Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Exposure Experiment
2.2. Exposure Experiment
2.3. Eugenol Detection
2.4. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jiang, S.; Zhou, F.L.; Yang, W.L.; Wu, Z.G.; Le, Y.; Yang, Q.B.; Yu, Y.B.; Jiang, S.G. Anaesthetic effect of eugenol at different concentrations and temperatures on blank tiger shrimp (Penaeus monodon). Aquac. Res. 2020, 51, 3268–3273. [Google Scholar] [CrossRef]
- Li, Y.D.; She, Q.X.; Han, Z.B.; Sun, N.; Liu, X.; Li, X.D. Anaesthetic Effects of Eugenol on Grass Shrimp (Palaemonetes sinensis) of Different Sizes at Different Concentrations and Temperatures. Sci. Rep. 2018, 8, 11007. [Google Scholar] [CrossRef] [Green Version]
- Ghanawi, J.; Saoud, G.; Zakher, C.; Monzer, S.; Saoud, I.P. Clove oil as an anaesthetic for Australian redclaw crayfish Cherax quadricarinatus. Aquac. Res. 2019, 50, 3628–3632. [Google Scholar] [CrossRef]
- Xu, J.H.; Liu, Y.; Zhou, X.W.; Ding, H.T.; Dong, X.J.; Qu, L.T.; Xia, T.; Chen, X.N.; Cheng, H.L.; Ding, Z.J. Anaesthetic effects of eugenol on preservation and transportation of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2021, 52, 3796–3803. [Google Scholar] [CrossRef]
- Viegas, R.M.; Franca, C.L.; Castro, J.S.; Castro, J.J.P.; Santana, T.C.; Costa-Lima, M.P.G.; Neta, R.N.F.C.; Carreiro, C.R.P.; Teixeira, E.G. Eugenol as an efficient anesthetic for neotropical fish Prochilodus nigricans (Teleostei, Prochilodontidae). Arq. Bras. Med. Veterinária Zootec. 2020, 72, 1813–1820. [Google Scholar] [CrossRef]
- He, R.; Lei, B.; Su, Y.; Wang, A.; Cui, K.; Shi, X.; Chen, X. Effectiveness of eugenol as an anesthetic for adult spotted sea bass (Lateolabrax maculatus). Aquaculture 2020, 523, 735180. [Google Scholar] [CrossRef]
- Barbosa de Oliveira, C.P.; da Paixao Lemos, C.H.; Felix e Silva, A.; de Souza, S.A.; Luscher Albinati, A.C.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Tarkhani, R.; Imani, A.; Jamali, H.; Moghanlou, K.S. Anaesthetic efficacy of eugenol on Flowerhorn (Amphilophus labiatus × Amphilophus trimaculatus). Aquac. Res. 2017, 48, 3207–3215. [Google Scholar] [CrossRef]
- Cowing, D.; Powell, A.; Johnson, M. Evaluation of different concentration doses of eugenol on the behaviour of Nephrops norvegicus. Aquaculture 2015, 442, 78–85. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries of Japan. The 28th Report on Usage of Fisheries Drug. Available online: https://www.maff.go.jp/j/syouan/suisan/suisan_yobo/pdf/28_suiyaku.pdf (accessed on 3 June 2021).
- Ministry for Primary Industries of New Zealand. Food Notice: Maximum Residue Levels for Agricultural Compounds. Available online: https://www.mpi.govt.nz/dmsdocument/19550-Maximum-Residue-Levels-for-Agricultural-Compounds (accessed on 3 June 2021).
- Secretariat of Association of Southeast Asian Nations. Guidelines for the Use of Chemicals in Aquaculture and Measures to Eliminate the Use of Harmful Chemicals; Association of Southeast Asian Nations: Jakarta, Indonesia, 2013. [Google Scholar]
- Medicines Control Council, Department of Health, South Africa. MRLs and Withdrawal Periods. Available online: http://www.sahpra.org.za/wp-content/uploads/2020/01/a0a7ad443.07MRLandwithdrawalperiodsJan04v1.pdf (accessed on 3 June 2021).
- National Institutes of Health. National Toxicology Program Technical Report on the Carcinogenesis Studies of Eugenol (CAS No. 97-53-0) in F344/N Rates and B6C3F Mice (Feeding Studies); NTP/TR No. 223, NIH No. 84-1779; US Department of Health and Human Services: Washington, DC, USA, 1983.
- The Food and Drug Administration’s Center for Veterinary Medicine. Guidance for Industry 150: Concerns Related to the use Clove Oil as an Anesthetic for Fish. Available online: https://www.fda.gov/media/69954/download (accessed on 3 June 2021).
- The Japan Food Chemical Research Foundation. Positive List System for Agricultural Chemical Residues in Foods, Maximum Residue Limits (MRLs) List of Agricultural Chemicals in Foods. 2016. Available online: http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/TrueMainE?OpenFrameset (accessed on 3 June 2021).
