Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update
Abstract
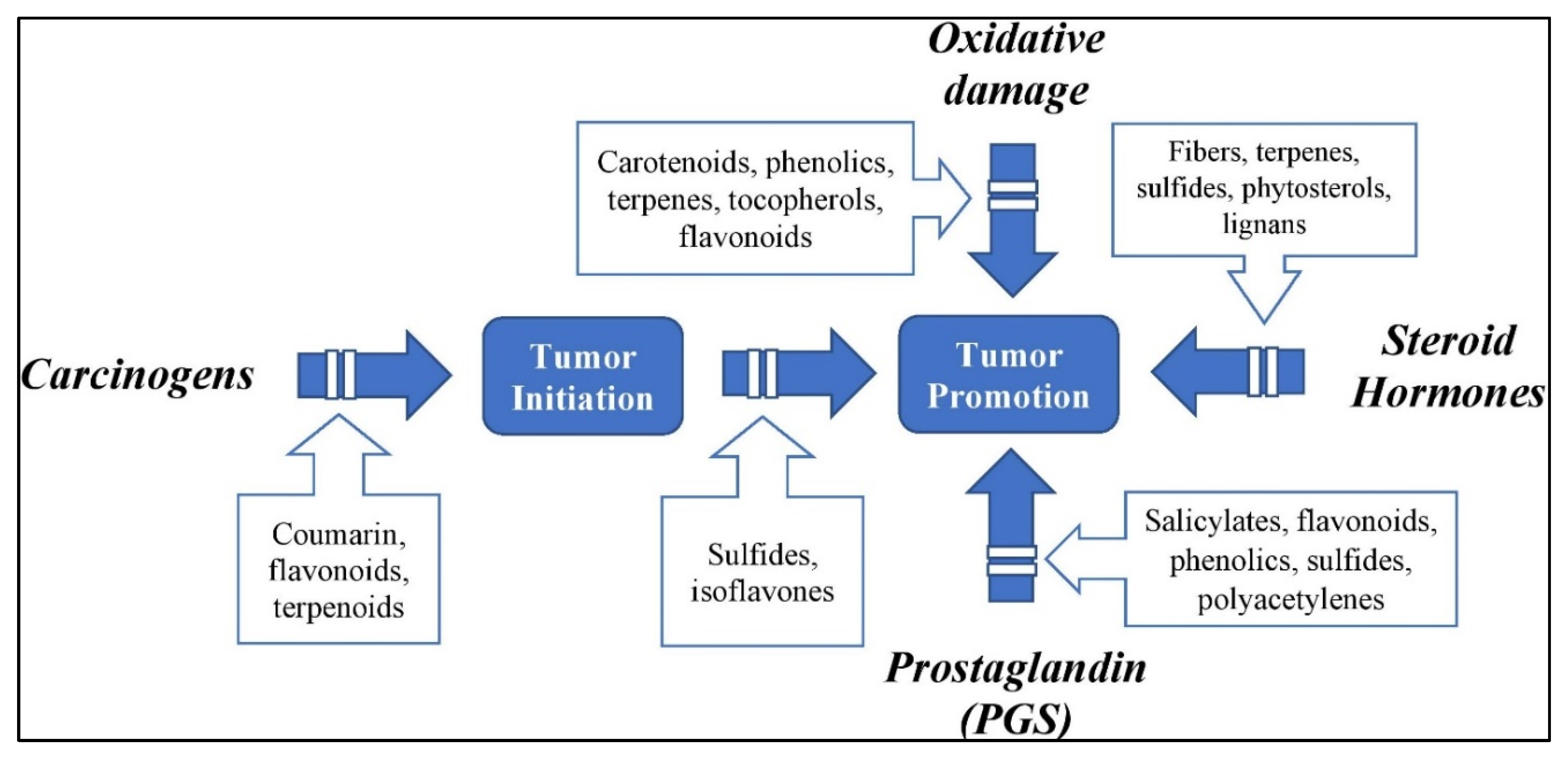
:1. Introduction
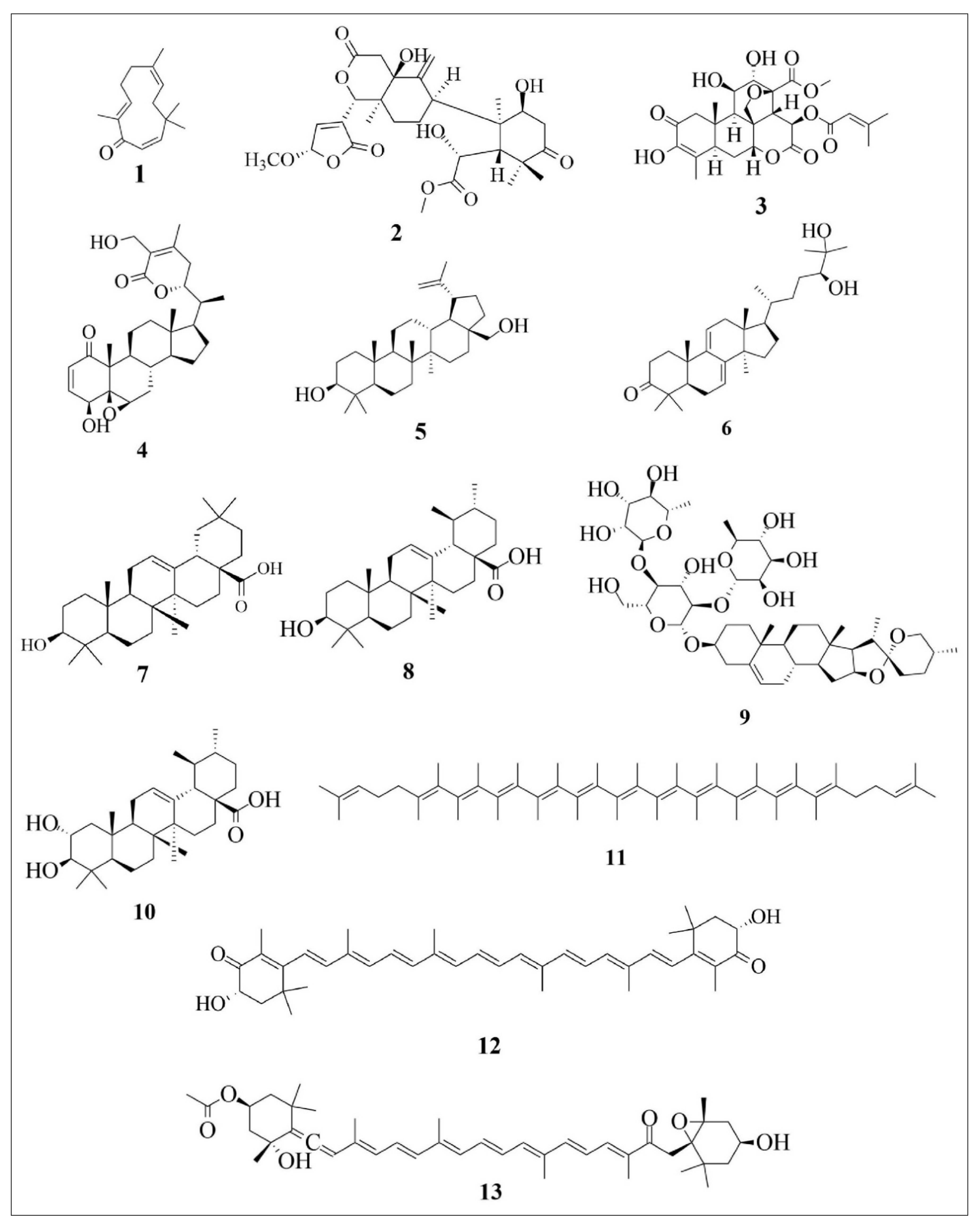
2. Terpenoids
| Compound Number | Compound Name | Chemical Formula | Sub-Chemical Class | Major Chemical Class |
|---|---|---|---|---|
| 1 | Zerumbone | C15H22O | Sesquiterpenoid | Sesquiterpenoid |
| 2 | Khayandirobilide A | C22H27O11 | Andirobin-type limonoid | Triterpenoid |
| 3 | Brusatol | C26H32O11 | Quassinoid compound | Triterpenoid |
| 4 | Withaferin A | C28H38O6 | Steroid | Triterpenoid |
| 5 | Betulin | C30H50O2 | Steroid | Triterpenoid |
| 6 | Ganodermanondiol | C30H48O3 | Steroid | Triterpenoid |
| 7 | Oleanolic acid | C30H48O3 | Steroid | Triterpenoid |
| 8 | Ursolic acid | C30H48O3 | Steroid | Triterpenoid |
| 9 | Dioscin | C45H72O16 | Saponin | Triterpenoid |
| 10 | Corosolic acid | C30H48O4 | Steroid | Triterpene acid |
| 11 | Lycopene | C40H56 | Carotenoid | Tetraterpenoid |
| 12 | Astaxanthin | C40H52O4 | Carotenoid | Tetraterpenoid |
| 13 | Fucoxanthin | C42H58O6 | Carotenoid | Tetraterpenoid |
| Compound Number | Compound Name | Common Dietary Sources | Scientific Name | References |
|---|---|---|---|---|
| 1 | Zerumbone | Ginger | Zingiber zerumbet Smith. | [39] |
| 2 | Khayandirobilide A | Stem barks, fruits, and leaves of African mahogany | Khaya senegalensis | [40] |
| 3 | Brusatol | Dried ripe fruits of Brucea javanica | Brucea javanica | [41] |
| Seeds of Brucea sumatrana | Brucea sumatrana | [42] | ||
| 4 | Withaferin A | Ashwagandha | Withania somnifera | [43] |
| 5 | Betulin | Silver Birch tree bark | Betula pendula | [44] |
| 6 | Ganodermanondiol | Lingzhi mushrooms | Ganoderma lucidum | [45] |
| 7 | Oleanolic acid | Olive leaves | Olea europaea | [46] |
| Jujube | Ziziphus jujube Mill. | |||
| Ginseng | Panex sp. | |||
| 8 | Ursolic acid | Apple peels | Malus domestica | [47] |
| 9 | Dioscin | Leaves and rhizomes of plants from Dioscoreaceae family | Dioscorea opposita | [48] |
| Dioscorea alata | ||||
| Dioscorea japonica | ||||
| 10 | Corosolic acid | Kosam | Schisandra chinensis | [49] |
| Loquat | Eriobotrya japonica | |||
| Banaba | Lagerstroemia speciosa L. | |||
| Java tea | Orthosiphon stamineus | |||
| Java tea | Orthosiphon aristatus | |||
| Korean weigela | Weigela subsessilis | |||
| 11 | Lycopene | Papaya | Carica papaya | [50] |
| Tomato | Solanum lycopersicum | |||
| Watermelon | Citrullus lanatus | |||
| 12 | Astaxanthin | Salmon | Salmo salar | [51] |
| Trout | Oncorhynchus mykiss | |||
| 13 | Fucoxanthin | Microalgae | Phaeodactylum tricornutum | [52] |
| seaweeds | Undaria pinnatifida |
3. Keap1-Nrf2-ARE Signaling System
4. Role of Dietary Terpenoids in Modulating the Keap1-Nrf2-ARE Signaling System
4.1. Zerumbone (ZB)
4.2. Khayandirobilide A (KLA)
4.3. Brusatol (BT)
4.4. Withaferin A (WFA)
4.5. Betulin (BE)
4.6. Ganodermanondiol (GD)
4.7. Oleanolic Acid (OA)
4.8. Ursolic Acid (UA)
4.9. Dioscin (DC)
4.10. Corosolic acid (CA)
4.11. Lycopene (LP)
4.12. Astaxanthin (AX)
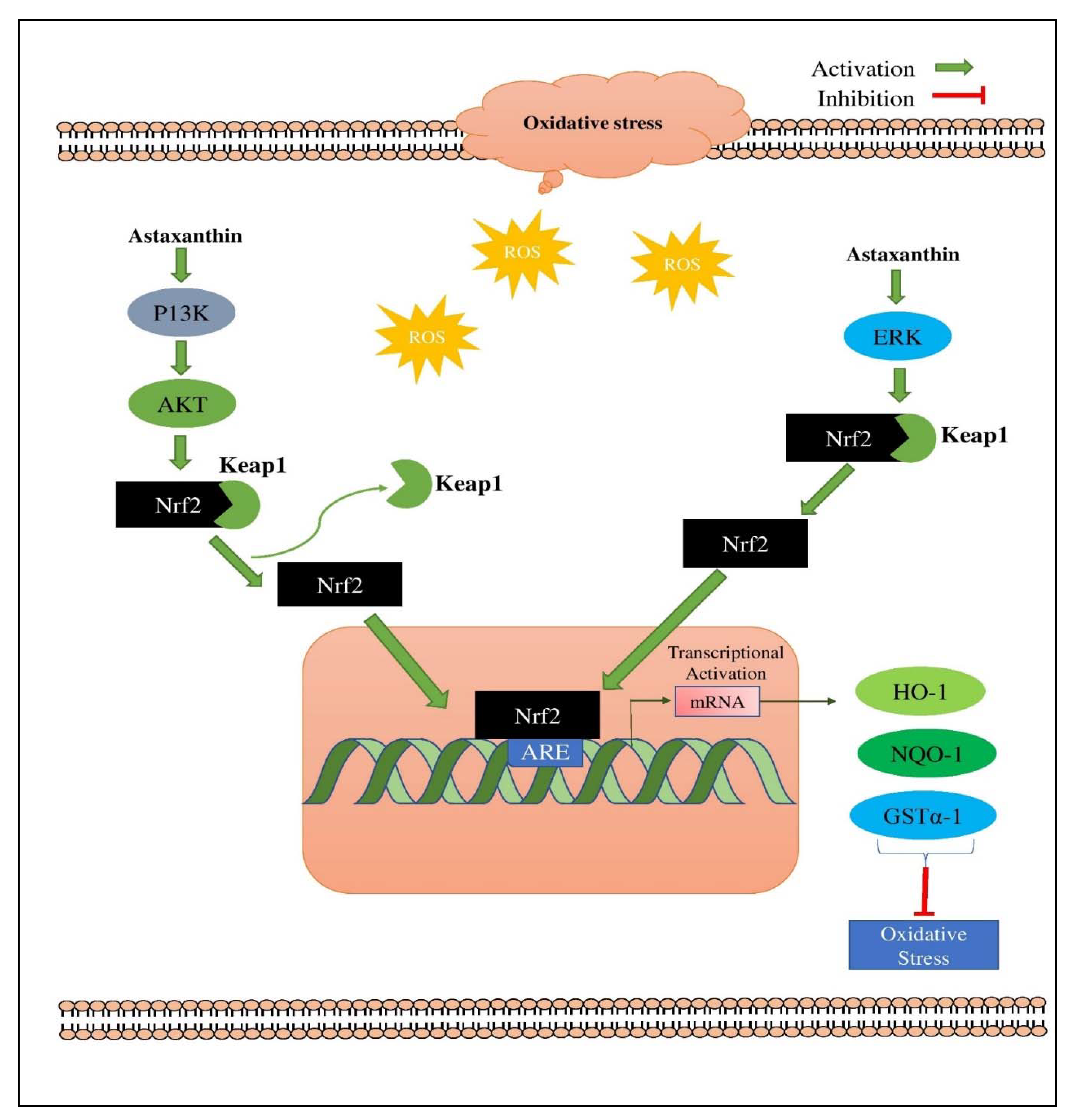
4.13. Fucoxanthin (FX)
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Spira, A.; Garber, J.E.; Szabo, E.; Lee, J.J.; Dong, Z.; Dannenberg, A.J.; Hait, W.N.; Blackburn, E.; Davidson, N.E. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev. Res. 2016, 9, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Beeken, R.; Williams, K.; Wardle, J.; Croker, H. “What about diet?” A qualitative study of cancer survivors’ views on diet and cancer and their sources of information. Eur. J. Cancer Care 2016, 25, 774–783. [Google Scholar] [CrossRef]
- Kłósek, M.; Kuropatnicki, A.K.; Szliszka, E.; Korzonek-Szlacheta, I.; Król, W. Chalcones Target the Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand (TRAIL) Signaling Pathway for Cancer Chemoprevention. In Nutrition and Functional Foods for Healthy Aging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–244. [Google Scholar]
- Ruddon, R.W. Cancer Biology; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse screening methods to search for the protein targets of chemopreventive compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517. [Google Scholar] [PubMed]
- Rather, R.A.; Bhagat, M. Cancer chemoprevention and piperine: Molecular mechanisms and therapeutic opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, X.; Saud, S.M.; Zhang, X.; Li, W.; Hua, B. Protective effect of Shaoyao Decoction against colorectal cancer via the Keap1–Nrf2–ARE signaling pathway. J. Ethnopharmacol. 2019, 241, 111981. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Papagiannakopoulos, T. The pleiotropic role of the KEAP1/NRF2 pathway in cancer. Annu. Rev. Cancer Biol. 2020, 4, 413–435. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Liang, X.; Huang, Y.; Lan, Z.; Zhang, Z.; Su, Z.; Fang, Z.; Lai, Y.; Yao, W.; Liu, T. p62 promotes proliferation, apoptosis-resistance and invasion of prostate cancer cells through the Keap1/Nrf2/ARE axis. Oncol. Rep. 2020, 43, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Jaglan, S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci. Technol. 2016, 54, 114–126. [Google Scholar] [CrossRef]
