Metal Titanate (ATiO3, A: Ni, Co, Mg, Zn) Nanorods for Toluene Photooxidation under LED Illumination
Abstract
:1. Introduction
2. Materials and Methods
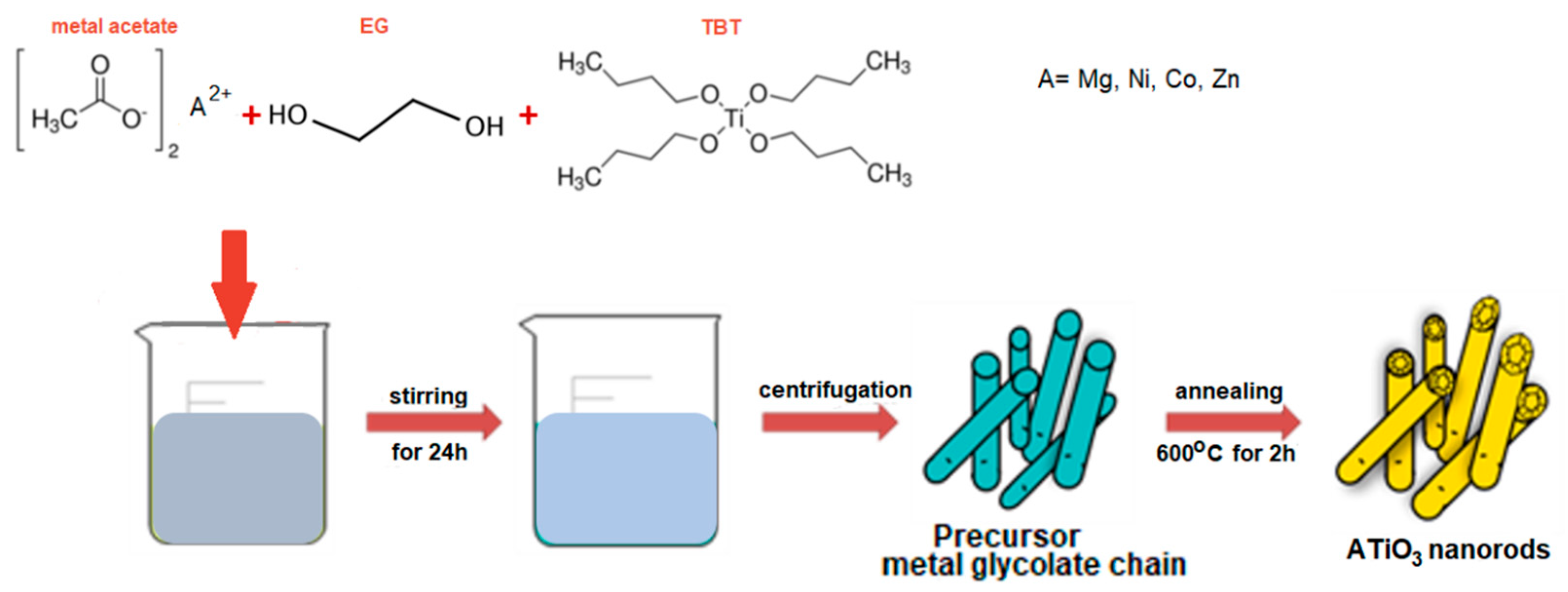
2.1. Synthesis of Metal Titanate Nanorods
2.2. Characterization
2.3. Measurement of Photocatalytic Activity
3. Results
3.1. Structural Characterization of Metal Titanate Nanorods
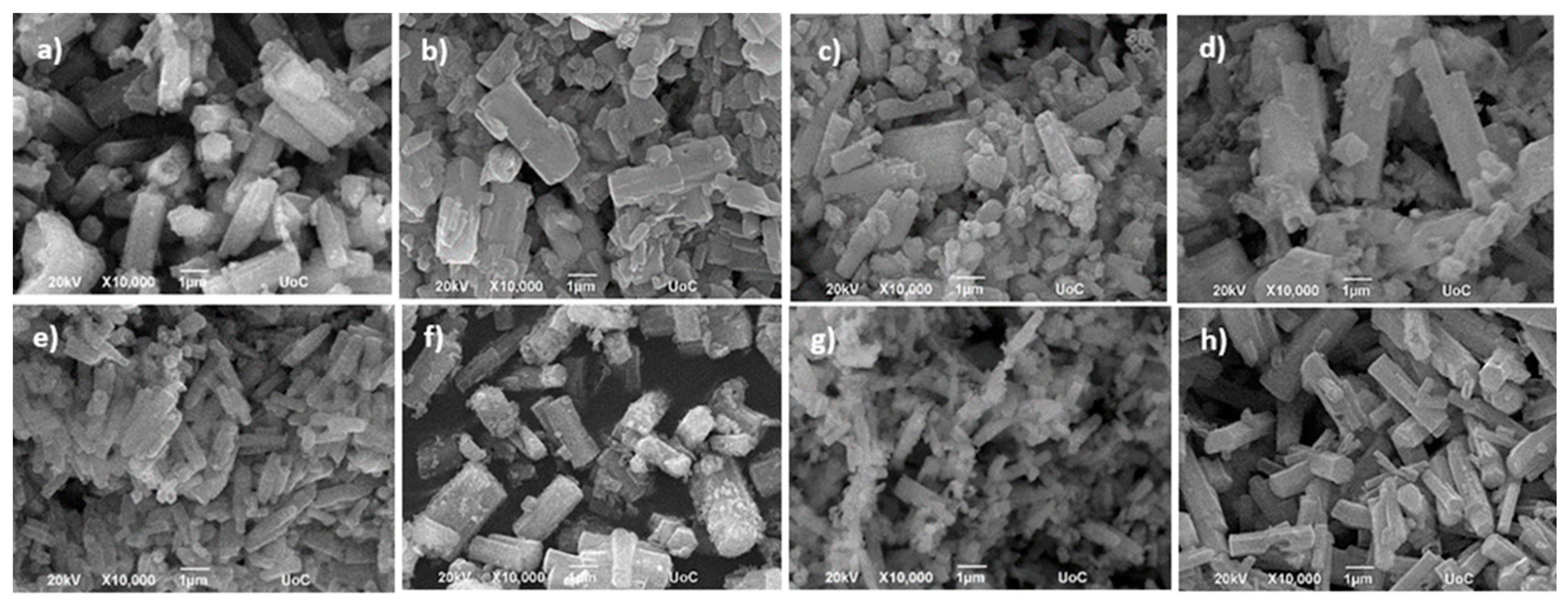
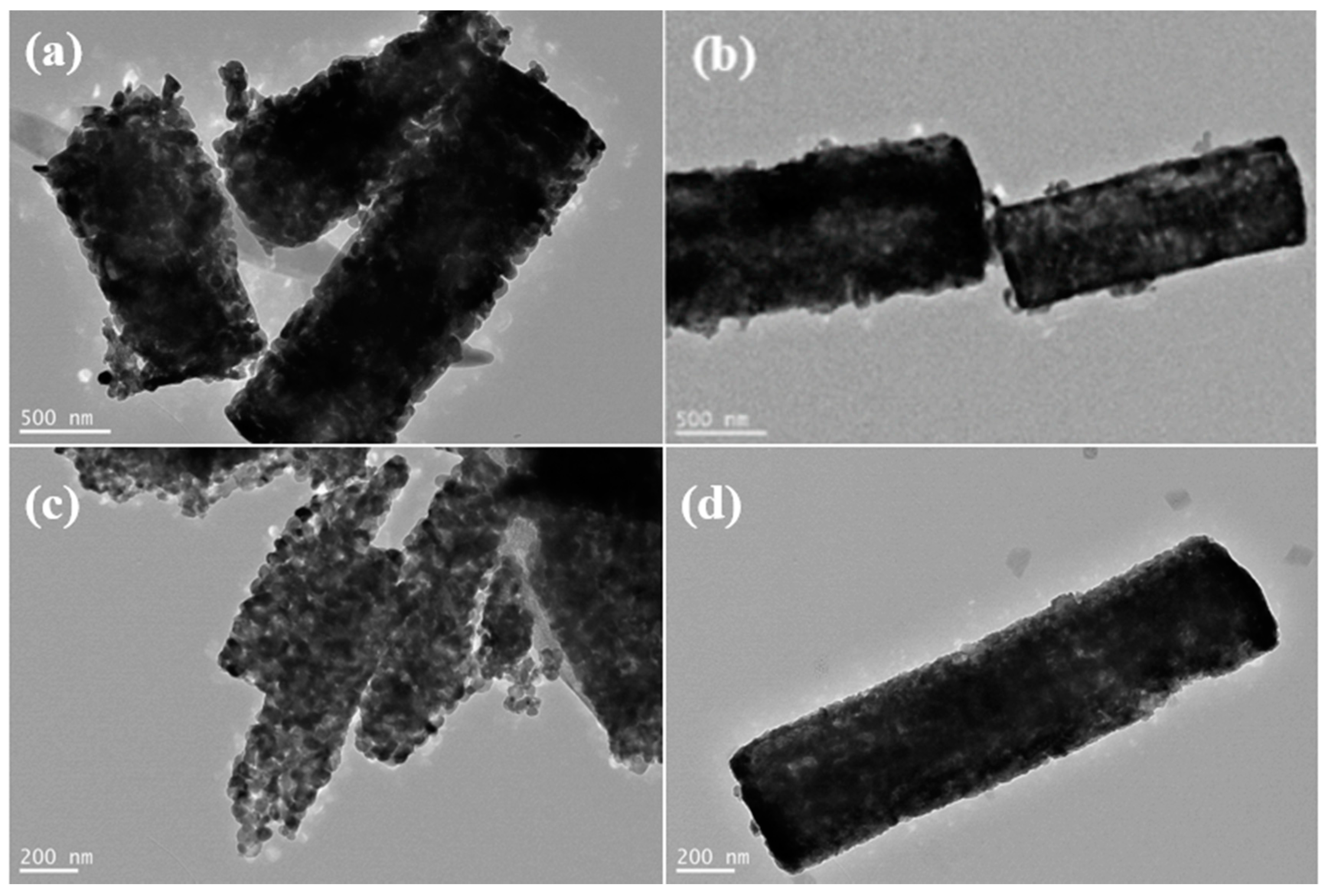
3.2. Morphological Characterization of Metal Titanate Nanorods
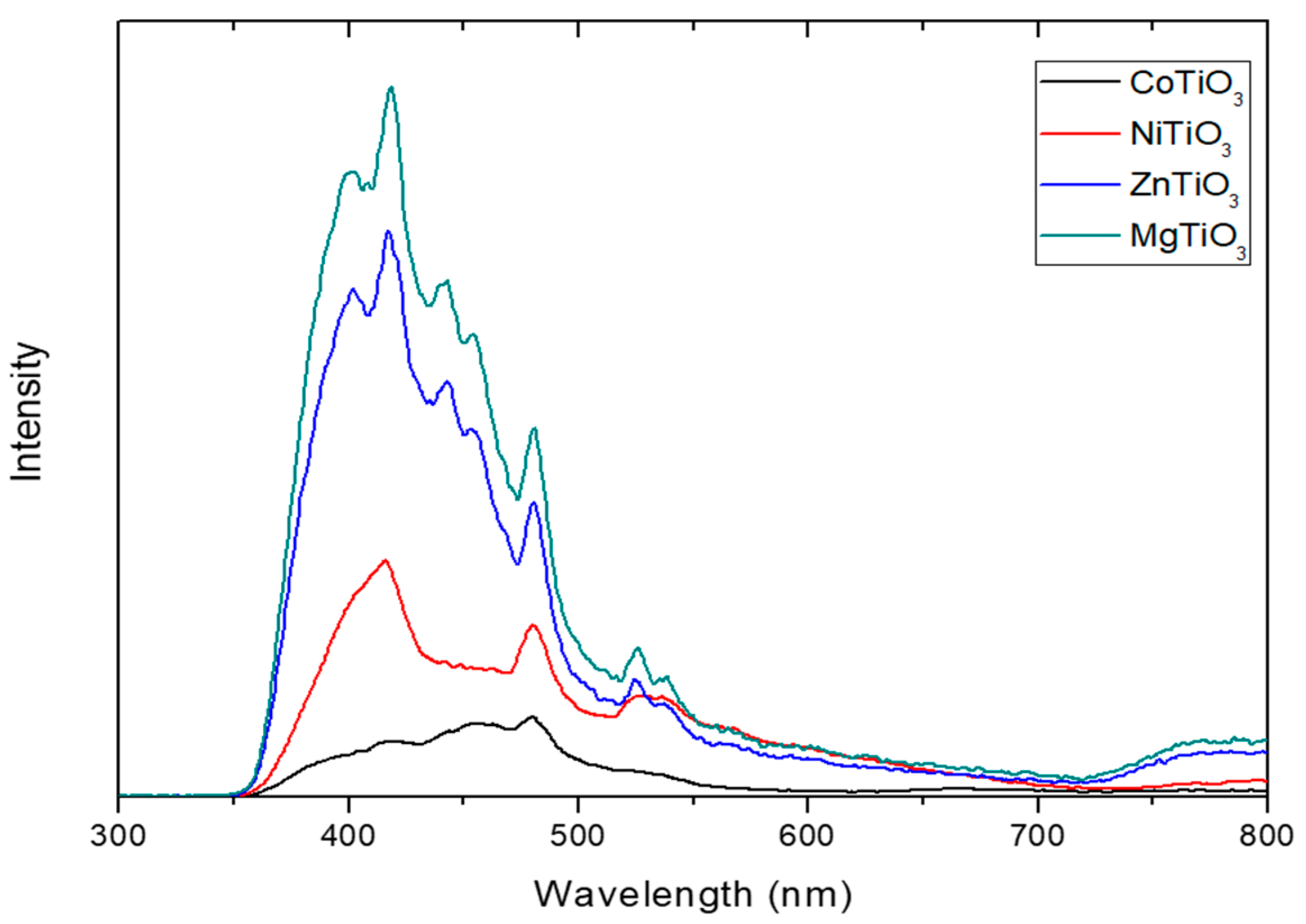
3.3. Optical Characterization of Metal Titanate Nanorods
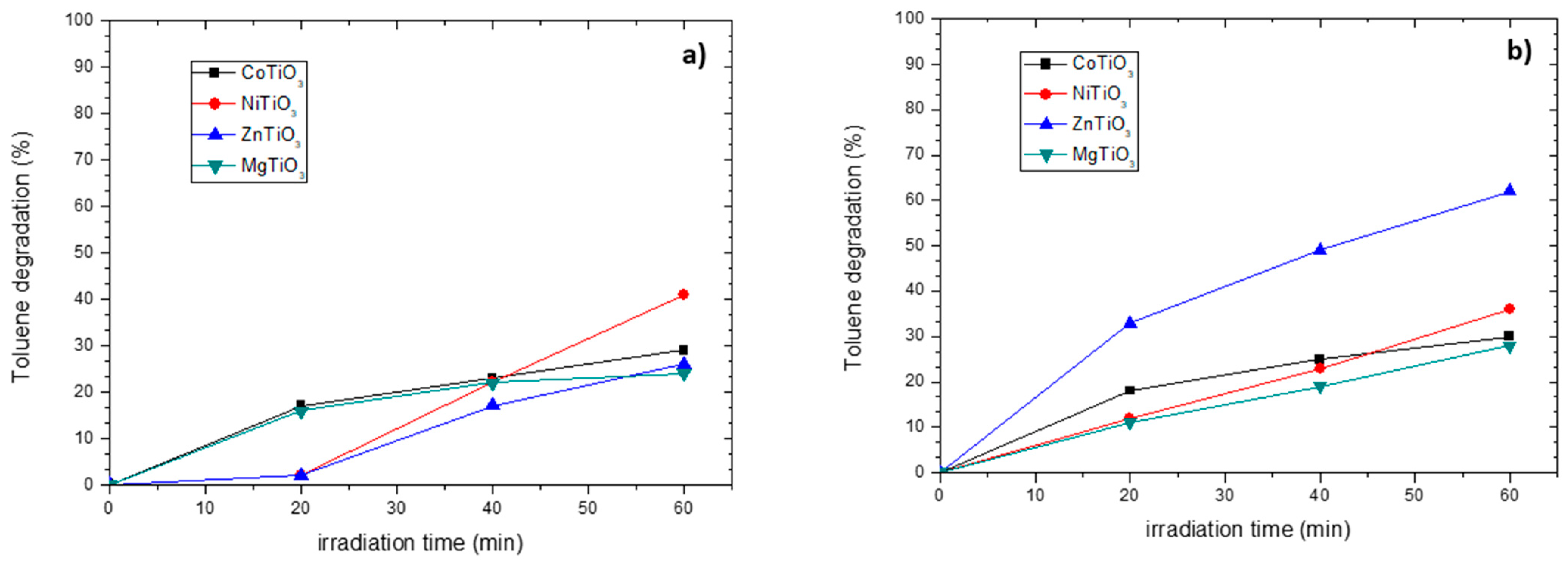
3.4. Photocatalytic Activity in Toluene Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CDC. Agency for Toxic Substances and Disease Registry Public Health Statement for Ethylbenzene; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2015. [Google Scholar]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Li, C.; Soh, K.C.K.; Wu, P. Formability of ABO3 perovskites. J. Alloy. Compd. 2004, 372, 40–48. [Google Scholar] [CrossRef]
- Ishihara, T.; Properties, T. Springer Handbook of Electronic and Photonic Materials. In Springer Handbook of Electronic and Photonic Materials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1405–1420. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Giorgi, G.; Yamashita, K. MgTaO2N Photocatalysts: Perovskite vs. Ilmenite Structure. A Theoretical Investigation. J. Phys. Chem. C 2017, 121, 27813–27821. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Dos Santos-García, A.J.; Solana-Madruga, E.; Ritter, C.; Ávila-Brande, D.; Fabelo, O.; Sáez-Puche, R. Synthesis, structures and magnetic properties of the dimorphic Mn2CrSbO6 oxide. Dalton Trans. 2015, 44, 10665–10672. [Google Scholar] [CrossRef]
- Hossain, A.; Bandyopadhyay, P.; Roy, S. An overview of double perovskites A2B′B″O6 with small ions at A site: Synthesis, structure and magnetic properties. J. Alloy. Compd. 2018, 740, 414–427. [Google Scholar] [CrossRef]
- Liu, X.; Hong, R.; Tian, C. Tolerance factor and the stability discussion of ABO3-type ilmenite. J. Mater. Sci. Mater. Electron. 2009, 20, 323–327. [Google Scholar] [CrossRef]
- Wilson, N.; Muscat, J.; Mkhonto, D.; Ngoepe, P.E.; Harrison, N. Structure and properties of ilmenite from first principles. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 075202. [Google Scholar] [CrossRef]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis of MgTiO3 Nanoparticles for Photocatalytic Applications. ChemistrySelect 2019, 4, 788–796. [Google Scholar] [CrossRef]