- Joint FAO/WHO Expert Committee on food Additives. WHO Technical Report Series 934: Evaluation of Certain Food Additives; World Health Organization: Geneva, Switzerland, 2006; pp. 49–54. [Google Scholar]
- Javahery, S.; Nekoubin, H.; Moradlu, A.H. Effect of anaesthesia with clove oil in fish (review). Fish. Physiol. Biochem. 2012, 38, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Tago, A.; Yokoyama, S.; Ishikawa, M.; Koshio, S. Pharmacokinetics of Eugenol in Japanese Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 49, 780–787. [Google Scholar] [CrossRef]
- Guenette, S.A.; Uhland, F.C.; Helie, P.; Beaudry, F.; Vachon, P. Pharmacokinetics of eugenol in rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 266, 262–265. [Google Scholar] [CrossRef]
- Zhao, D.H.; Ke, C.L.; Liu, Q.; Wang, X.F.; Wang, Q.; Li, L.D. Elimination kinetics of eugenol in grass carp in a simulated transportation setting. BMC Vet. Res. 2017, 13, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.T.; Ai, X.H.; Li, L.; Li, J.C.; Yang, H. A fast and accurate isotope dilution GC-IT-MS/MS method for de-termination of eugenol in different tissues of fish: Application to a depletion study in mandarin fish. Biomed. Chromatogr. 2018, 32, e4163. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Schreier, T.M.; Porcher, S.T.; Smerud, J.R.; Gaikowski, M.P. Depletion of eugenol residues from the skin-on fillet tissue of rainbow trout exposed to 14C-labeled eugenol. Aquaculture 2014, 430, 74–78. [Google Scholar] [CrossRef]
- Ke, C.; Liu, Q.L.; Li, L.; Chen, J.W.; Zhao, C.H.; Xu, J.P.; Huang, K.; Mo, M.S.; Li, L.D. Residual levels and risk assessment of eugenol and its isomers in fish from China markets. Aquaculture 2018, 484, 338–342. [Google Scholar] [CrossRef]
- Ke, C.L.; Liu, Q.; Li, L.D.; Chen, J.W.; Wang, X.U.; Huang, K. Simultaneous determination of eugenol, isoeugenol and methyleugenol in fish fillet using gas chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2016, 1031, 189–194. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Guidance for Assessing Chemical Contaminant Data for Use in Fish. Advisories. Vol 2: Risk Assessment and Fish. Consumption Limits, 3rd ed.; EPA 823-B-00-008; Office of Water: Washington, DC, USA, 2000.
- Chinese Nutrition Society. Chinese Dietary Guidelines Society; People’s Medical Publishing House Co., Ltd.: Beijing, China, 2016. [Google Scholar]
- Bureau of Disease Prevention and Control of National Health Commission of P.R.C. 2015 Report on the Nutrition and Chronic Disease Status of Chinese Residents; People’s Medical Publishing House Co., Ltd.: Beijing, China, 2015.