- Gingras, D.; Béliveau, R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011, 4, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, A.; Krasnov, G.; Lipatova, A.; Alekseev, B.; Maganova, F.; Shaposhnikov, M.; Fedorova, M.; Snezhkina, A.; Moskalev, A. Effects of Abies sibirica terpenes on cancer-and aging-associated pathways in human cells. Oncotarget 2016, 7, 83744. [Google Scholar] [CrossRef] [Green Version]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, pharmacokinetics and anti-cancer activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. In Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 333–359. [Google Scholar]
- Li, A.-L.; Shen, T.; Wang, T.; Zhou, M.-X.; Wang, B.; Song, J.-T.; Zhang, P.-L.; Wang, X.-L.; Ren, D.-M.; Lou, H.-X. Novel diterpenoid-type activators of the Keap1/Nrf2/ARE signaling pathway and their regulation of redox homeostasis. Free Radic. Biol. Med. 2019, 141, 21–33. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as anti-colon cancer agents—A comprehensive review on its mechanistic perspectives. Eur. J. Pharmacol. 2017, 795, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Burgos, E.; Gómez-Serranillos, M. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Holstein, S.A.; Tong, H.; Kuder, C.H.; Hohl, R.J. Quantitative determination of geranyl diphosphate levels in cultured human cells. Lipids 2009, 44, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Neighbors, J.D. The mevalonate pathway and terpenes: A diversity of chemopreventatives. Curr. Pharmacol. Rep. 2018, 4, 157–169. [Google Scholar] [CrossRef]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Burda, P.; Aebi, M. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1426, 239–257. [Google Scholar] [CrossRef]
- Norat, T.; Scoccianti, C.; Boutron-Ruault, M.-C.; Anderson, A.; Berrino, F.; Cecchini, M.; Espina, C.; Key, T.; Leitzmann, M.; Powers, H. European code against cancer 4th edition: Diet and cancer. Cancer Epidemiol. 2015, 39, S56–S66. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, V.; Negi, A.S.; Kumar, J.; Gupta, M.; Khanuja, S.P. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–5908. [Google Scholar] [CrossRef]
- Cragg, G.; Newman, D. Plants as a source of anti-cancer and anti-HIV agents. Ann. Appl. Biol. 2003, 143, 127–133. [Google Scholar] [CrossRef]
- Weindruch, R.; Albanes, D.; Kritchevsky, D. The role of calories and caloric restriction in carcinogenesis. Hematol./Oncol. Clin. 1991, 5, 79–89. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Dia, V.P. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev. 2010, 29, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yoshida, C.; Murakami, A.; Ohigashi, H.; Osawa, T.; Uchida, K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004, 572, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.-M.; Zhang, W.-Y.; Li, R.-J.; Guo, C.; Wei, S.-S.; Tian, X.-M.; Luo, J.; Kong, L.-Y. Anti-inflammatory activity of Khayandirobilide A from Khaya senegalensis via NF-κB, AP-1 and p38 MAPK/Nrf2/HO-1 signaling pathways in lipopolysaccharide-stimulated RAW 264.7 and BV-2 cells. Phytomedicine 2018, 42, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.L.; Liu, K.X.; Mao, X.Y.; Li, Y.L.; Li, J.; Zhang, M.M. Effect of injection of Brucea javanica oil emulsion plus chemoradiotherapy for lung cancer: A review of clinical evidence. J. Evid.-Based Med. 2012, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019, 9, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, G.L.; Rana, A. Withania somnifera (Ashwagandha): A review. Pharmacogn. Rev. 2007, 1, 129–136. [Google Scholar]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Li, B.; Lee, D.-S.; Kang, Y.; Yao, N.-Q.; An, R.-B.; Kim, Y.-C. Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes. Food Chem. Toxicol. 2013, 53, 317–324. [Google Scholar] [CrossRef]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic acid alters multiple cell signaling pathways: Implication in cancer prevention and therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [Green Version]
- Frighetto, R.T.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Yang, L.; Ren, S.; Xu, F.; Ma, Z.; Liu, X.; Wang, L. Recent advances in the pharmacological activities of dioscin. BioMed Res. Int. 2019, 2019, 5763602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Lee, Y.; Kim, M.; Yoon, J.-H.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Corosolic acid induces apoptotic cell death in HCT116 human colon cancer cells through a caspase-dependent pathway. Int. J. Mol. Med. 2014, 33, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Kwak, M.-K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef] [Green Version]
- Satoh, H.; Moriguchi, T.; Taguchi, K.; Takai, J.; Maher, J.M.; Suzuki, T.; Winnard, P.T., Jr.; Raman, V.; Ebina, M.; Nukiwa, T. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis 2010, 31, 1833–1843. [Google Scholar] [CrossRef] [Green Version]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef]
