CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.2. Synthesis Procedure
2.3. Characterization
3. Results and Discussion
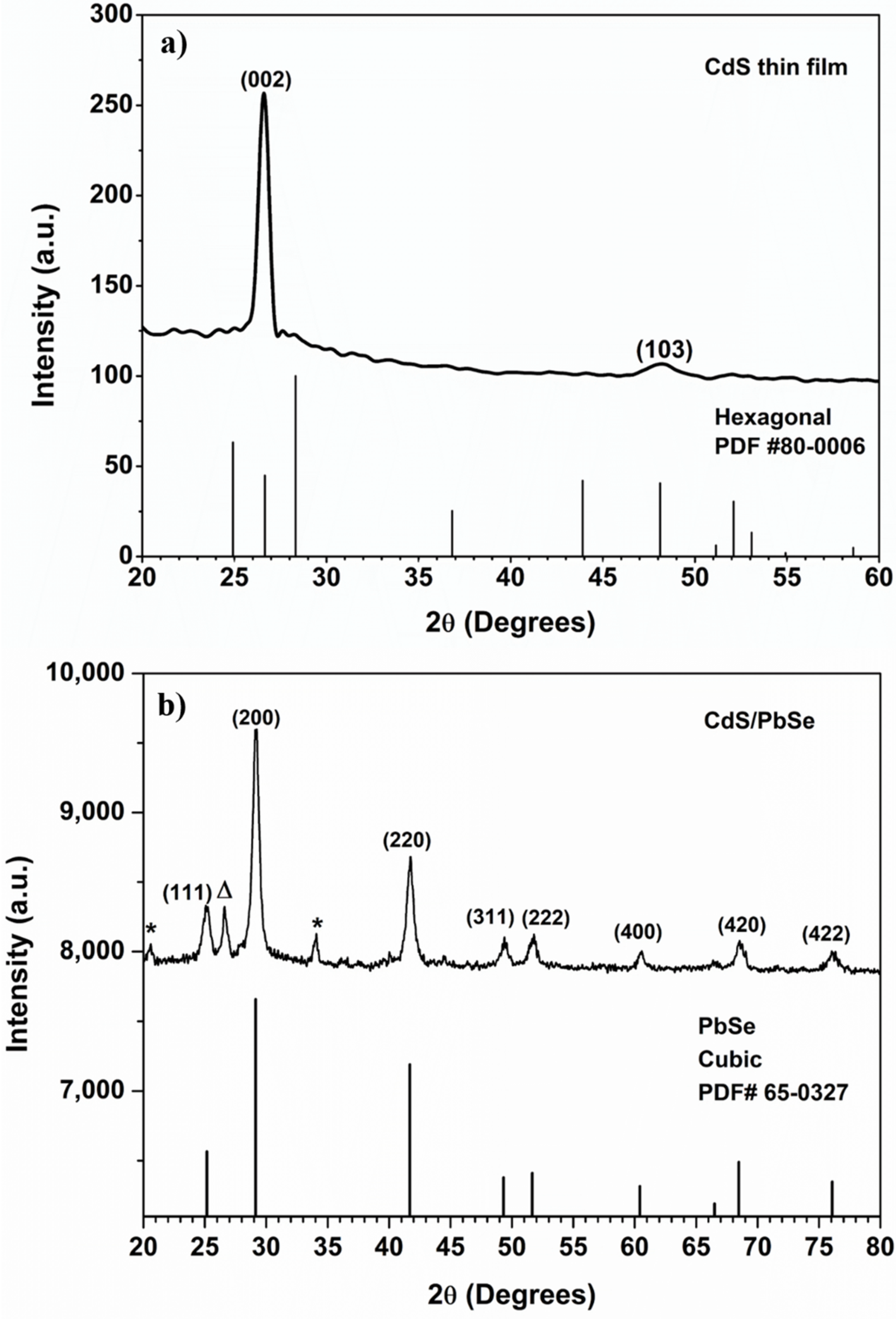
