Comparison of Initial pH Adjustment Prior to Thermophilic Anaerobic Digestion of Lime-Treated Corn Stover via Liquid Digestate or CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Lime Pretreatment
2.3. Neutralization of Lime-Treated Corn Stover
2.4. Biomethane Potential Test
2.5. Batch Liquid Anaerobic Digestion Test
2.6. Batch Solid-State Anaerobic Digestion Test
2.7. Analytical Methods
2.8. Data Analyses
3. Results and Discussion
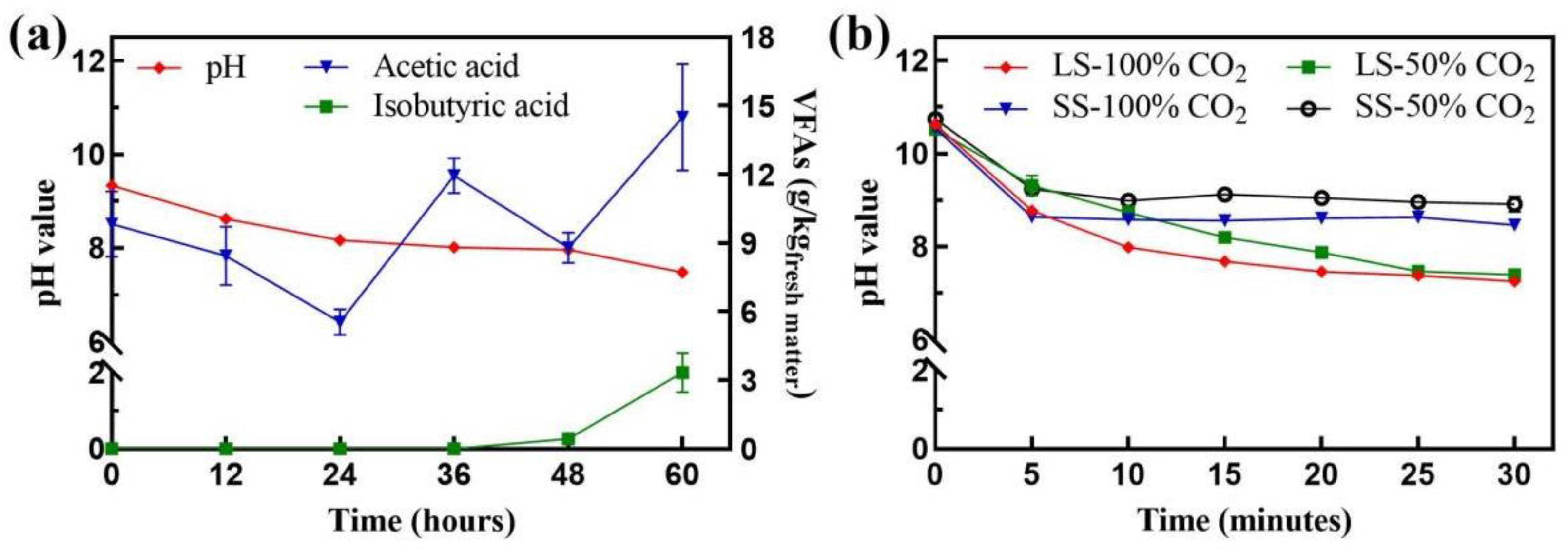
3.1. Reduction in pH and Accumulation of Volatile Fatty Acids during Liquid Digestate Neutralization
3.2. Reduction in pH during Gas-Neutralization
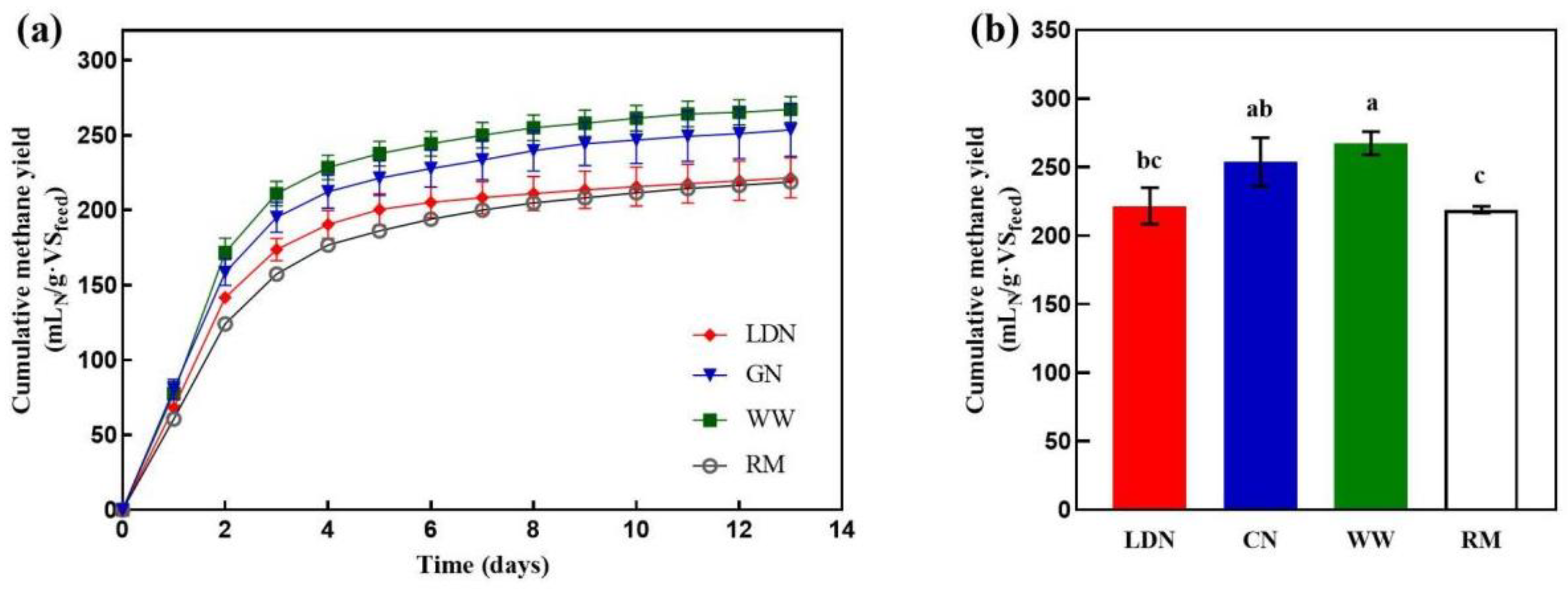
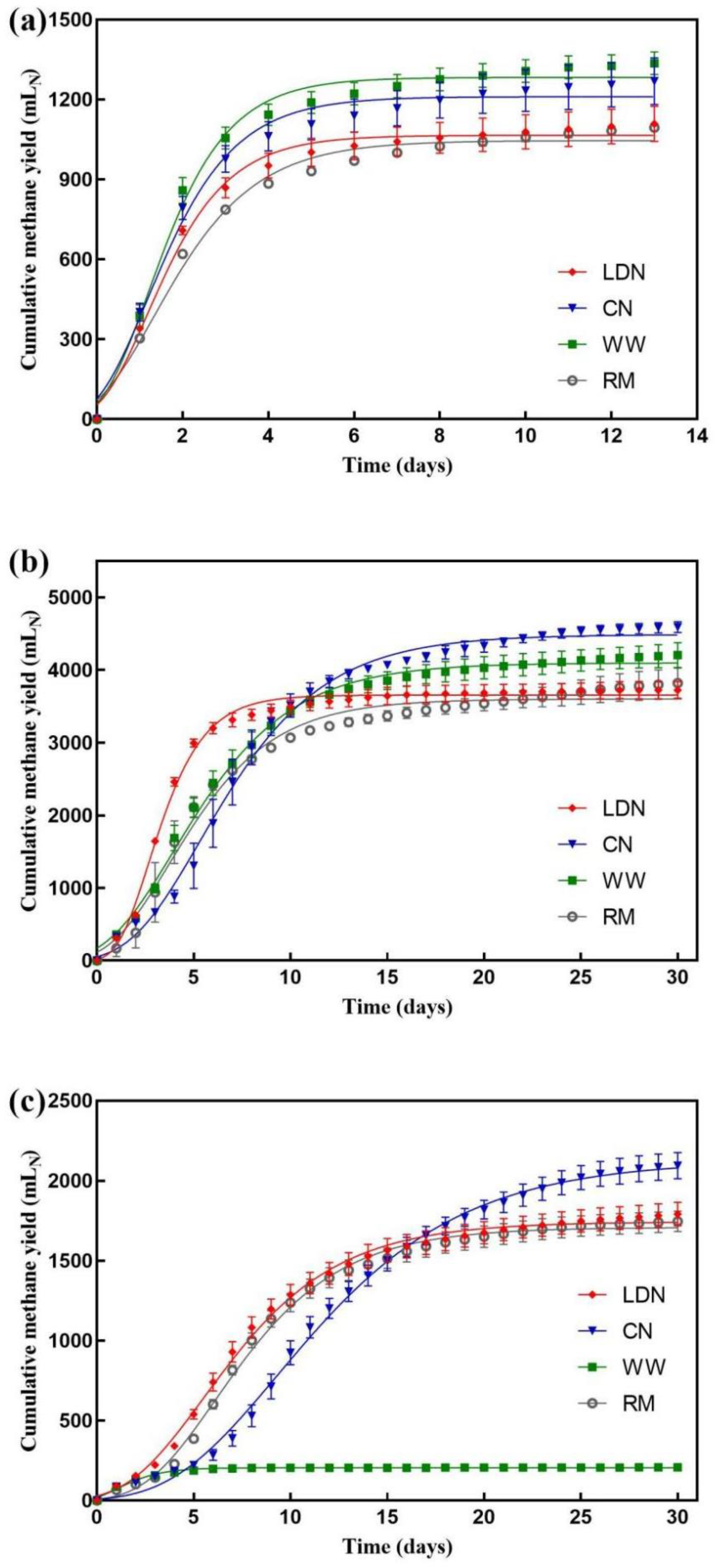
3.3. Effect of Neutralization on Biomethane Potential Test
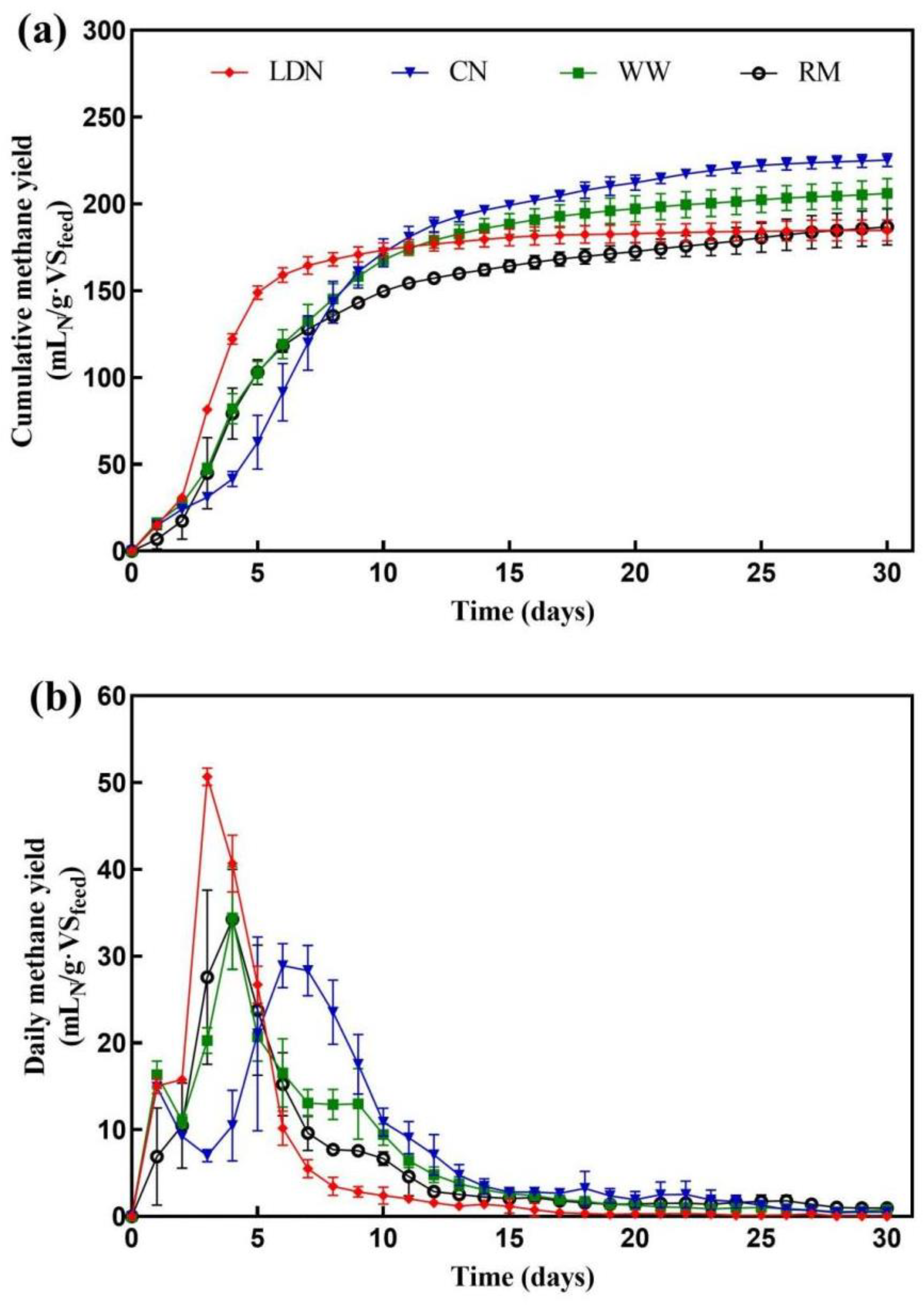
3.4. Effect of Neutralization on Thermophilic Liquid Anaerobic Digestion
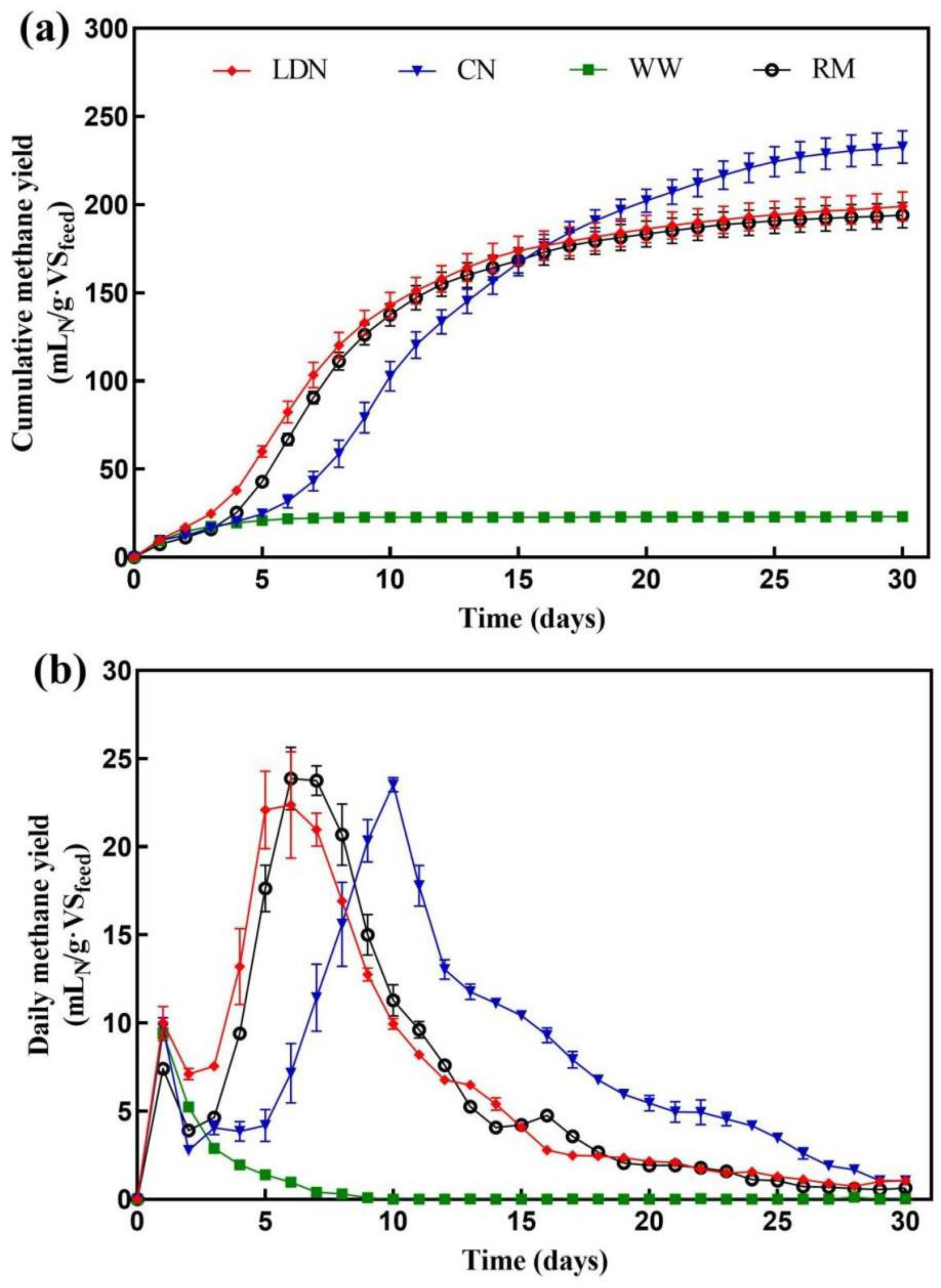
3.5. Effect of Neutralization on Thermophilic Solid-State Anaerobic Digestion
3.6. Kinetics of the Anaerobic Digestion Process
3.7. Economic Evaluation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AD | anaerobic digestion |