- Alammar, T.; Hamm, I.; Wark, M.; Mudring, A.-V. Low-temperature route to metal titanate perovskite nanoparticles for photocatalytic applications. Appl. Catal. B Environ. 2015, 178, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Wattanawikkam, C.; Kansa-Ard, T.; Pecharapa, W. X-ray absorption spectroscopy analysis and photocatalytic behavior of ZnTiO3 nanoparticles doped with Co and Mn synthesized by sonochemical method. Appl. Surf. Sci. 2019, 474, 169–176. [Google Scholar] [CrossRef]
- Wattanawikkam, C.; Phoohinkong, W.; Pecharapa, W. Structural, Optical and Magnetic Properties of Diluted Magnetic Perovskite ZnTiO3 Doped with Co and Mn Prepared by Sonochemical Method. J. Nanosci. Nanotechnol. 2017, 17, 3620–3628. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of the graphene-ZnTiO3 nanocomposite for solar light assisted photodegradation of methylene blue. J. Phys. D Appl. Phys. 2015, 48, 415305. [Google Scholar] [CrossRef]
- Debnath, A.; Srivastava, V.; Sunny, S.; Singh, S. Fabrication and characterization of metal-ferroelectric-semiconductor non-volatile memory using BaTiO3 film prepared through sol-gel process. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Adhami, S.; Esfahany, M.N.; Eränen, K.; Peurla, M.; Mäkilä, E.; Murzin, D.Y.; Salmi, T. Influence of the specific surface area and silver crystallite size of mesoporous Ag/SrTiO3 on the selectivity enhancement of ethylene oxide production. J. Chem. Technol. Biotechnol. 2019, 94, 3839–3849. [Google Scholar] [CrossRef]
- Bautista-Ruiz, J.; Chaparro, A.; Sánchez, J. Characterization of the anticorrosive properties in bismuth-titanate films obtained by the sol-gel method. J. Phys. Conf. Ser. 2019, 1386. [Google Scholar] [CrossRef]
- Boudjellal, L.; Belhadi, A.; Brahimi, R.; Boumaza, S.; Trari, M. Physical and photoelectrochemical properties of the ilmenite NiTiO3 prepared by wet chemical method and its application for O2 evolution under visible light. Mater. Sci. Semicond. Process. 2018, 75, 247–252. [Google Scholar] [CrossRef]
- Suresh, M.; Thomas, J.; Sreemoolanadhan, H.; George, C.; John, A.; Solomon, S.; Wariar, P.; Koshy, J. Synthesis of nanocrystalline magnesium titanate by an auto-igniting combustion technique and its structural, spectroscopic and dielectric properties. Mater. Res. Bull. 2010, 45, 761–765. [Google Scholar] [CrossRef]
- Liu, F.; Qu, J.; Yuan, C.; Huang, X.; Chen, G.; Wang, D. Correlation between dielectric loss, microstructures and phase structures in a novel Mgn+1TinO3n+1 microwave ceramic system. Mater. Chem. Phys. 2017, 198, 35–41. [Google Scholar] [CrossRef]