3.1. XRD Analysis
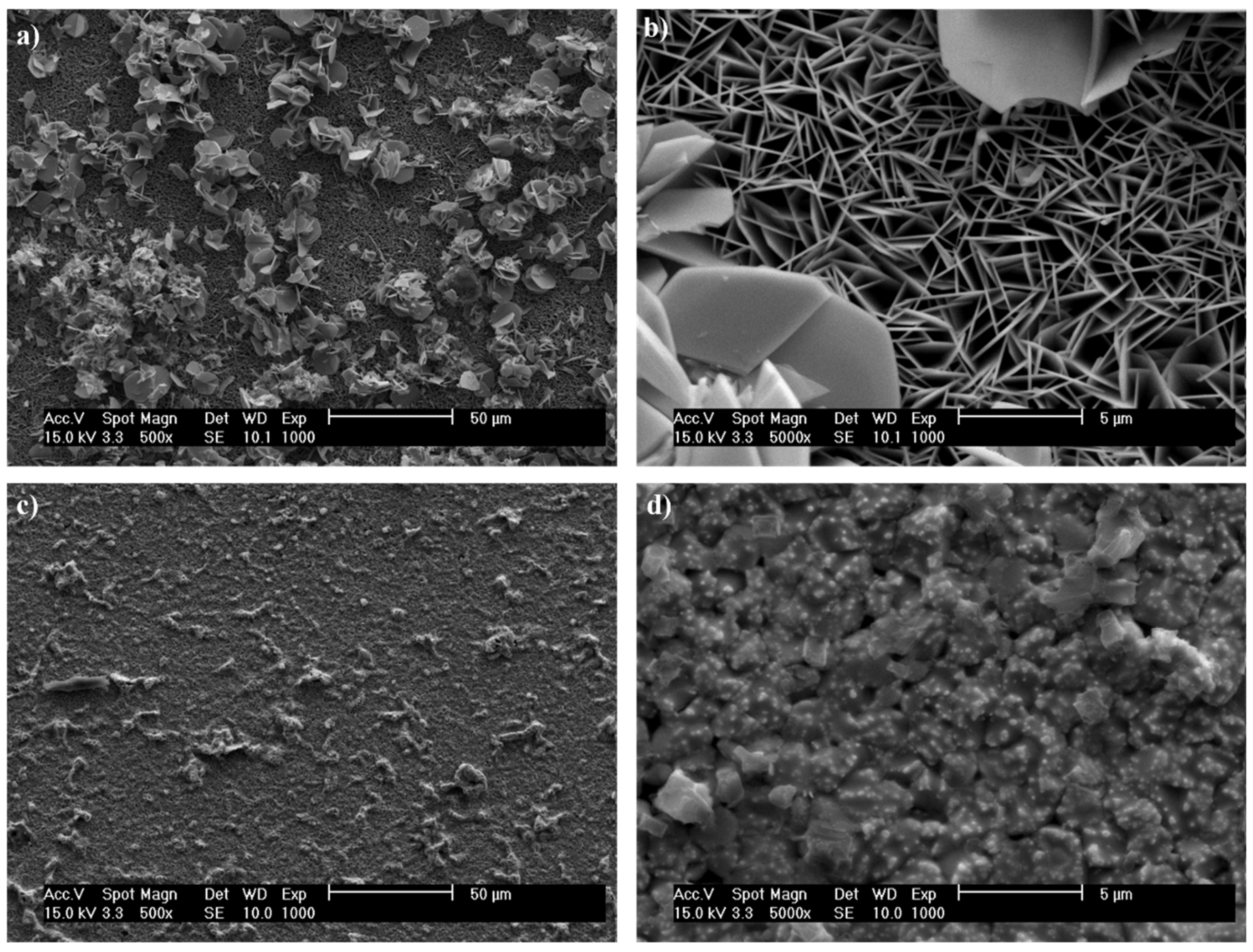
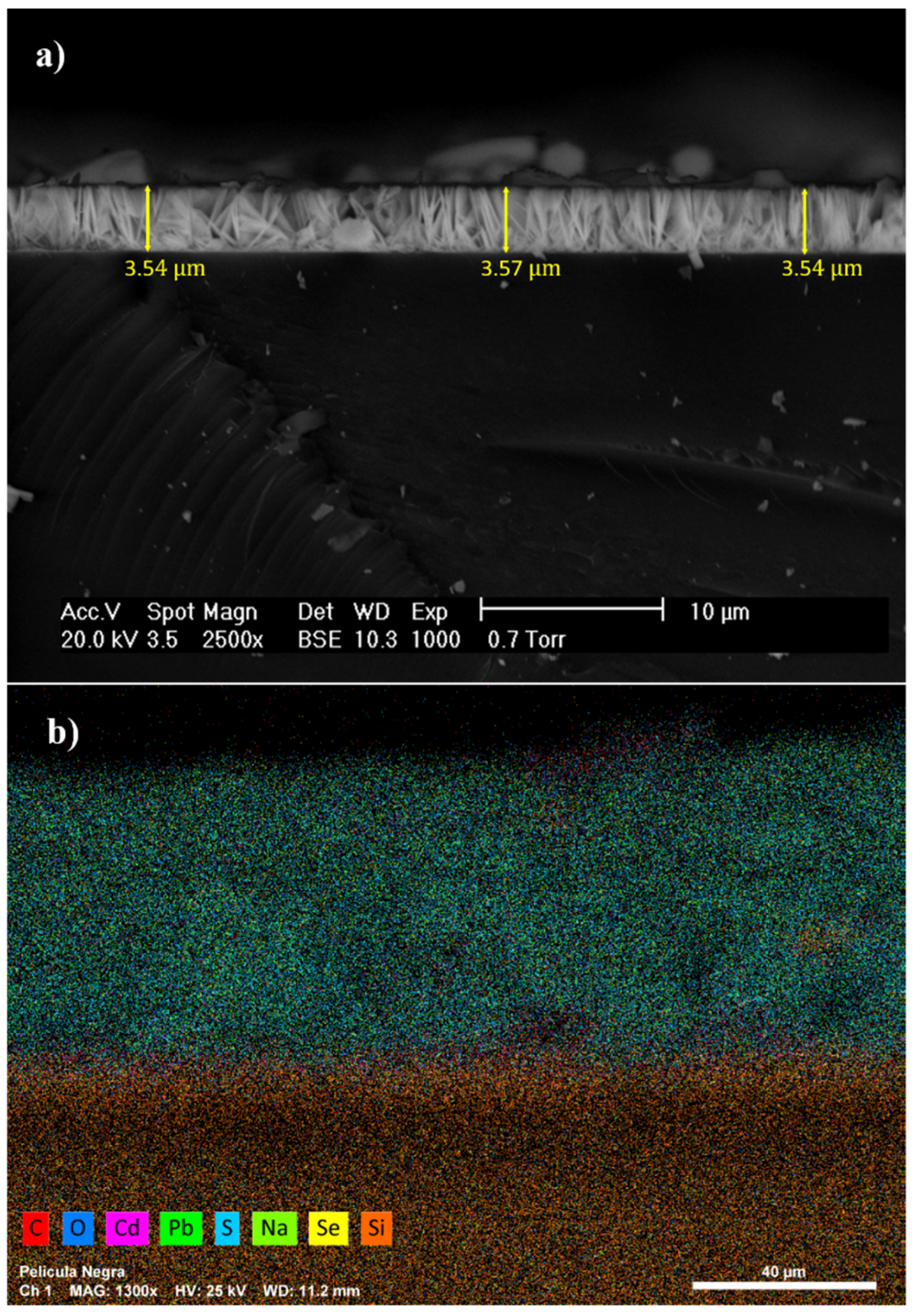
3.2. SEM and EDS