| CN | CO2 neutralization |
| C/N ratio | carbon/nitrogen ratio |
| CS | corn stover |
| I/S ratio | inoculum/substrate ratio |
| L-AD | liquid anaerobic digestion |
| LD | Liquid digestate |
| LDN | liquid digestate neutralization |
| S/I ratio | substrate/inoculum ratio |
| SS-AD | solid-state anaerobic digestion |
| TS | total solids |
| VFAs | volatile fatty acids |
| VS | volatile solids |
| WW | washed with tap water |
References
- Sun, H.; Cui, X.; Stinner, W.; Shah, G.M.; Cheng, H.; Shan, S.; Guo, J.; Dong, R. Synergetic effect of combined ensiling of freshly harvested and excessively wilted maize stover for efficient biogas production. Bioresour. Technol. 2019, 285, 121338. [Google Scholar] [CrossRef]
- Bhatia, P.; Fujiwara, M.; Salangsang, M.; Qian, J.; Liu, X.; Ban, S.; Myojin, M.; Toda, T. Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora. Appl. Sci. 2021, 11, 4452. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Stiverson, J.; Yu, Z.; Li, Y. Reactor performance and microbial community dynamics during solid-state anaerobic digestion of corn stover at mesophilic and thermophilic conditions. Bioresour. Technol. 2013, 136, 574–581. [Google Scholar] [CrossRef]