- Li, C.; Cheng, L.; Wu, H.; He, P.; Zhang, Y.; Yang, Y.; Chen, J.; Chen, M. Activation of the KEAP1-NRF2-ARE signaling pathway reduces oxidative stress in Hep2 cells. Mol. Med. Rep. 2018, 18, 2541–2550. [Google Scholar] [CrossRef] [Green Version]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.-I.; Kobayashi, A.; Wakabayashi, N.; Kim, S.-G.; Yamamoto, M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA 2004, 101, 2046–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, S.J.; Siow, R.C.; Mann, G.E. Crosstalk between Nrf2 and the proteasome: Therapeutic potential of Nrf2 inducers in vascular disease and aging. Int. J. Biochem. Cell Biol. 2012, 44, 1315–1320. [Google Scholar] [CrossRef]
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci. Biotechnol. Biochem. 2007, 71, 0612070208. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Priestley, J.R.; Kautenburg, K.E.; Casati, M.C.; Endres, B.T.; Geurts, A.M.; Lombard, J.H. The NRF2 knockout rat: A new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H478–H487. [Google Scholar] [CrossRef] [Green Version]
- Zeraik, M.; Yariwake, J. Quantification of isoorientin and total flavonoids in Passiflora edulis fruit pulp by HPLC-UV/DAD. Microchem. J. 2010, 96, 86–91. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2–Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Wakabayashi, N.; Kensler, T.W. Chemoprevention through the Keap1–Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2004, 555, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, K.-M.; Lee, H.J.; Yun, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, J.; Li, D.; Ma, H.; Mu, Y.; Huang, X.; Li, L. Guavinoside B from Psidium guajava alleviates acetaminophen-induced liver injury via regulating the Nrf2 and JNK signaling pathways. Food Funct. 2020, 11, 8297–8308. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Gladwell, W.; Wang, X.; Chorley, B.; Bell, D.; Reddy, S.P.; Kleeberger, S.R. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2010, 182, 170–182. [Google Scholar] [CrossRef]
- Liu, G.-H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotblat, B.; Melino, G.; Knight, R.A. NRF2 and p53: Januses in cancer? Oncotarget 2012, 3, 1272. [Google Scholar] [CrossRef] [Green Version]
- Naidu, S.D.; Kostov, R.V.; Dinkova-Kostova, A.T. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol. Sci. 2015, 36, 6–14. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, K.; Irie, K.; Murakami, A. In vitro covalent binding proteins of zerumbone, a chemopreventive food factor. Biosci. Biotechnol. Biochem. 2009, 73, 0907061526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, N.; Khoshnazar, A.; Saidijam, M.; Azizi Jalilian, F.; Najafi, R.; Mahdavinezhad, A.; Ezati, R.; Sotanian, A.; Amini, R. Zerumbone Suppresses Human Colorectal Cancer Invasion and Metastasis via Modulation of FAk/PI3k/NFκB-uPA Pathway. Nutr. Cancer 2019, 71, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, J.-L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.R.; Hsia, D.A.; Schlaepfer, D.D. The focal adhesion kinase—A regulator of cell migration and invasion. IUBMB Life 2002, 53, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Matsuo, Y.; Shamoto, T.; Shibata, T.; Koide, S.; Morimoto, M.; Guha, S.; Sung, B.; Aggarwal, B.B.; Takahashi, H. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol. Rep. 2014, 31, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Jantan, I.; Arshad, L.; Bukhari, S.N.A. Exploring the immunomodulatory and anticancer properties of zerumbone. Food Funct. 2017, 8, 3410–3431. [Google Scholar] [CrossRef]
- Tang, C.; Bi, M.; Yu, H.; Chen, W.; Wang, J. Zerumbone protects HEK 293 cells from irradiation-induced DNA damage via activating Keap1/Nrf2/ARE pathway. Afr. J. Pharm. Pharmacol. 2011, 5, 2247–2254. [Google Scholar]
- Kim, M.-J.; Yun, J.-M. Molecular mechanism of the protective effect of zerumbone on lipopolysaccharide-induced inflammation of THP-1 cell-derived macrophages. J. Med. Food 2019, 22, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Lee, C.-L.; Korivi, M.; Liao, J.-W.; Rajendran, P.; Wu, J.-J.; Hseu, Y.-C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Lo, H.-W.; Korivi, M.; Tsai, Y.-C.; Tang, M.-J.; Yang, H.-L. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated Human keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Kawachi, Y.; Itoh, K.; Nakamura, Y.; Xu, X.; Banno, T.; Takahashi, T.; Yamamoto, M.; Otsuka, F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: Protective role in UVA-induced apoptosis. J. Investig. Dermatol. 2005, 124, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimta, A.-A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The role of Nrf2 activity in cancer development and progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Y.-C.; Chang, C.-T.; Gowrisankar, Y.V.; Chen, X.-Z.; Lin, H.-C.; Yen, H.-R.; Yang, H.-L. Zerumbone Exhibits Antiphotoaging and Dermatoprotective Properties in Ultraviolet A-Irradiated Human Skin Fibroblast Cells via the Activation of Nrf2/ARE Defensive Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4098674. [Google Scholar] [CrossRef]