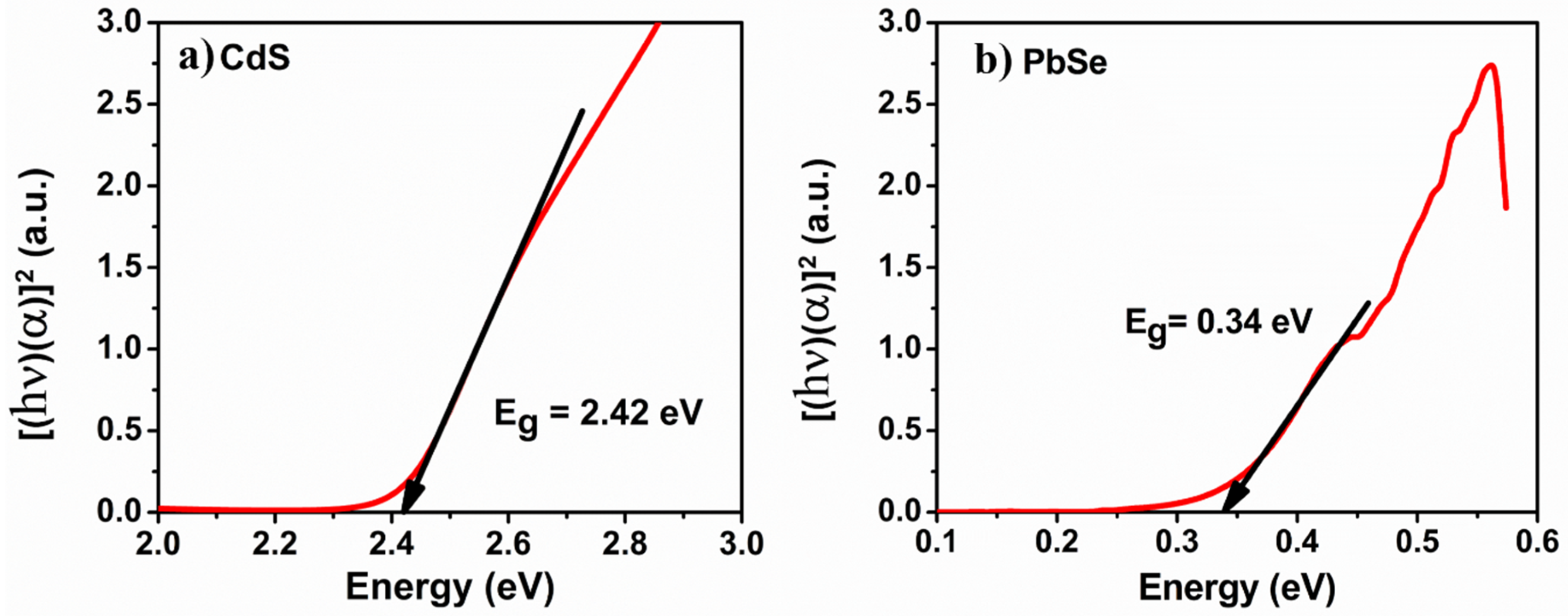
3.3. Optical Absorption
3.4. Electrical Response
4. Conclusions
- The heterojunction CdS/Pb5(CO3)3O(OH)2 was deposited on glass and glass/ITO substrates via CBD, to which an ion exchange process with selenium ions was applied to obtain the heterojunction CdS/PbSe.
