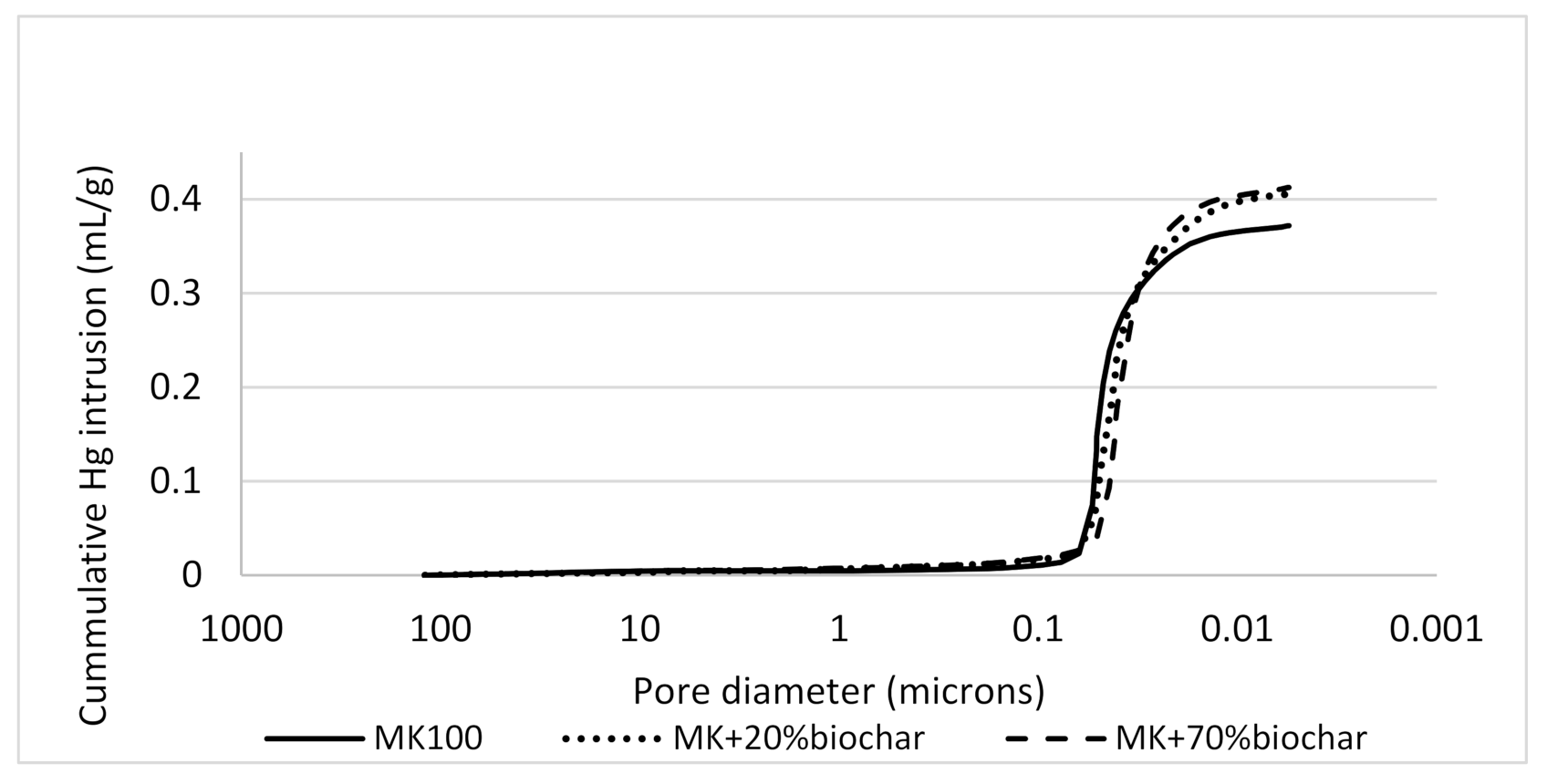
| Sample | Total Intrusion Hg Volume (m3 g−1) | Total Pore Area (m2 g−1) | Average Pore Diameter (μm) | Total Porosity (%) |
|---|---|---|---|---|
| MK100 28 d | 399.5 × 10−6 ± 1 × 10−6 | 41.552 | 0.0385 ± 0.0001 | 45.2198 |
| MK100 90 d | 371.9 × 10−6 ± 1 × 10−6 | 39.841 | 0.0373 ± 0.0001 | 43.9117 |
| MK + 20%biochar 90 d | 406.2 × 10−6 ± 1 × 10−6 | 49.290 | 0.0330 ± 0.00015 | 45.4361 |
| MK + 70%biochar 90 d | 412.5 × 10−6 ± 1 × 10−6 | 51.127 | 0.0323 ± 0.0001 | 46.1124 |
| 50MK-50BC 28 d | 459.5 × 10−6 ± 1 × 10−6 | 58.196 | 0.0316 ± 0.0001 | 49.7563 |
| MK + 50%biochar 28 d | 371.6 × 10−6 ± 1 × 10−6 | 53.040 | 0.0280 ± 0.00007 | 43.9968 |
| SAMPLE | BET (m2 g−1) |
|---|---|
| 50% MK-50% IBA | 19.42 ± 0.1773 |
| 50% MK-50% IBA + 50%biochar | 30.43 ± 0.3201 |
| 50% MK-50% IBA + 70%biochar | 38.72 ± 0.6309 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, F.; Andreola, F.; Barbieri, L.; Lancellotti, I. Synthesis and Characterization of Biochar-Based Geopolymer Materials. Appl. Sci. 2021, 11, 10945. https://doi.org/10.3390/app112210945
Piccolo F, Andreola F, Barbieri L, Lancellotti I. Synthesis and Characterization of Biochar-Based Geopolymer Materials. Applied Sciences. 2021; 11(22):10945. https://doi.org/10.3390/app112210945
Chicago/Turabian StylePiccolo, Federica, Fernanda Andreola, Luisa Barbieri, and Isabella Lancellotti. 2021. "Synthesis and Characterization of Biochar-Based Geopolymer Materials" Applied Sciences 11, no. 22: 10945. https://doi.org/10.3390/app112210945
APA StylePiccolo, F., Andreola, F., Barbieri, L., & Lancellotti, I. (2021). Synthesis and Characterization of Biochar-Based Geopolymer Materials. Applied Sciences, 11(22), 10945. https://doi.org/10.3390/app112210945