- Marking, L.L.; Meyer, F.P.J.F. Are Better Anesthetics Needed in Fisheries? Fisheries 1985, 10, 2–5. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Gingerich, W.H.; Allen, J.L.J.X. Metabolism and elimination of benzocaine by rainbow trout, Oncorhynchus mykiss. Xenobiotica 1991, 21, 525–533. [Google Scholar] [CrossRef]
- Hayton, W.L.; Szoke, A.; Kemmenoe, B.H.; Vick, A.M.J.A.T. Disposition of benzocaine in channel catfish. Aquat. Toxicol. 1996, 36, 99–113. [Google Scholar] [CrossRef]
- Kiessling, A.; Johansson, D.; Zahl, I.H.; Samuelsen, O.B. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) fol-lowing bath administration. Aquaculture 2009, 286, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Patra, R.W.; Chapman, J.C.; Lim, R.P.; Gehrke, P.C.; Sunderam, R.M. 2009 Effects of temperature on ventilatory behavior of fish exposed to sublethal concentrations of endosulfan and chlorpyrifos. Environ. Toxicol. Chem. 2009, 28, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Meinertz, J.R.; Greseth, S.L.; Schreier, T.M.; Bernardy, J.A.; Gingerich, W.H. Isoeugenol concentrations in rainbow trout (Oncorhynchus mykiss) skin-on fillet tissue after exposure to AQUI-S (TM) at different temperatures, durations, and concentrations. Aquaculture 2006, 254, 347–354. [Google Scholar] [CrossRef]
- Kildea, M.A.; Allan, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277. [Google Scholar] [CrossRef]
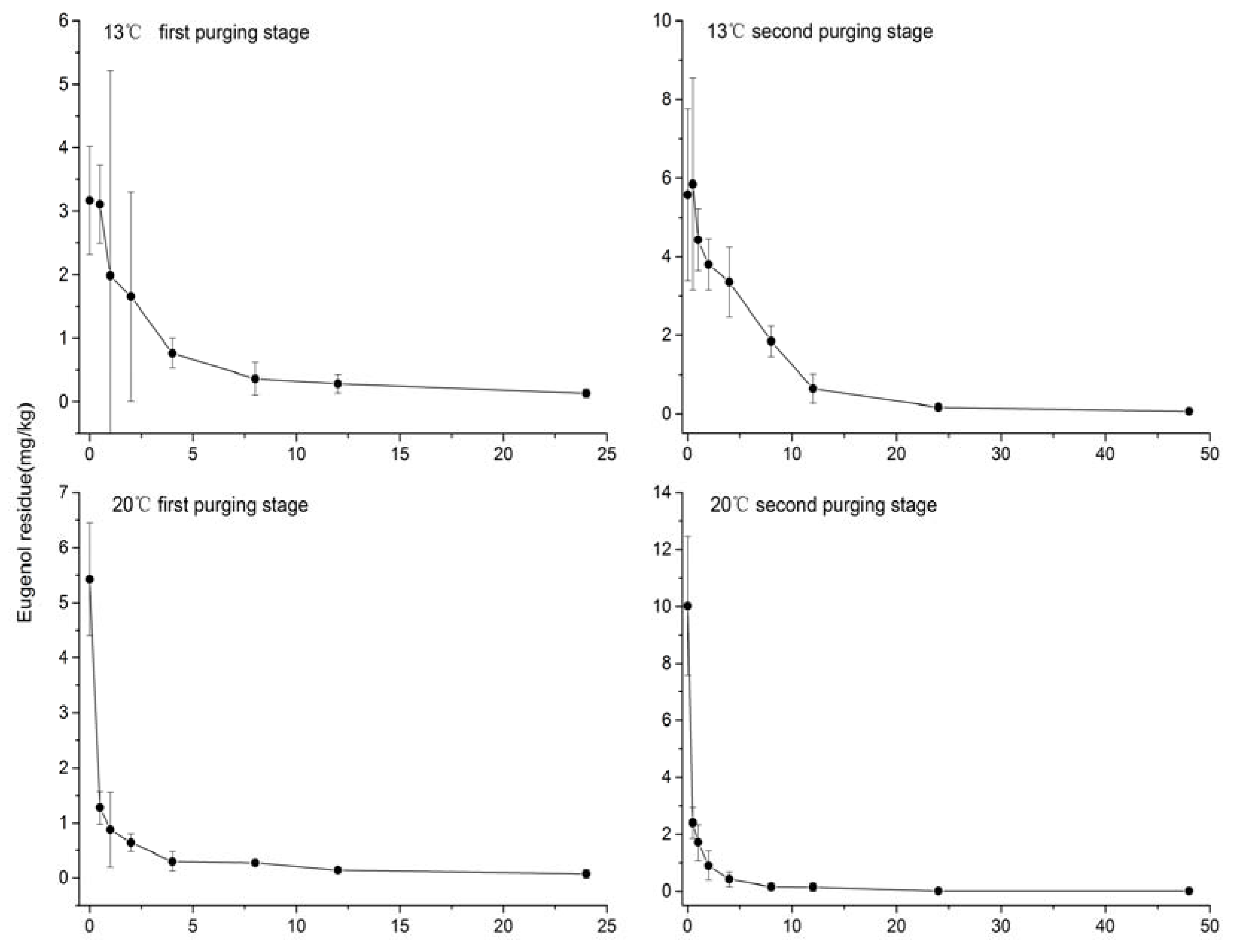
| Exposure Temperature | Purging Stage | Model Equation | R2 | Half-Life (h) |
|---|---|---|---|---|
| 13 °C | First | Yt = 3255 × e−0.34t | 0.968 | 2.0 |
| Second | Yt = 5667 × e−0.154t | 0.978 | 4.5 | |
| 20 °C | First | Yt = 5377 × e−2.444t | 0.963 | 0.28 |
| Second | Yt = 9932 × e−2.405t | 0.977 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, C.; Liu, Q.; Huang, K.; Mo, M.; Chen, H.; Cheng, B. Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport. Appl. Sci. 2021, 11, 10882. https://doi.org/10.3390/app112210882
Ke C, Liu Q, Huang K, Mo M, Chen H, Cheng B. Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport. Applied Sciences. 2021; 11(22):10882. https://doi.org/10.3390/app112210882
Chicago/Turabian StyleKe, Changliang, Qi Liu, Ke Huang, Mengsong Mo, Haigang Chen, and Bo Cheng. 2021. "Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport" Applied Sciences 11, no. 22: 10882. https://doi.org/10.3390/app112210882
APA StyleKe, C., Liu, Q., Huang, K., Mo, M., Chen, H., & Cheng, B. (2021). Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport. Applied Sciences, 11(22), 10882. https://doi.org/10.3390/app112210882