- Liang, K.; He, H.; Ren, Y.; Wang, H.; Liao, Y.; Huang, X. Porous lithium titanate nanosheets as an advanced anode material for sodium ion batteries. J. Mater. Sci. 2020, 55, 4372–4381. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, C.; Wang, J.; Chew, J.W.; Gao, G.; Pan, B.-C. Multifunctional Piezoelectric Heterostructure of BaTiO3@Graphene: Decomplexation of Cu-EDTA and Recovery of Cu. Environ. Sci. Technol. 2019, 53, 8342–8351. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Fu, H. Porous Cobalt Titanate Nanorod: A New Candidate for Visible Light-Driven Photocatalytic Water Oxidation. ChemCatChem 2014, 6, 265–270. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Ramos-Sanchez, J.E.; Camposeco, R.; Lee, S.-W.; Rodríguez-González, V. Sustainable synthesis of AgNPs/strontium-titanate-perovskite-like catalysts for the photocatalytic production of hydrogen. Catal. Today 2020, 341, 112–119. [Google Scholar] [CrossRef]
- Li, X.; Ge, Z.; Xue, F.; Liu, H.; Lyu, B.; Liu, M. Lattice-oriented contact in Pd/SrTiO3 heterojunction for rapid electron transfer during photocatalytic H2 production. Mater. Res. Bull. 2020, 123, 110722. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Zhang, Y.; Jiang, M.; Xu, S.; Liang, Q.; Li, Z. Shape-controlled synthesis of golf-like, star-like, urchin-like and flower-like SrTiO3 for highly efficient photocatalytic degradation and H2 production. J. Alloy. Compd. 2020, 817, 152796. [Google Scholar] [CrossRef]
- Chung, K.; Park, Y.; Kim, H.; Kim, B.; Jung, S. Effect of Liquid Phase Plasma Irradiation on Production by Photocatalytic Water Splitting over SrTiO3 Photocatalysts. ChemCatChem 2019, 11, 6451–6459. [Google Scholar] [CrossRef]
- Li, N.; Wu, J.; Fang, H.-B.; Zhang, X.-H.; Zheng, Y.-Z.; Tao, X. Au-nanorod-anchored {0 0 1} facets of Bi4Ti3O12 nanosheets for enhanced visible-light-driven photocatalysis. Appl. Surf. Sci. 2018, 448, 41–49. [Google Scholar] [CrossRef]
- El Rouby, W.; Comesana-Hermo, M.; Testa-Anta, M.; Carbó-Argibay, E.; Salgueiriño, V.; Pérez-Lorenzo, M.; Correa-Duarte, M.A. Au-decorated sodium titanate nanotubes as high-performance selective photocatalysts for pollutant degradation. J. Phys. D Appl. Phys. 2017, 50, 144002. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef]
- Selvarajan, S.; Malathy, P.; Suganthi, A.; Rajarajan, M. Fabrication of mesoporous BaTiO3/SnO2 nanorods with highly enhanced photocatalytic degradation of organic pollutants. J. Ind. Eng. Chem. 2017, 53, 201–212. [Google Scholar] [CrossRef]