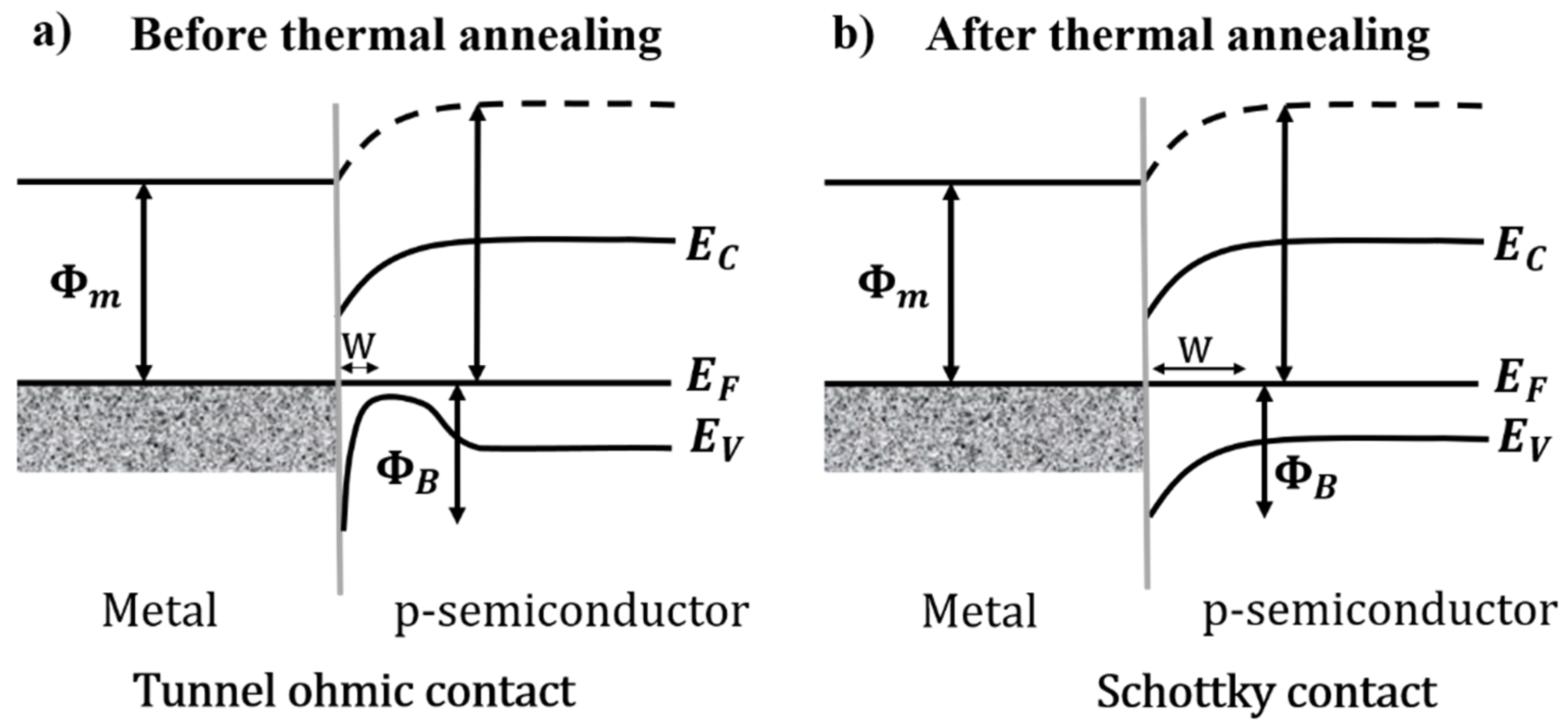
- The films evolved were uniform and had a good adhesion on the heterostructure ITO/CdS/PbSe, which was characterized by I vs. V, obtaining an ohmic response.
- Thermal annealing was necessary to improve the diffusion between the layers of the ITO/CdS/PbSe heterostructure and to obtain well-interlaced interfaces, changing from an ohmic to a diode response in the I vs. V characterization.
- The turn-on voltage and ideality factor under the darkness condition of the diode after thermal annealing were 0.8 V and 2.4, respectively. The ideality factor value may be due to diffusion between ITO/CdS caused by the annealing treatment, which behaved as a MOS hybrid layer, suggesting a Schottky diode response.