- Shin, J.-W.; Murakami, A.; Ohigashi, H.; Johnson, D.; Johnson, J.; Na, H.-K.; Surh, Y.-J. Zerumbone, a Sesquiterpene Derived from Tropical Ginger, Induces Heme Oxygenase-1 Expression via Activation of Nrf2 Signaling in Mouse Epidermal Cells and Hairless Mouse Skin In Vivo; American Association for Cancer Rresearch: Philadelphia, PA, USA, 2008. [Google Scholar]
- Leung, W.-S.; Yang, M.-L.; Lee, S.-S.; Kuo, C.-W.; Ho, Y.-C.; Huang-Liu, R.; Lin, H.-W.; Kuan, Y.-H. Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int. Immunopharmacol. 2017, 46, 194–200. [Google Scholar] [CrossRef]
- Wang, M.; Niu, J.; Ou, L.; Deng, B.; Wang, Y.; Li, S. Zerumbone protects against carbon tetrachloride (CCl4)-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019, 24, 1964. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-Y.; Chen, S.-P.; Su, C.-H.; Ho, Y.-C.; Yang, M.-L.; Lee, S.-S.; Huang-Liu, R.; Yang, C.-P.; Chen, C.-J.; Kuan, Y.-H. Zerumbone from Zingiber zerumbet ameliorates lipopolysaccharide-induced ICAM-1 and cytokines expression via p38 MAPK/JNK-IκB/NF-κB pathway in mouse model of acute lung injury. Chin. J. Physiol. 2018, 61, 171–180. [Google Scholar] [CrossRef]
- Shin, J.-W.; Ohnishi, K.; Murakami, A.; Lee, J.-S.; Kundu, J.K.; Na, H.-K.; Ohigashi, H.; Surh, Y.-J. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev. Res. 2011, 4, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bours, V.; Dejardin, E.; Goujon-Letawe, F.; Merville, M.-P.; Castronovo, V. The NF-κB transcription factor and cancer: High expression of NF-κB-and IκB-related proteins in tumor cell lines. Biochem. Pharmacol. 1994, 47, 145–149. [Google Scholar] [CrossRef]
- Dejardin, E.; Bonizzi, G.; Bellahcene, A.; Castronovo, V.; Merville, M.-P.; Bours, V. Highly-expressed p100/p52 (NFKB2) sequesters other NF-κB-related proteins in the cytoplasm of human breast cancer cells. Oncogene 1995, 11, 1835–1841. [Google Scholar] [PubMed]
- Nakshatri, H.; Bhat-Nakshatri, P.; Martin, D.A.; Goulet, R.J.; Sledge, G.W. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 1997, 17, 3629–3639. [Google Scholar] [CrossRef] [Green Version]
- Young, M.R.; Yang, H.-S.; Colburn, N.H. Promising molecular targets for cancer prevention: AP-1, NF-κB and Pdcd4. Trends Mol. Med. 2003, 9, 36–41. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tian, Z.; Guo, R.; Ren, F. Nrf2 Inhibitor, Brusatol in Combination with Trastuzumab Exerts Synergistic Antitumor Activity in HER2-Positive Cancers by Inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 Pathways. Oxid. Med. Cell. Longev. 2020, 2020, 9867595. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Wang, Y.; Wang, Y.; Wei, L.; Zheng, W. Mechanism of progestin resistance in endometrial precancer/cancer through Nrf2-survivin pathway. Am. J. Transl. Res. 2017, 9, 1483. [Google Scholar]
- Wu, T.; Harder, B.G.; Wong, P.K.; Lang, J.E.; Zhang, D.D. Oxidative stress, mammospheres and Nrf2–new implication for breast cancer therapy? Mol. Carcinog. 2015, 54, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Murakami, Y.; Sugiyama, K.; Ebinuma, H.; Nakamoto, N.; Ojiro, K.; Chu, P.-S.; Taniki, N.; Saito, Y.; Teratani, T.; Koda, Y. Dual effects of the Nrf2 inhibitor for inhibition of hepatitis C virus and hepatic cancer cells. BMC Cancer 2018, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shi, G.; Bian, C.; Nisar, M.F.; Guo, Y.; Wu, Y.; Li, W.; Huang, X.; Jiang, X.; Bartsch, J.W. UVA irradiation enhances Brusatol-mediated inhibition of melanoma growth by downregulation of the Nrf2-mediated antioxidant response. Oxid. Med. Cell. Longev. 2018, 2018, 9742154. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Wei, C.; Lu, Z.; Cai, R.; Pan, D.; Zhang, H.; Liang, B.; Zhang, Z. Anticancer effects of brusatol in nasopharyngeal carcinoma through suppression of the Akt/mTOR signaling pathway. Cancer Chemother. Pharmacol. 2020, 85, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yuan, F.; Chen, X.-P.; Zhang, W.; Zhao, X.-L.; Jiang, Z.-P.; Zhou, H.-H.; Zhou, G.; Cao, S. Inhibition of Nrf2-mediated glucose metabolism by brusatol synergistically sensitizes acute myeloid leukemia to Ara-C. Biomed. Pharmacother. 2021, 142, 111652. [Google Scholar] [CrossRef]
- Olayanju, A.; Copple, I.M.; Bryan, H.K.; Edge, G.T.; Sison, R.L.; Wong, M.W.; Lai, Z.-Q.; Lin, Z.-X.; Dunn, K.; Sanderson, C.M. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity—Implications for therapeutic targeting of Nrf2. Free Radic. Biol. Med. 2015, 78, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tan, L.; Xie, J.; Lai, Z.; Huang, Y.; Qu, C.; Luo, D.; Lin, Z.; Huang, P.; Su, Z. Characterization of brusatol self-microemulsifying drug delivery system and its therapeutic effect against dextran sodium sulfate-induced ulcerative colitis in mice. Drug Deliv. 2017, 24, 1667–1679. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-H.; Kim, J.H.; Ko, E.; Kim, J.-Y.; Park, M.-J.; Kim, M.J.; Seo, H.; Li, S.; Lee, J.-Y. Resistance to gefitinib and cross-resistance to irreversible EGFR-TKIs mediated by disruption of the Keap1-Nrf2 pathway in human lung cancer cells. FASEB J. 2018, 32, 5862–5873. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Huang, C.; Lou, B.; Zhang, J.; Yu, D.; Huang, X.; Chen, B.; Zhou, M. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-κb/Stat3/Bcl-2 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 820–826. [Google Scholar] [CrossRef]