- Kasinski, S. Mesophilic and Thermophilic Anaerobic Digestion of Organic Fraction Separated during Mechanical Heat Treatment of Municipal Waste. Appl. Sci. 2020, 10, 2412. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; He, Y.; Liu, X.; Chen, C.; Liu, G. Thermophilic Solid-State Anaerobic Digestion of Alkaline-Pretreated Corn Stover. Energy Fuels 2014, 28, 3759–3765. [Google Scholar] [CrossRef]
- Rusin, J.; Chamradova, K.; Grycova, B. The influence of biomass agitation on biogas and methane production using the high-solids thermophilic anaerobic digestion. Green Process. Synth. 2017, 6, 273–279. [Google Scholar] [CrossRef]
- Meng, L.; Xie, L.; Riya, S.; Terada, A.; Hosomi, M. Impact of turning waste on performance and energy balance in thermophilic solid-state anaerobic digestion of agricultural waste. Waste Manag. 2019, 87, 183–191. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Mu, X. Review of Alkali-Based Pretreatment to Enhance Enzymatic Saccharification for Lignocellulosic Biomass Conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- You, Z.; Zhang, S.; Kim, H.; Chiang, P.-C.; Sun, Y.; Guo, Z.; Xu, H. Effects of Corn Stover Pretreated with NaOH and CaO on Anaerobic Co-Digestion of Swine Manure and Corn Stover. Appl. Sci. 2018, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Qian, Y.; Xi, Y.; Lü, X. Hydrothermal and alkaline thermal pretreatment at mild temperature in solid state for physicochemical properties and biogas production from anaerobic digestion of rice straw. Renew. Energy 2019, 139, 261–267. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, Q.; Zhang, R.; Chen, C.; Liu, G. Co-pretreatment of wheat straw by potassium hydroxide and calcium hydroxide: Methane production, economics, and energy potential analysis. J. Environ. Manag. 2019, 236, 720–726. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, Q.; Zhou, X.; Chen, Z.; Keener, H.; Li, Y. Comparison of sodium hydroxide and calcium hydroxide pretreatments of giant reed for enhanced enzymatic digestibility and methane production. Bioresour. Technol. 