- da Silva, L.; Lopes, O.; de Mendonça, V.; Carvalho, K.T.G.; Longo, E.; Ribeiro, C.; Mastelaro, V.R. An Understanding of the Photocatalytic Properties and Pollutant Degradation Mechanism of SrTiO3 Nanoparticles. Photochem. Photobiol. 2016, 92, 371–378. [Google Scholar] [CrossRef]
- Naudin, G.; Entradas, T.; Barrocas, B.; Monteiro, O. Titanate Nanorods Modified with Nanocrystalline ZnS Particles and Their Photocatalytic Activity on Pollutant Removal. J. Mater. Sci. Technol. 2016, 32, 1122–1128. [Google Scholar] [CrossRef]
- Patil, R.; Hiragond, C.; Jain, G.; Khanna, P.K.; Gaikwad, V.; More, P.V. La doped BaTiO3 nanostructures for room temperature sensing of NO2/NH3: Focus on La concentration and sensing mechanism. Vacuum 2019, 166, 37–44. [Google Scholar] [CrossRef]
- Wang, J.; Liu, A.; Wang, C.; You, R.; Liu, F.; Li, S.; Yang, Z.; He, J.; Yan, X.; Sun, P.; et al. Solid state electrolyte type gas sensor using stabilized zirconia and MTiO3 (M: Zn, Co and Ni)-SE for detection of low concentration of SO2. Sens. Actuators B Chem. 2019, 296, 126644. [Google Scholar] [CrossRef]
- Chen, Y.-B. Dielectric properties and crystal structure of Mg2TiO4 ceramics substituting Mg2+ with Zn2+ and Co2+. J. Alloy. Compd. 2012, 513, 481–486. [Google Scholar] [CrossRef]
- Al-Hobaib, A.; El Ghoul, J.; El Mir, L. Fabrication of polyamide membrane reached by MgTiO3 nanoparticles for ground water purification. Desalination Water Treat. 2016, 57, 8639–8648. [Google Scholar] [CrossRef]
- Hao, F.X.; Zhang, C.; Liu, X.; Yin, Y.W.; Sun, Y.Z.; Li, X.G. Photovoltaic effect in YBa2Cu3O7−δ/Nb-doped SrTiO3 heterojunctions. Appl. Phys. Lett. 2016, 109, 131104. [Google Scholar] [CrossRef]
- Sharma, S.; Tomar, M.; Kumar, A.; Puri, N.; Gupta, V. Photovoltaic effect in BiFeO3/BaTiO3 multilayer structure fabricated by chemical solution deposition technique. J. Phys. Chem. Solids 2016, 93, 63–67. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, V.; Tomar, M. Ferroelectric PZT thin films for photovoltaic application. Mater. Sci. Semicond. Process. 2020, 105, 104723. [Google Scholar] [CrossRef]
- Sidheswaran, M.; Tavlarides, L.L. Visible Light Photocatalytic Oxidation of Toluene Using a Cerium-Doped Titania Catalyst. Ind. Eng. Chem. Res. 2008, 47, 3346–3357. [Google Scholar] [CrossRef]
- Tasbihi, M.; Lavrencic Stangar, U.; Černigoj, U.; Jirkovsky, J.; Bakardjieva, S.; Novak Tušar, N. Photocatalytic oxidation of gaseous toluene on titania/mesoporous silica powders in a fluidized-bed reactor. Catal. Today 2011, 161, 181–188. [Google Scholar] [CrossRef]