- An ITO/CdS/PbSe device made by aqueous solution methods represents a good, scalable, and promising alternative to produce cost-effective devices.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chusnutdinow, S.; Szot, M.; Wojtowicz, T.; Karczewski, G. PbSe/CdTe single quantum well infrared detectors. AIP Adv. 2017, 7, 035111. [Google Scholar] [CrossRef]
- He, B.; Ren, Y.X.; Dai, T.J.; Hou, S.; Liu, X.Z. Characterization and performance of graphene-PbSe thin film heterojunction. Rare Metals 2021, 40, 219–224. [Google Scholar] [CrossRef]
- Jang, M.H.; Yoo, S.S.; Kramer, M.T.; Dhar, N.K.; Gupta, M.C. Properties of chemical bath deposited and sensitized PbSe thin films for IR detection. Semicond. Sci. Technol. 2019, 34, 115010. [Google Scholar] [CrossRef]
- Ren, Y.X.; Dai, T.J.; Luo, W.B.; Liu, X.Z. Evidences of sensitization mechanism for PbSe thin films photoconductor. Vacuum 2018, 149, 190–194. [Google Scholar] [CrossRef]
- Sierra, C.; Torquemada, M.C.; Vergara, G.; Rodrigo, M.T.; Gutierrez, C.; Perez, G.; Génova, I.; Catalán, I.; Gómez, L.; Villamayor, V.; et al. Multicolour PbSe sensors for analytical applications. Sens. Actuators B-Chem. 2014, 190, 464–471. [Google Scholar] [CrossRef]
- Torquemada, M.C.; Villamayor, V.; Gomez, L.J.; Vergara, G.; Rodrigo, M.T.; Perez, G.; Génova, I.; Catalán, I.; Fernández, D.; Almazán, R.; et al. Monolithic integration of uncooled PbSe bicolor detectors. Sens. Actuators A-Phys. 2013, 199, 297–303. [Google Scholar] [CrossRef]
- Caliskan, M.; Yesilkaya, S.S.; Canlica, M. Characteristics of CoPc/CdS hybrid diode device. Bull. Mater. Sci. 2015, 38, 1439–1442. [Google Scholar] [CrossRef][Green Version]
- Hong, C.W.; Shin, S.W.; Suryawanshi, M.P.; Gang, M.G.; Heo, J.; Kim, J.H. Chemically Deposited CdS Buffer/Kesterite Cu2ZnSnS4 Solar Cells: Relationship between CdS Thickness and Device Performance. ACS Appl. Mater. Interfaces 2017, 9, 36733–36744. [Google Scholar] [CrossRef]
- Shao, Z.B.; Jie, J.S.; Sun, Z.; Xia, F.F.; Wang, Y.M.; Zhang, X.H.; Ding, K.; Lee, S.-T. MoO3 Nanodots Decorated CdS Nanoribbons for High-Performance, Homojunction Photovoltaic Devices on Flexible Substrates. Nano Lett. 2015, 15, 3590–3596. [Google Scholar] [CrossRef]
- Pal, K.; Thomas, S.; Mohan, M. Evaluation of Versatile CdS Nanomaterials Based Liquid Crystals Switchable Device. J. Nanosci. Nanotechnol. 2017, 17, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Xu, J.; Ning, H.P.; Xing, H.Z.; Wang, C.R.; Zhang, X.D.; Mo, G.K.; Shen, X.M. Preparation of nanocrystalline GZO/CdS bilayer films using magnetron sputtering and GZO/CdS/p-Si heterojunction photovoltaic device. J. Infrared Millim. Waves 2019, 38, 44–49. [Google Scholar]
- Wu, X.L.; Zhang, D.X.; Cai, H.K.; Zhou, Y.; Ni, J.; Zhang, J.J. Device design of GaSb/CdS thin film thermal photovoltaic solar cells. Acta Phys. Sin. 2015, 64, 096102. [Google Scholar]
- Wondmagegn, W.; Mejia, I.; Salas-Villasenor, A.; Stiegler, H.J.; Quevedo-Lopez, M.A.; Pieper, R.J.; Gnade, B. CdS Thin Film Transistor for Inverter and Operational Amplifier Circuit Applications. Microelectron. Eng. 2016, 157, 64–70. [Google Scholar] [CrossRef]