2017, 244, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Sympson, W.; Nokes, S.; Hickman, A. Recirculating calcium hydroxide solution: A practical choice for on-farm high solids lignocellulose pretreatment. Ind. Crops Prod. 2017, 97, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Falls, M.; Holtzapple, M.T. Oxidative Lime Pretreatment of Alamo Switchgrass. Appl. Biochem. Biotechnol. 2011, 165, 506–522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Shiroma, R.; Al-Haq, M.I.; Zhang, Y.; Ike, M.; Arai-Sanoh, Y.; Ida, A.; Kondo, M.; Tokuyasu, K. A novel lime pretreatment for subsequent bioethanol production from rice straw—Calcium capturing by carbonation (CaCCO) process. Bioresour. Technol. 2010, 101, 6805–6811. [Google Scholar] [CrossRef]
- Sun, C.; Xie, Y.; Hou, F.; Yu, Q.; Wang, Y.; Wang, X.; Miao, C.; Ma, J.; Ge, W.; Zhang, T.; et al. Enhancement on methane production and anaerobic digestion stability via co-digestion of microwave-Ca(OH)2 pretreated sugarcane rind slurry and kitchen waste. J. Clean. Prod. 2020, 264, 121731. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Promoting anaerobic biogasification of corn stover through biological pretreatment by liquid fraction of digestate (LFD). Bioresour. Technol. 2015, 175, 167–173. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D.; Zuo, X.; Gu, J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637–638, 9–17. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Li, Z.; Wang, X.; Sun, J. Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw. Bioresour. Technol. 2019, 280, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z.; Zhou, X.; Wang, X.; Liu, T.; Cheng, S. Investigation on methane yield of wheat husk anaerobic digestion andits enhancement effect by liquid digestate pretreatment. Anaerobe 2019, 59, 92–99. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving Biodegradability and Biogas Production of Corn Stover through Sodium Hydroxide Solid State Pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, L.; Li, Y. Comparison of premixing methods for solid-state anaerobic digestion of corn stover. Bioresour. Technol. 2015, 175, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Federation, W.E.; APH Association. Standard Methods for the Examination of Water and Wastewater; AWWA/WEF/APHA: Washington, DC, USA, 2005. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2010; TP–510–42618.