- Shu, Y.; Xu, Y.; Huang, H.; Ji, J.; Liang, S.; Wu, M.; Leung, D.Y. Catalytic oxidation of VOCs over Mn/TiO2/activated carbon under 185 nm VUV irradiation. Chemosphere 2018, 208, 550–558. [Google Scholar] [CrossRef]
- Guo, C.; Wu, X.; Yan, M.; Dong, Q.; Yin, S.; Sato, T.; Liu, S. The visible-light driven photocatalytic destruction of NOx using mesoporous TiO2 spheres synthesized via a “water-controlled release process”. Nanoscale 2013, 5, 8184–8191. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhao, W.; Guan, B. Photocatalytic oxidation of nitrogen oxides using TiO2 loading on woven glass fabric. Chemosphere 2007, 66, 185–190. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Chen, Z.; Veksha, A.; Lisak, G.; You, C. Interaction between SO2 and NO in their adsorption and photocatalytic conversion on TiO2. Chemosphere 2020, 249, 126136. [Google Scholar] [CrossRef]
- Vildozo, D.; Portela, R.; Ferronato, C.; Chovelon, J.-M. Photocatalytic oxidation of 2-propanol/toluene binary mixtures at indoor air concentration levels. Appl. Catal. B Environ. 2011, 107, 347–354. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Huang, Y.; Yao, J.; Li, Y.; Lee, S.-C.; Ho, W.; Huang, T.; Chen, K. Synthesis and characterization of Bi-BiPO4 nanocomposites as plasmonic photocatalysts for oxidative NO removal. Appl. Surf. Sci. 2020, 513, 145775. [Google Scholar] [CrossRef]
- Rao, F.; Zhu, G.; Wang, M.; Zubairu, S.M.; Peng, J.; Gao, J.; Hojamberdiev, M. Constructing the Pd/PdO/β-Bi2O3 microspheres with enhanced photocatalytic activity for Bisphenol A degradation and NO removal. J. Chem. Technol. Biotechnol. 2019, 95, 862–874. [Google Scholar] [CrossRef]
- Wang, C.-B.; Tang, C.-W.; Tsai, H.-C.; Chien, S.-H. Characterization and Catalytic Oxidation of Carbon Monoxide Over Supported Cobalt Catalysts. Catal. Lett. 2006, 107, 223–230. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Li, P.; Zhang, Y.; Kako, T.; Ye, J. A new heterojunction Ag3PO4/Cr-SrTiO3 photocatalyst towards efficient elimination of gaseous organic pollutants under visible light irradiation. Appl. Catal. B Environ. 2013, 134–135, 286–292. [Google Scholar] [CrossRef]
- Irie, H.; Maruyama, Y.; Hashimoto, K. Ag+- and Pb2+-Doped SrTiO3 Photocatalysts. A Correlation Between Band Structure and Photocatalytic Activity. J. Phys. Chem. C 2007, 111, 1847–1852. [Google Scholar] [CrossRef]