- Lee, J.H.; Rangappa, S.; Mohan, C.D.; Sethi, G.; Lin, Z.-X.; Rangappa, K.S.; Ahn, K.S. Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jiang, T.; Chen, H.; Su, J.; Wang, X.; Cao, Y.; Li, Q. Brusatol reverses lipopolysaccharide-induced epithelial-mesenchymal transformation and induces apoptosis through PI3K/Akt/NF-кB pathway in human gastric cancer SGC-7901 cells. Anticancer Drugs 2021, 32, 394–404. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Balasubramaniam, J.; Sellamuthu, A.; Ravi, A. An in vitro study on the reversal of epithelial to mesenchymal transition by brusatol and its synergistic properties in triple-negative breast cancer cells. J. Pharm. Pharmacol. 2021, 73, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Hou, J.; Wang, J.; Wang, J.; Gao, J. Brusatol Inhibits Laryngeal Cancer Cell Proliferation and Metastasis via Abrogating JAK2/STAT3 Signaling Mediated Epithelial-Mesenchymal Transition; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Lee, J.H.; Mohan, C.D.; Deivasigamani, A.; Jung, Y.Y.; Rangappa, S.; Basappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Garg, M. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J. Adv. Res. 2020, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Illangeswaran, R.S.S.; Karathedath, S.; Rajamani, B.M.; Balasubramanian, P. Global Gene Expression Analysis Reveals Genotoxic Effect of Nrf2 Pharmacological Inhibitor Brusatol in Myeloid Leukemia Cells-a Promising Novel Anticancer Agent. Blood 2018, 132, 5150. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Huang, C.; Yu, D.; Chen, H.; Deng, T.; Zhang, F.; Lou, B.; Zhang, J.; Shi, K. Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxid. Med. Cell. Longev. 2018, 2018, 2360427. [Google Scholar] [CrossRef]
- Evans, J.P.; Winiarski, B.K.; Sutton, P.A.; Jones, R.P.; Ressel, L.; Duckworth, C.A.; Pritchard, D.M.; Lin, Z.-X.; Vicky, F.L.; Tweedle, E.M. The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget 2018, 9, 27104. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-Q.; Shang, X.-Y.; Huang, X.-X.; Yao, G.-D.; Song, S.-J. Brusatol: A potential anti-tumor quassinoid from Brucea javanica. Chin. Herb. Med. 2020, 12, 359–366. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, Y.; Wang, P.; Gao, W. Brusatol inhibits amyloid-β-induced neurotoxicity in U-251 cells via regulating the Nrf2/HO-1 pathway. J. Cell. Biochem. 2019, 120, 10556–10563. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: A review. Drug Des. Dev. Ther. 2018, 12, 3181. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.; Hammers, H.; Bargagna-Mohan, P.; Zhan, X.; Herbstritt, C.; Ruiz, A.; Zhang, L.; Hanson, A.; Conner, B.; Rougas, J. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Kim, S.-H.; Singh, K.B.; Singh, K.; Singh, S.V. A comprehensive review and perspective on anticancer mechanisms of withaferin A in breast cancer. Cancer Prev. Res. 2020, 13, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Sivasankarapillai, V.S.; Madhu Kumar Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of Withaferin A, an active constituent of the Indian Ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef]
- Lee, I.-C.; Choi, B.Y. Withaferin-A—A natural anticancer agent with pleitropic mechanisms of action. Int. J. Mol. Sci. 2016, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Dom, M.; Berghe, W.V.; Van Ostade, X. Broad-spectrum antitumor properties of Withaferin A: A proteomic perspective. RSC Med. Chem. 2020, 11, 30–50. [Google Scholar] [CrossRef]
- Hahm, E.R.; Kim, S.H.; Singh, K.B.; Singh, S.V. RNA-seq reveals novel cancer-selective and disease subtype-independent mechanistic targets of withaferin A in human breast cancer cells. Mol. Carcinog. 2021, 60, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.-J.; Tang, J.-Y.; Ou-Yang, F.; Wang, Y.-Y.; Yuan, S.-S.F.; Tseng, K.; Lin, L.-C.; Chang, H.-W. Low concentration of withaferin A inhibits oxidative stress-mediated migration and invasion in oral cancer cells. Biomolecules 2020, 10, 777. [Google Scholar] [CrossRef]
- Chien, T.-M.; Wu, K.-H.; Chuang, Y.-T.; Yeh, Y.-C.; Wang, H.-R.; Yeh, B.-W.; Yen, C.-H.; Yu, T.-J.; Wu, W.-J.; Chang, H.-W. Withaferin a Triggers Apoptosis and DNA Damage in Bladder Cancer J82 Cells through Oxidative Stress. Antioxidants 2021, 10, 1063. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Li, R.; Cui, J.; Hannink, M.; Gu, Z.; Fritsche, K.L.; Lubahn, D.B.; Simonyi, A. Withania somnifera and its withanolides attenuate oxidative and inflammatory responses and up-regulate antioxidant responses in BV-2 microglial cells. Neuromol. Med. 2016, 18, 241–252. [Google Scholar] [CrossRef]