- Meza-Arroyo, J.; Reddy, K.C.S.; Rao, M.G.S.; Garibay-Martinez, F.; de Urquijo-Ventura, M.S.; Ramirez-Bon, R. Solution-based CdS thin film transistors with low temperature-processed Al2O3-GPTMS-PMMA as hybrid dielectric gate. Semicond. Sci. Technol. 2021, 36, 045015. [Google Scholar] [CrossRef]
- Woollins, J.; Afzaal, M.; O’Brien, P.; Hua, G.; Woollins, J.D. Morphological Evolution of PbSe Crystals via the CVD Route. Chem. Mater. 2010, 22, 4619–4624. [Google Scholar]
- Hmood, A.; Kadhim, A.; Abu Hassan, H. Composition-dependent structural and electrical properties of PbSe1−xTex thin films. Superlattices Microstruct. 2012, 51, 825–833. [Google Scholar] [CrossRef]
- Huang, N.M.; Basirun, W.J.B.; Yousefi, R.; Jamali-Sheini, F.; Hoomi, S.; Cheraghizade, M.; Basirun, W.J.; Huang, N.M. Large-scale and facile fabrication of PbSe nanostructures by selenization of a Pb sheet. Funct. Mater. Lett. 2015, 8, 1550063. [Google Scholar]
- Weng, B.; Qiu, J.; Zhao, L.; Chang, C.; Shi, Z. CdS/PbSe heterojunction for high temperature mid-infrared photovoltaic detector applications. Appl. Phys. Lett. 2014, 104, 121111. [Google Scholar] [CrossRef]
- Ling, P.; Zhang, X.; Zhang, Q.; Su, J.; Zhang, X.; Li, J. Chemical vapor deposition of a PbSe/CdS/nitrogen-doped TiO2 nanorod array photoelectrode and its band-edge level structure. New J. Chem. 2012, 36, 2302–2307. [Google Scholar]
- Qiu, J.J.; Weng, B.B.; McDowell, L.L.; Shi, Z.S. Low-cost uncooled MWIR PbSe quantum dots photodiodes. RSC Adv. 2019, 9, 42516–42523. [Google Scholar] [CrossRef]
- Mohammadi, S.; Zavvari, M. High performance n-ZnO/p-metal-oxides UV detector grown in low-temperature aqueous solution bath. Thin Solid Film. 2017, 626, 173–177. [Google Scholar] [CrossRef]
- Yunus, S.H.A.; Sahdan, M.Z.; Kamaruddin, S.A.; Rahim, M.S.; Supee, A.; Ichimura, M. ZnO Growth Properties in ZnO/SnS Heterojunction by a Combination of ECD and CBD Method. In Proceedings of the 2016 World Symposium on Computer Applications & Research (WSCAR), Cairo, Egypt, 12–14 March 2016; pp. 130–133. [Google Scholar]
- Terasako, T.; Hambali, N.A.; Jayah, N.A.; Wakisaka, T.; Hashim, A.M.; Yagi, M. Shape controlled growth of ZnO nanorods and fabrication of ZnO/CuO heterojunctions by chemical bath deposition using zinc nitrate hexahydrate and copper (III) nitrate trihydrate. Thin Solid Film. 2015, 596, 201–208. [Google Scholar] [CrossRef]
- Mwankemwa, B.S.; Akinkuade, S.; Maabong, K.; Nel, J.M.; Diale, M. Effects of surface morphology on the optical and electrical properties of Schottky diodes of CBD deposited ZnO nanostructures. Phys. B Condens. Matter 2018, 535, 175–180. [Google Scholar] [CrossRef]
- Ochoa-Landin, R.; Sastre-Hernandez, J.; Vigil-Galan, O.; Ramirez-Bon, R. Chemically deposited CdS by an ammonia-free process for solar cells window layers. Sol. Energy 2010, 84, 208–214. [Google Scholar] [CrossRef]
- Heredia-Cancino, J.A.; Mendivil-Reynoso, T.; Ochoa-Landin, R.; Ramírez-Bon, R.; Castillo, S.J. Optical and structural properties of PbSe films obtained by ionic exchange of lead oxyhydroxicarbonate in a selenium-rongalite solution. Mater. Sci. Semicond. Process. 2016, 56, 90–93. [Google Scholar] [CrossRef]