- Miyauchi, M.; Takashio, M.; Tobimatsu, H. Photocatalytic Activity of SrTiO3 Codoped with Nitrogen and Lanthanum under Visible Light Illumination. Langmuir 2004, 20, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, S.; Li, J.; Chen, J.; Rui, Z. Promotion effect of strong metal-support interaction to thermocatalytic, photocatalytic, and photothermocatalytic oxidation of toluene on Pt/SrTiO3. Chemosphere 2020, 249, 126096. [Google Scholar] [CrossRef]
- Ji, W.; Shen, T.; Kong, J.; Rui, Z.; Tong, Y. Synergistic Performance between Visible-Light Photocatalysis and Thermocatalysis for VOCs Oxidation over Robust Ag/F-Codoped SrTiO3. Ind. Eng. Chem. Res. 2018, 57, 12766–12773. [Google Scholar] [CrossRef]
- Marchelek, M.; Grabowska, E.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Various types of semiconductor photocatalysts modified by CdTe QDs and Pt NPs for toluene photooxidation in the gas phase under visible light. Appl. Surf. Sci. 2017, 393, 262–275. [Google Scholar] [CrossRef]
- Suárez-Vázquez, S.; Gil, S.; García-Vargas, J.; Cruz-López, A.; Giroir-Fendler, A. Catalytic oxidation of toluene by SrTi1-XBXO3 (B = Cu and Mn) with dendritic morphology synthesized by one pot hydrothermal route. Appl. Catal. B Environ. 2018, 223, 201–208. [Google Scholar] [CrossRef]
- Liferovich, R.P.; Mitchell, R.H. Geikielite–ecandrewsite solid solutions: Synthesis and crystal structures of the Mg1−x ZnxTiO3 (0 ≤ x ≤ 0.8) series. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D.; Prewitt, C.T. Revised values of effective ionic radii. Acta Cryst. Sect. B Struct. Cryst. Cryst. Chem. 1970, 26, 1046–1048. [Google Scholar] [CrossRef]
- Busca, G.; Ramis, G.; Escribano, V.S.; Piaggio, P. FT Raman and FTIR studies of titanias and metatitanate powders. J. Chem. Soc. Faraday Trans. 1994, 90, 3181–3190. [Google Scholar] [CrossRef]
- Raveendra, R.S.; Prashanth, P.A.; Hari Krishna, R.; Bhagya, N.P.; Sathyanarayani, S.; Na-gabhushana, B.M. Carbothermal Synthesis and Photoluminescence Characteristics of Pure Un-doped ZnTiO3 Nanocrystals. J. Adv. Phys. Sci. 2016, 6, 4–7. [Google Scholar]
- Chai, Y.-L.; Chang, Y.-S.; Chen, G.-J.; Hsiao, Y.-J. The effects of heat-treatment on the structure evolution and crystallinity of ZnTiO3 nano-crystals prepared by Pechini process. Mater. Res. Bull. 2008, 43, 1066–1073. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, J.; Liu, J.; Liu, L.; Liang, S. Facile synthesis of cobalt vanadium oxides and their applications in lithium batteries. Int. J. Electrochem. Sci. 2013, 8, 1138–1145. [Google Scholar]
- Lakhera, S.K.; Hafeez, H.Y.; Veluswamy, P.; Ganesh, V.; Khan, A.; Ikeda, H.; Neppolian, B. Enhanced photocatalytic degradation and hydrogen production activity of in situ grown TiO2 coupled NiTiO3 nanocomposites. Appl. Surf. Sci. 2018, 449, 790–798. [Google Scholar] [CrossRef]