- Eggler, A.L.; Gay, K.A.; Mesecar, A.D. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2008, 52, S84–S94. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Urrunaga, N.H.; Dash, S.; Khurana, S.; Saxena, N.K. Withaferin-A reduces acetaminophen-induced liver injury in mice. Biochem. Pharmacol. 2015, 97, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Logie, E.; Vanden Berghe, W. Tackling Chronic Inflammation with Withanolide Phytochemicals—A Withaferin A Perspective. Antioxidants 2020, 9, 1107. [Google Scholar] [CrossRef]
- Shiragannavar, V.D.; Gowda, N.G.S.; Kumar, D.P.; Mirshahi, F.; Santhekadur, P.K. Withaferin A Acts as a Novel Regulator of Liver X Receptor-α in HCC. Front. Oncol. 2021, 10, 3124. [Google Scholar] [CrossRef]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.; Gupta, R.C. Withaferin a inhibits epithelial to mesenchymal transition in non-small cell lung cancer cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and withaferin A inhibited the signaling in colorectal cancer cells. Mediat. Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. BioMed Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef]
- Yim, N.-H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, H.M.; Eom, H.J.; Lee, D.; Kim, S.; Kang, K.S.; Lee, I.K.; Baek, K.-H.; Park, J.Y.; Kim, K.H. Bioactivity evaluations of betulin identified from the bark of Betula platyphylla var. japonica for cancer therapy. Arch. Pharm. Res. 2018, 41, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in LPS-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/Nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Kim, I.-S.; More, S.V.; Kim, B.-W.; Choi, D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Liu, W.; Han, X.; Zhao, M. Betulin attenuates lung and liver injuries in sepsis. Int. Immunopharmacol. 2016, 30, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, B.; Sun, J.; Chen, H.; Yang, Z. Betulin ameliorates 7,12-dimethylbenz (a) anthracene-induced rat mammary cancer by modulating MAPK and AhR/Nrf-2 signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22779. [Google Scholar] [CrossRef]
- Dai, J.; Miller, M.A.; Everetts, N.J.; Wang, X.; Li, P.; Li, Y.; Xu, J.-H.; Yao, G. Elimination of quiescent slow-cycling cells via reducing quiescence depth by natural compounds purified from Ganoderma lucidum. Oncotarget 2017, 8, 13770. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Kim, J.-H.; Song, C.-H.; Jang, K.-J. Ethanol extract of Ganoderma lucidum augments cellular anti-oxidant defense through activation of Nrf2/HO-1. J. Pharm. 2016, 19, 59. [Google Scholar]
- Li, P.; Liu, L.; Huang, S.; Zhang, Y.; Xu, J.; Zhang, Z. Anti-cancer effects of a neutral triterpene fraction from Ganoderma lucidum and its active constituents on SW620 human colorectal cancer cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2020, 20, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.T.T.; Cuong, T.D.; Thu, N.V.; Woo, M.H.; Min, B.S. Cytotoxic triterpenoids from the fruiting bodies of Ganoderma lucidum. Nat. Prod. Sci. 2014, 20, 7–12. [Google Scholar]
- Jedinak, A.; Thyagarajan-Sahu, A.; Jiang, J.; Sliva, D. Ganodermanontriol, a lanostanoid triterpene from Ganoderma lucidum, suppresses growth of colon cancer cells through ss-catenin signaling. Int. J. Oncol. 2011, 38, 761–767. [Google Scholar] [PubMed] [Green Version]
- Jiang, J.; Jedinak, A.; Sliva, D. Ganodermanontriol (GDNT) exerts its effect on growth and invasiveness of breast cancer cells through the down-regulation of CDC20 and uPA. Biochem. Biophys. Res. Commun. 2011, 415, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Yong, T.; Wang, Z.; Su, J.; Jiao, C.; Xie, Y.; Yang, B.B. Cytotoxic lanostane-type triterpenoids from the fruiting bodies of Ganoderma lucidum and their structure–activity relationships. Oncotarget 2017, 8, 10071. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.S.; Chen, L.S. Triterpenoids from Ganoderma lucidum inhibit the activation of EBV antigens as telomerase inhibitors. Exp. Ther. Med. 2017, 14, 3273–3278. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Shen, C.-E.; Lin, Q.-F.; Zhong, J.-Y.; Zhou, Y.-F.; Liu, B.-C.; Xu, J.-H.; Zhang, Z.-Q.; Li, P. Sterols and triterpenoids from Ganoderma lucidum and their reversal activities of tumor multidrug resistance. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Kim, H.-I.; Kim, J.-H.; Kwon, O.; Son, E.-S.; Lee, C.-S.; Park, Y.-J. Effects of ganodermanondiol, a new melanogenesis inhibitor from the medicinal mushroom Ganoderma lucidum. Int. J. Mol. Sci. 2016, 17, 1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Liese, J.; Abhari, B.A.; Fulda, S. Smac mimetic and oleanolic acid synergize to induce cell death in human hepatocellular carcinoma cells. Cancer Lett. 2015, 365, 47–56. [Google Scholar] [CrossRef]
- Lúcio, K.A.; da Graça Rocha, G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yang, C.; Guo, C.; Li, X.; Yang, N.; Zhao, L.; Hang, H.; Liu, S.; Chu, P.; Sun, Z. SZC015, a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MCF-7 breast cancer cells. Chem.-Biol. Interact. 2016, 244, 94–104. [Google Scholar] [CrossRef]