- Lay, J.; Li, Y.; Noike, T.; Endo, J.; Ishimoto, S. Analysis of environmental factors affecting methane production from high solids organic waste. Water Sci. Technol. 1997, 36, 493–500. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 79, 744–751. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Capela, I.; Tarelho, L.A. Over-acidification control strategies for enhanced biogas production from anaerobic digestion: A review. Biomass-Bioenergy 2020, 143, 105833. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Braz, G.H.R.; Fernandez-Gonzalez, N.; Lema, J.; Carballa, M. The time response of anaerobic digestion microbiome during an organic loading rate shock. Appl. Microbiol. Biotechnol. 2018, 102, 10285–10297. [Google Scholar] [CrossRef]
- Reilly, M.; Dinsdale, R.; Guwy, A. Mesophilic biohydrogen production from calcium hydroxide treated wheat straw. Int. J. Hydrogen Energy 2014, 39, 16891–16901. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.A.; Rahman, S.; Cihacek, L.; Nahar, N. Comparison of the Reactor Performance of Alkaline-Pretreated Corn Stover Co-digested with Dairy Manure under Solid-State. Waste Biomass-Valorization 2020, 11, 5211–5222. [Google Scholar] [CrossRef]
- Halil, Ş. Enhancement in methane yield from anaerobic co-digestion of walnut shells and cattle manure. Environ. Prog. Sustain. 2020, 39, e13524. [Google Scholar]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Yeganeh, L.P.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Meng, L.; Xie, L.; Kinh, C.T.; Suenaga, T.; Hori, T.; Riya, S.; Terada, A.; Hosomi, M. Influence of feedstock-to-inoculum ratio on performance and microbial community succession during solid-state thermophilic anaerobic co-digestion of pig urine and rice straw. Bioresour. Technol. 2018, 252, 127–133. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, T.; Chang, J.; Tang, Q.; Luo, T.; Cui, Z. Effect of Substrate to Inoculum Ratio on Biogas Production and Microbial Community during Hemi-Solid-State Batch Anaerobic Co-digestion of Rape Straw and Dairy Manure. Appl. Biochem. Biotechnol. 2019, 189, 884–902. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Corn Stover | Inoculum | Liquid Digestate |
|---|---|---|---|
| Total solids (%) | 91.48 ± 0.10 | 13.54 ± 0.05 | N/D |
| Volatile solids (%) | 84.55 ± 0.19 | 6.51 ± 0.04 | N/D |
| Total organic carbon (%) | 43.11 ± 0.77 | 25.77 ± 0.36 | N/D |
| Total nitrogen (%) | 1.02 ± 0.03 | 2.61 ± 0.07 | 0.15 ± 0.00 |
| C/N ratio | 42.28 ± 0.74 | 9.89 ± 0.29 | N/D |
| Alkalinity (g CaCO3/kg) | 8.54 ± 0.02 | 10.23 ± 0.35 | 1.87 ± 0.11 |