- Murcia-López, S.; Moschogiannaki, M.; Binas, V.; Andreu, T.; Tang, P.; Arbiol, J.; Biendicho, J.J.; Kiriakidis, G.; Morante, J.R. Insights into the Performance of CoxNi1–xTiO3 Solid Solutions as Photocatalysts for Sun-Driven Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 40290–40297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materials Data on MgTiO3 (SG:148) by Materials Project. Available online: https://materialsproject.org/materials/mp-3771/ (accessed on 12 November 2021). [CrossRef]
- Li, M.-W.; Gao, X.-M.; Hou, Y.-L.; Wang, C.-Y. Characterization of CoTiO3 Nanocrystallites Prepared by Homogeneous Precipitation Method. J. Nano Electron. Phys. 2013, 5, 03022. [Google Scholar]
- Li, Y.; Xu, J.; Peng, M.; Liu, Z.; Li, X.; Zhao, S. MoS2/NiTiO3 Heterojunctions as Photocatalysts: Improved Charge Separation for Promoting Photocatalytic Hydrogen Production Activity. Catal. Surv. Asia 2019, 23, 277–289. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.; Medina, F.; González, S. DFT Study of Methylene Blue Adsorption on ZnTiO3 and TiO2 Surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef]
- Bellam, J.B.; Ruiz-Preciado, M.A.; Edely, M.; Szade, J.; Jouanneaux, A.; Kassiba, A.H. Visible-light photocatalytic activity of nitrogen-doped NiTiO3 thin films prepared by a co-sputtering process. RSC Adv. 2015, 5, 10551–10559. [Google Scholar] [CrossRef]
- Wangkawong, K.; Suntalelat, S.; Tantraviwat, D.; Inceesungvorn, B. Novel CoTiO3/Ag3VO4 Composite: Synthesis, Characterization and Visible-light-driven Photocatalytic Activity. Mater. Lett. 2014, 133, 119–122. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Kaviyarasan, K.; Sivasankar, T.; Emeline, A.; Bahnemann, D.; Mangalaraja, R.; Anandan, S. Sonochemical synthesis of porous NiTiO3 nanorods for photocatalytic degradation of ceftiofur sodium. Ultrason. Sonochem. 2017, 35, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.L.; Rivera-García, M.T.; Woodward, J.J. Review of Toluene Actions: Clinical Evidence, Animal Studies, and Molecular Targets. J. Drug Alcohol Res. 2014, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
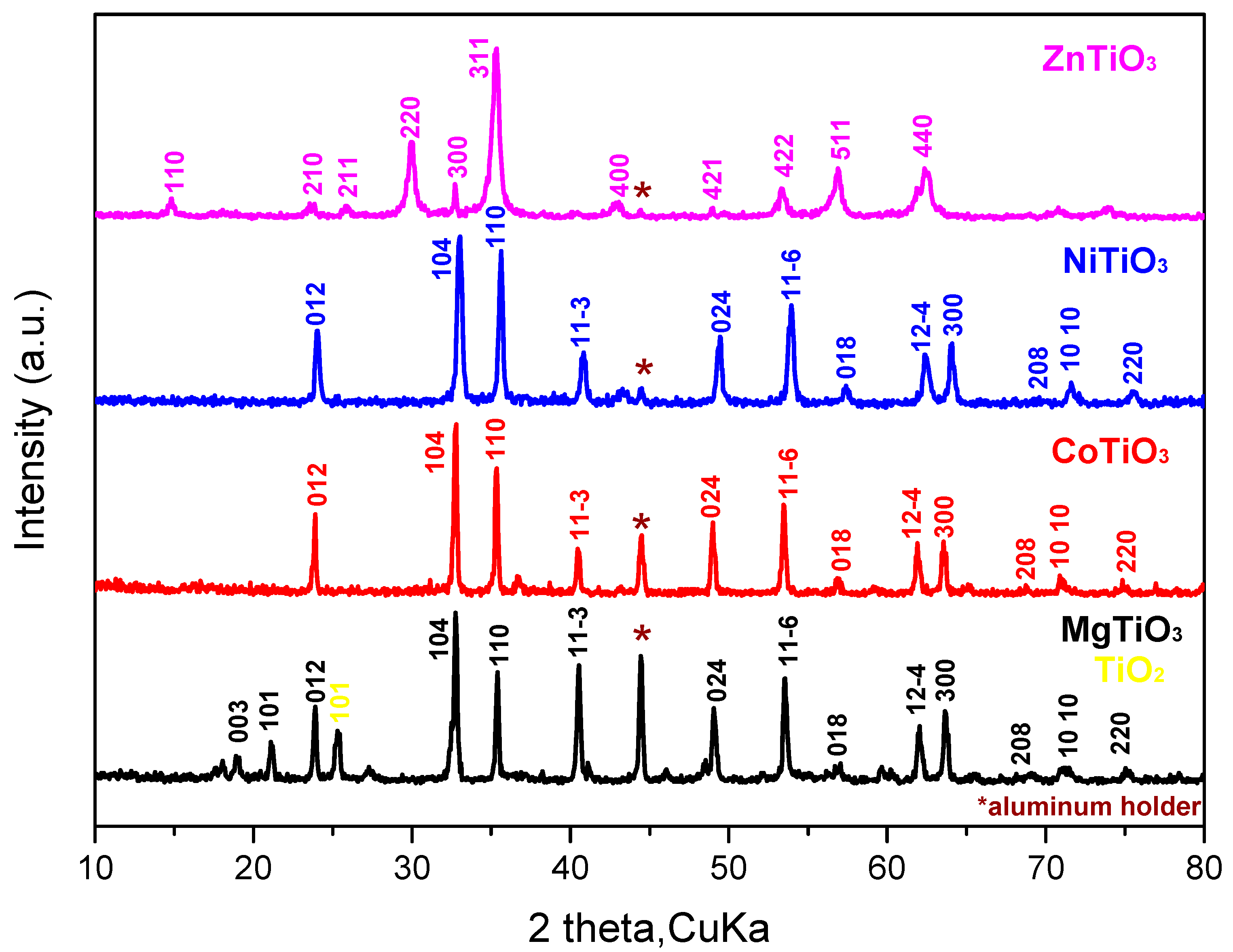
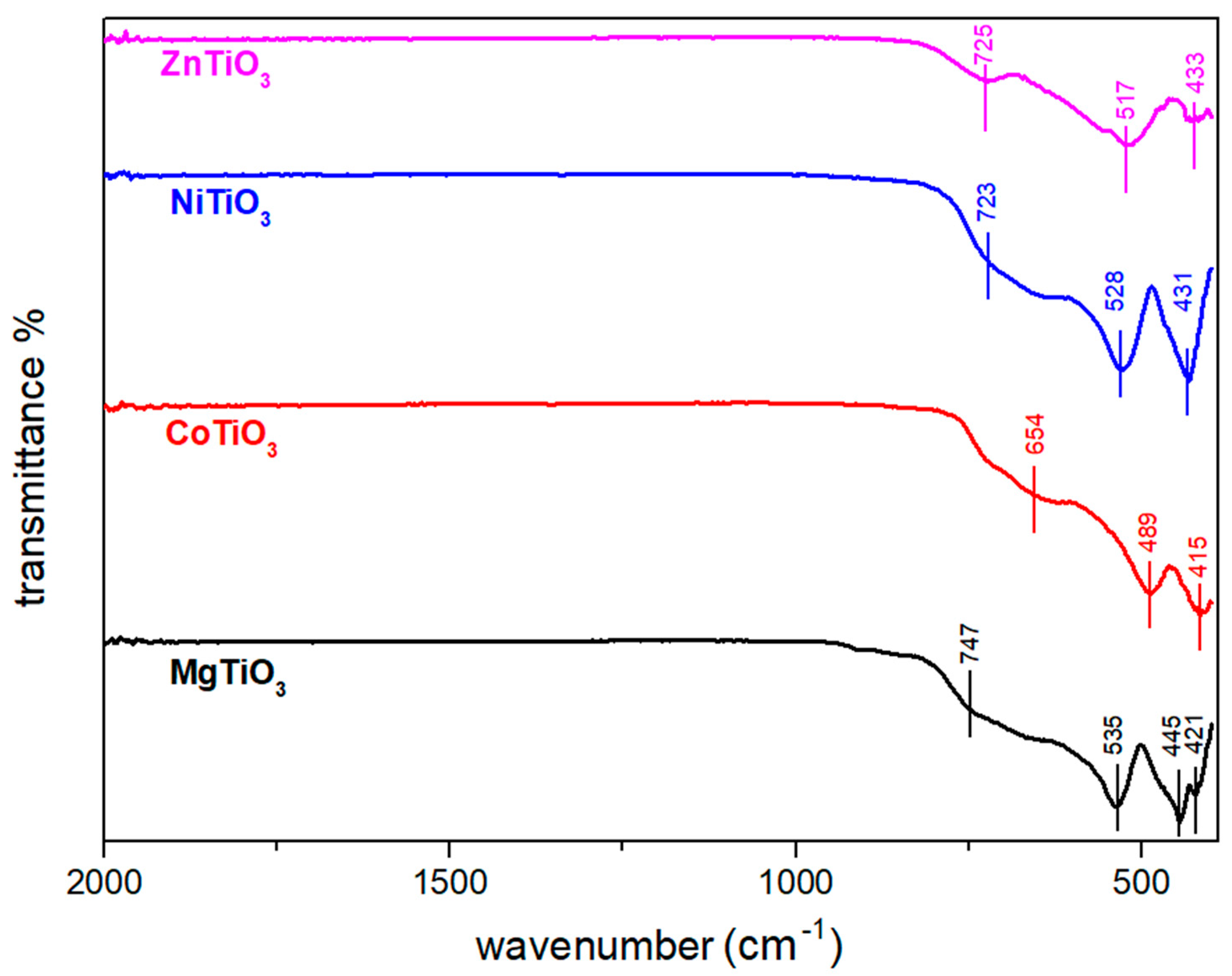
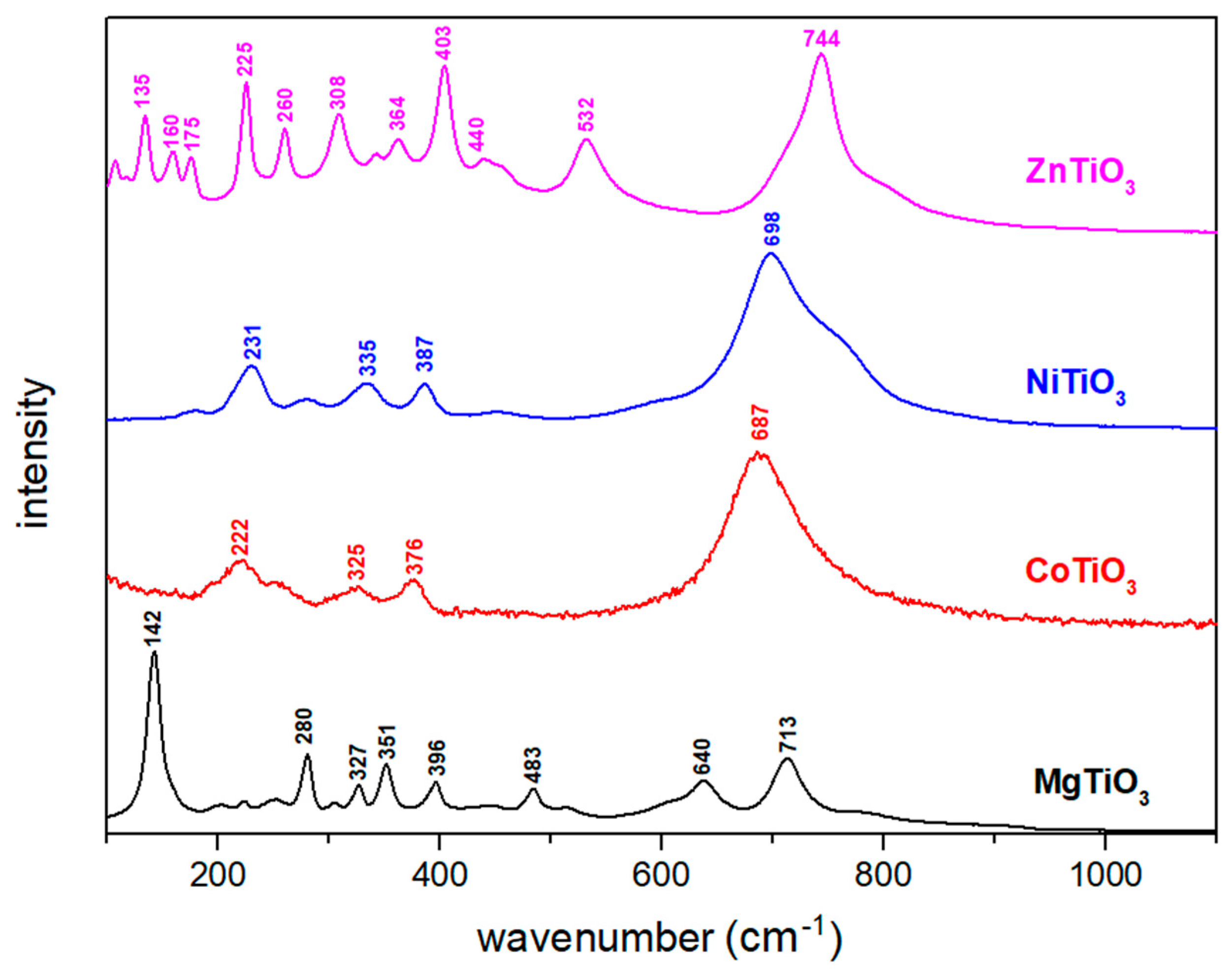
| Samples | Lattice Parameters (Å) | Volume Cell (Å3) | Crystallite Size (nm) | Ionic Radius of A-Site Metal (Å) | Tolerance Factor | ||
|---|---|---|---|---|---|---|---|
| a (ath) | b (bth) | c (cth) | |||||