- Mu, D.-W.; Guo, H.-Q.; Zhou, G.-B.; Li, J.-Y.; Su, B. Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 13864. [Google Scholar] [PubMed]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol. 2013, 33, 756–765. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Chen, D.; Ni, J.; Kang, Y.; Wang, S. Oleanolic acid induces apoptosis in human leukemia cells through caspase activation and poly (ADP-ribose) polymerase cleavage. Acta Biochim. Biophys. Sin. 2007, 39, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Yao, W.; Zhang, Q.; Bo, Y. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway. PLoS ONE 2013, 8, e72079. [Google Scholar]
- Li, H.-F.; Wang, X.-A.; Xiang, S.-S.; Hu, Y.-P.; Jiang, L.; Shu, Y.-J.; Li, M.-L.; Wu, X.-S.; Zhang, F.; Ye, Y.-Y. Oleanolic acid induces mitochondrial-dependent apoptosis and G0/G1 phase arrest in gallbladder cancer cells. Drug Des. Dev. Ther. 2015, 9, 3017. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Sun, L.; Pei, Y.; Wang, D. Oleanolic acid inhibits colorectal cancer angiogenesis by blocking the VEGFR2 signaling pathway. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2018, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Lu, Y.-F.; Pi, J. New insights into generalized hepatoprotective effects of oleanolic acid: Key roles of metallothionein and Nrf2 induction. Biochem. Pharmacol. 2008, 76, 922–928. [Google Scholar] [CrossRef]
- Castrejón-Jiménez, N.S.; Leyva-Paredes, K.; Baltierra-Uribe, S.L.; Castillo-Cruz, J.; Campillo-Navarro, M.; Hernández-Pérez, A.D.; Luna-Angulo, A.B.; Chacón-Salinas, R.; Coral-Vázquez, R.M.; Estrada-García, I. Ursolic and oleanolic acids induce mitophagy in A549 human lung cancer cells. Molecules 2019, 24, 3444. [Google Scholar] [CrossRef] [Green Version]
- Narożna, M.; Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Kucińska, M.; Kleszcz, R.; Kujawski, J.; Piotrowska-Kempisty, H.; Plewiński, A.; Murias, M.; Baer-Dubowska, W. Conjugation of Diclofenac with Novel Oleanolic Acid Derivatives Modulate Nrf2 and NF-κB Activity in Hepatic Cancer Cells and Normal Hepatocytes Leading to Enhancement of Its Therapeutic and Chemopreventive Potential. Pharmaceuticals 2021, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Narożna, M.; Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Kleszcz, R.; Baer-Dubowska, W. The Effect of Novel Oleanolic Acid Oximes Conjugated with Indomethacin on the Nrf2-ARE And NF-κB Signaling Pathways in Normal Hepatocytes and Human Hepatocellular Cancer Cells. Pharmaceuticals 2021, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Narożna, M.; Krajka-Kuźniak, V.; Kleszcz, R.; Bednarczyk-Cwynar, B.; Szaefer, H.; Baer-Dubowska, W. Activation of the Nrf2 response by oleanolic acid oxime morpholide (3-hydroxyiminoolean-12-en-28-oic acid morpholide) is associated with its ability to induce apoptosis and inhibit proliferation in HepG2 hepatoma cells. Eur. J. Pharmacol. 2020, 883, 173307. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L.; Wu, N.; Ma, L.; Zhong, J.; Liu, G.; Lin, X. Oleanolic acid induces metabolic adaptation in cancer cells by activating the AMP-activated protein kinase pathway. J. Agric. Food Chem. 2014, 62, 5528–5537. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.-L.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.-L.; Hai, C.-X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem.-Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-H.; Lee, J.; Jeon, J.; Kim, K.-J.; Yun, J.-H.; Jeong, H.-S.; Lee, E.H.; Koh, Y.J.; Cho, C.-H. Oleanolic acids inhibit vascular endothelial growth factor receptor 2 signaling in endothelial cells: Implication for anti-angiogenic therapy. Mol. Cells 2018, 41, 771. [Google Scholar]
- Hosny, S.; Sahyon, H.; Youssef, M.; Negm, A. Oleanolic acid suppressed dmba-induced liver carcinogenesis through induction of mitochondrial-mediated apoptosis and autophagy. Nutr. Cancer 2021, 73, 968–982. [Google Scholar] [CrossRef]
- Xin, C.; Liu, S.; Qu, H.; Wang, Z. The novel nanocomplexes containing deoxycholic acid-grafted chitosan and oleanolic acid displays the hepatoprotective effect against CCl4-induced liver injury in vivo. Int. J. Biol. Macromol. 2021, 185, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 2009, 77, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, Y.F.; Wu, Q.; Xu, S.F.; Shi, F.G.; Klaassen, C.D. Oleanolic acid reprograms the liver to protect against hepatotoxicants, but is hepatotoxic at high doses. Liver Int. 2019, 39, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ma, L.; Chen, X.; Wang, J.; Yu, T.; Gong, Y.; Ma, A.; Zheng, L.; Liang, H. ERK inhibition sensitizes cancer cells to oleanolic acid-induced apoptosis through ERK/Nrf2/ROS pathway. Tumor Biol. 2016, 37, 8181–8187. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Song, J.; Kim, H.-R.; Hwang, K.-A. Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells. BMB Rep. 2014, 47, 524. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; He, H.; Zhang, X.; Wu, R.; Gan, L.; Li, D.; Lu, Y.; Wu, P.; Wong, W.-L.; Zhang, K. The in vitro and in vivo study of oleanolic acid indole derivatives as novel anti-inflammatory agents: Synthesis, biological evaluation, and mechanistic analysis. Bioorg. Chem. 2021, 113, 104981. [Google Scholar] [CrossRef] [PubMed]