- Luque, A.; Hegedus, S. Handbook of Photovoltaic Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Parker, D.; Singh, D.J. High-temperature thermoelectric performance of heavily doped PbSe. Phys. Rev. B 2010, 82, 035204. [Google Scholar] [CrossRef]
- El Kissani, A.; Nkhaili, L.; Ammar, A.; Elassali, K.; Outzourhit, A. Synthesis, annealing, characterization, and electronic properties of thin films of a quaternary semiconductor; copper zinc tin sulfide. Spectrosc. Lett. 2016, 49, 343–347. [Google Scholar] [CrossRef]
- Murali, B.; Krupanidhi, S.B. Transport properties of CuIn(1-x)Al(x)Se2/AZnO heterostructure for low cost thin film photovoltaics. Dalton Trans. 2014, 43, 1974–1983. [Google Scholar] [CrossRef]
- Begum, A.; Rahman, A. Studies of current–voltage characteristics of nanocrystalline Al/(p)PbSe Schottky barrier junctions. Mater. Sci. Semicond. Process. 2014, 25, 231–237. [Google Scholar] [CrossRef]
- Breitenstein, O.; Altermatt, P.; Ramspeck, K.; Schenk, A. The Origin of Ideality factors n > 2 of Shunts and Surfaces in the Dark I-V Curves of Si Solar Cells. In Proceedings of the 21st European Photovoltaic Solar Energy Conference, Dresden, Germany, 4–8 September 2006; pp. 625–628. [Google Scholar]
- Chavali, R.V.; Wilcox, J.R.; Ray, B.; Gray, J.L.; Alam, M.A. Correlated Nonideal Effects of Dark and Light I–V Characteristics in a-Si/c-Si Heterojunction Solar Cells. IEEE J. Photovolt. 2014, 4, 763–771. [Google Scholar] [CrossRef]
- Roccaforte, F.; La Via, F.; Raineri, V. Ohmic contacts to SiC. Int. J. High Speed Electron. Syst. 2005, 15, 781–820. [Google Scholar] [CrossRef]
- DasGupta, N.; DasGupta, A. Semiconductor Devices: Modelling and Technology; PHI Learning Pvt. Ltd.: New Delhi, India, 2004. [Google Scholar]


| Ideality Factor | Saturation Current (eV) | Barrier Height | Series Resistance |
|---|---|---|---|
| η | Is (10−7 mA) | (eV) | Rs (Ω) |
| 2.47 | 0.36 | 1.08 | 712 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Cancino, J.A.; Salcido, O.; Britto-Hurtado, R.; Ruvalcaba-Manzo, S.G.; Ochoa-Landín, R.; Castillo, S.J. CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices. Appl. Sci. 2021, 11, 10914. https://doi.org/10.3390/app112210914
Heredia-Cancino JA, Salcido O, Britto-Hurtado R, Ruvalcaba-Manzo SG, Ochoa-Landín R, Castillo SJ. CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices. Applied Sciences. 2021; 11(22):10914. https://doi.org/10.3390/app112210914
Chicago/Turabian StyleHeredia-Cancino, José Antonio, Oscar Salcido, Ricardo Britto-Hurtado, Sayra Guadalupe Ruvalcaba-Manzo, Ramón Ochoa-Landín, and Santos Jesús Castillo. 2021. "CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices" Applied Sciences 11, no. 22: 10914. https://doi.org/10.3390/app112210914
APA StyleHeredia-Cancino, J. A., Salcido, O., Britto-Hurtado, R., Ruvalcaba-Manzo, S. G., Ochoa-Landín, R., & Castillo, S. J. (2021). CdS/PbSe Heterojunction Made via Chemical Bath Deposition and Ionic Exchange Processes to Develop Low-Cost and Scalable Devices. Applied Sciences, 11(22), 10914. https://doi.org/10.3390/app112210914