| Cellulose (%) a | 33.83 ± 0.36 | N/D | N/D |
| Hemicellulose (%) a | 26.10 ± 0.33 | N/D | N/D |
| Lignin (%) a | 19.10 ± 0.89 | N/D | N/D |
| MLSS (g/L) | N/D | N/D | 3.72 ± 0.13 |
| MLVSS (g/L) | N/D | N/D | 2.64 ± 0.14 |
| pH | N/D | 7.87 ± 0.08 | 7.53 ± 0.09 |
| Method | Rm (mLN·d−1) | λ (d) | Pm (mLN) | R2 |
|---|---|---|---|---|
| LDN-B a | 364.8 | 0.1 | 1065.6 | 0.9897 |
| CN-B a | 385.0 | 0.0 | 1210.7 | 0.9827 |
| WW-B a | 445.1 | 0.1 | 1283.2 | 0.9870 |
| RM-B a | 286.2 | 0.0 | 1045.8 | 0.9842 |
| LDN-L b | 721.0 | 1.1 | 3108.2 | 0.9942 |
| CN-L b | 453.2 | 1.8 | 4398.6 | 0.9946 |
| WW-L b | 431.8 | 0.6 | 4010.2 | 0.9955 |
| RM-L b | 432.3 | 0.8 | 3513.9 | 0.9832 |
| LDN-SS c | 163.9 | 1.6 | 1744.2 | 0.9962 |
| CN-SS c | 143.9 | 3.9 | 2128.1 | 0.9975 |
| WW-SS c | 58.9 | −0.2 | 205.1 | 0.9632 |
| RM-SS c | 172.8 | 2.5 | 1708.5 | 0.9962 |
| Iterms | Lime Pretreatment Reagent and Water Cost (RMB/t TS a) | Neutralization Cost (RMB/t TS a) | Wastewater Treatment Cost (RMB/t TS a) | Methane Profits (RMB/t TS a) | Net Profits (RMB/t TS a) |
|---|---|---|---|---|---|
| LDN-L b | 87.41 | 0.00 | 0.00 | −9.33 | −96.74 |
| CN-L b | 87.41 | 0.00 | 0.00 | 188.30 | 100.89 |
| WW-L b | 87.41 | 82.00 | 40.00 | 94.37 | −126.89 |
| RM-L b | - | - | - | - | - |
| LDN-SS c | 87.41 | 0.00 | 0.00 | 24.72 | −62.69 |
| CN-SS c | 87.41 | 0.00 | 0.00 | 186.15 | 98.74 |
| WW-SS c | 87.41 | 82.00 | 40.00 | −835.10 | −1056.36 |
| RM-SS c | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Yang, M.; Hu, D.; Mei, Y.; Zhao, S.; Liang, Y. Comparison of Initial pH Adjustment Prior to Thermophilic Anaerobic Digestion of Lime-Treated Corn Stover via Liquid Digestate or CO2. Appl. Sci. 2021, 11, 10884. https://doi.org/10.3390/app112210884
Xiao L, Yang M, Hu D, Mei Y, Zhao S, Liang Y. Comparison of Initial pH Adjustment Prior to Thermophilic Anaerobic Digestion of Lime-Treated Corn Stover via Liquid Digestate or CO2. Applied Sciences. 2021; 11(22):10884. https://doi.org/10.3390/app112210884
Chicago/Turabian StyleXiao, Lei, Man Yang, Di Hu, Yuxia Mei, Shumiao Zhao, and Yunxiang Liang. 2021. "Comparison of Initial pH Adjustment Prior to Thermophilic Anaerobic Digestion of Lime-Treated Corn Stover via Liquid Digestate or CO2" Applied Sciences 11, no. 22: 10884. https://doi.org/10.3390/app112210884
APA StyleXiao, L., Yang, M., Hu, D., Mei, Y., Zhao, S., & Liang, Y. (2021). Comparison of Initial pH Adjustment Prior to Thermophilic Anaerobic Digestion of Lime-Treated Corn Stover via Liquid Digestate or CO2. Applied Sciences, 11(22), 10884. https://doi.org/10.3390/app112210884