| NiTiO3 | 5.0229 (5.0312) | 5.0229 (5.0312) | 12.9012 (13.7881) | 976.48 | 19.28 | 0.69 | 0.73 |
| MgTiO3 | 5.0465 (5.0548) | 5.0465 (5.0548) | 13.8892 (13.8992) | 1061.17 | 19.50 | 0.72 | 0.75 |
| CoTiO3 | 5.0579 (5.0662) | 5.0579 (5.0662) | 13.9177 (13.9180) | 1068.14 | 28.50 | 0.74 | 0.76 |
| ZnTiO3 | 8.4044 (8.4080) | 8.4044 (8.4080) | 8.4044 (8.4080) | 593.65 | 10.40 | 1.39 | 0.99 |
| Sample | Energy Gap (eV) | Energy Gap (eV) Data from Literature |
|---|---|---|
| MgTiO3 | 3.15 | 3.51 [70] |
| CoTiO3 | 2.79 | 2.53 [71] |
| NiTiO3 | 2.6 | 2.33 [72] |
| ZnTiO3 | 3.5 | 3.16 [73] |
| Photocatalyst | k (10−3 min−1) under λmax = 375 nm | k (10−3 min−1) under λmax = 400 nm |
|---|---|---|
| NiTiO3 | 9.21 | 7.44 |
| MgTiO3 | 4.43 | 5.47 |
| CoTiO3 | 5.59 | 5.72 |
| ZnTiO3 | 5.37 | 16.08 |
| λ = 375 nm Illumination | λ = 400 nm Illumination | |||||
|---|---|---|---|---|---|---|
| Photocatalyst | Mass (mg) | Toluene Degradation (%) | Photoactivity per Mass (%/mg) | Mass (mg) | Toluene Degradation (%) | Photoactivity per Mass (%/mg) |
| NiTiO3 | 16.6 | 41 | 2.5 | 13.2 | 36 | 2.7 |
| MgTiO3 | 13.1 | 24 | 1.8 | 19.8 | 28 | 1.4 |
| CoTiO3 | 13.2 | 29 | 2.2 | 36.4 | 30 | 0.8 |
| ZnTiO3 | 30.8 | 26 | 0.8 | 4.1 | 62 | 15.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souri, A.P.; Andrigiannaki, N.; Moschogiannaki, M.; Faka, V.; Kiriakidis, G.; Malankowska, A.; Zaleska-Medynska, A.; Binas, V. Metal Titanate (ATiO3, A: Ni, Co, Mg, Zn) Nanorods for Toluene Photooxidation under LED Illumination. Appl. Sci. 2021, 11, 10850. https://doi.org/10.3390/app112210850
Souri AP, Andrigiannaki N, Moschogiannaki M, Faka V, Kiriakidis G, Malankowska A, Zaleska-Medynska A, Binas V. Metal Titanate (ATiO3, A: Ni, Co, Mg, Zn) Nanorods for Toluene Photooxidation under LED Illumination. Applied Sciences. 2021; 11(22):10850. https://doi.org/10.3390/app112210850
Chicago/Turabian StyleSouri, Anna P., Natalia Andrigiannaki, Marilena Moschogiannaki, Vasiliki Faka, George Kiriakidis, Anna Malankowska, Adriana Zaleska-Medynska, and Vassilios Binas. 2021. "Metal Titanate (ATiO3, A: Ni, Co, Mg, Zn) Nanorods for Toluene Photooxidation under LED Illumination" Applied Sciences 11, no. 22: 10850. https://doi.org/10.3390/app112210850
APA StyleSouri, A. P., Andrigiannaki, N., Moschogiannaki, M., Faka, V., Kiriakidis, G., Malankowska, A., Zaleska-Medynska, A., & Binas, V. (2021). Metal Titanate (ATiO3, A: Ni, Co, Mg, Zn) Nanorods for Toluene Photooxidation under LED Illumination. Applied Sciences, 11(22), 10850. https://doi.org/10.3390/app112210850