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cao, W.; Biswal, S.; Doré, S. Carbon monoxide–activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 2011, 42, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Fang, Q.; Li, Y.; Wang, J.; Sun, J.; Zhang, Y.; Hu, X.; Wang, P.; Zhou, S. Crucial role of heme oxygenase-1 in the sensitivity of acute myeloid leukemia cell line Kasumi-1 to ursolic acid. Anti-Cancer Drugs 2014, 25, 406–414. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Michaluart, P.; Sporn, M.B.; Dannenberg, A.J. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000, 60, 2399–2404. [Google Scholar]
- Liu, L.; Zhang, J.; Li, M.; Zhang, X.; Zhang, J.; Li, Z.; Wang, L.; Wu, J.; Luo, C. Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2. Mol. Med. Rep. 2014, 9, 2505–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shu, L.; Zhang, C.; Li, W.; Wu, R.; Guo, Y.; Yang, Y.; Kong, A.N. Histone methyltransferase Setd7 regulates Nrf2 signaling pathway by phenethyl isothiocyanate and ursolic acid in human prostate cancer cells. Mol. Nutr. Food Res. 2018, 62, 1700840. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Gong, E.S.; Liu, R.H. Antiproliferative Activity of Ursolic Acid in MDA-MB-231 Human Breast Cancer Cells through Nrf2 Pathway Regulation. J. Agric. Food Chem. 2020, 68, 7404–7415. [Google Scholar] [CrossRef]
- Yeh, C.T.; Wu, C.H.; Yen, G.C. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 2010, 54, 1285–1295. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, T.; Du, J. Ursolic acid sensitizes cisplatin-resistant HepG2/DDP cells to cisplatin via inhibiting Nrf2/ARE pathway. Drug Des. Dev. Ther. 2016, 10, 3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Shin, E.A.; Jung, J.H.; Park, J.E.; Kim, D.S.; Shim, B.S.; Kim, S.-H. Ursolic acid induces apoptosis in colorectal cancer cells partially via upregulation of MicroRNA-4500 and inhibition of JAK2/STAT3 phosphorylation. Int. J. Mol. Sci. 2019, 20, 114. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Meng, X.; Dong, Y. Ursolic acid nanoparticles inhibit cervical cancer growth in vitro and in vivo via apoptosis induction. Int. J. Oncol. 2017, 50, 1330–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.-Q.; Ding, J.; Zhang, L.; Liu, C.-M. Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Li, W.; Wu, R.; Guo, Y.; Cheng, D.; Yang, Y.; Androulakis, I.P.; Kong, A.-N. Pharmacokinetics and pharmacodynamics of the triterpenoid ursolic acid in regulating the antioxidant, anti-inflammatory, and epigenetic gene responses in rat leukocytes. Mol. Pharm. 2017, 14, 3709–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.N.; Li, W.; Zhang, C.; Wu, R.; Su, S.; Wang, C.; Gao, L.; Yin, R.; Kong, A.-N. In vitro-in vivo dose response of ursolic acid, sulforaphane, PEITC, and curcumin in cancer prevention. AAPS J. 2018, 20, 1–19. [Google Scholar]
- Li, W.; Sun, K.; Hu, F.; Chen, L.; Zhang, X.; Wang, F.; Yan, B. Protective effects of natural compounds against oxidative stress in ischemic diseases and cancers via activating the Nrf2 signaling pathway: A mini review. J. Biochem. Mol. Toxicol. 2021, 35, e22658. [Google Scholar] [CrossRef]
- Kim, H.; Ramirez, C.N.; Su, Z.-Y.; Kong, A.-N.T. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, C.N. Epigenetic Regulation of Nrf2 and Ursolic Acid in Skin Carcinogenesis; Rutgers University-School of Graduate Studies: New Brunswick, NJ, USA, 2018. [Google Scholar]
- Liu, W.; Tan, X.; Shu, L.; Sun, H.; Song, J.; Jin, P.; Yu, S.; Sun, M.; Jia, X. Ursolic acid inhibits cigarette smoke extract-induced human bronchial epithelial cell injury and prevents development of lung cancer. Molecules 2012, 17, 9104–9115. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R. DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef]
- Tao, X.; Xu, L.; Yin, L.; Han, X.; Qi, Y.; Xu, Y.; Song, S.; Zhao, Y.; Peng, J. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-β. Cell Death Dis. 2017, 8, e2989. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.; Yin, H.; Chen, W.; Zhao, T.; Wu, S.; Jin, H.; Du, B.; Tan, Y.; Zhang, R.; He, Y. Network pharmacology and experimental evidence reveal dioscin suppresses proliferation, invasion, and EMT via AKT/GSK3b/mTOR signaling in lung adenocarcinoma. Drug Des. Dev. Ther. 2020, 14, 2135. [Google Scholar] [CrossRef]
- Ma, T.; Wang, R.-P.; Zou, X. Dioscin inhibits gastric tumor growth through regulating the expression level of lncRNA HOTAIR. BMC Complement. Altern. Med. 2016, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, C.; Liu, C. Antitumor effects of dioscin in A431 cells via adjusting ATM/p53-mediated cell apoptosis, DNA damage and migration. Oncol. Lett. 2021, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Q.-Y.; Chiu, J.-F. Dioscin induced activation of p38 MAPK and JNK via mitochondrial pathway in HL-60 cell line. Eur. J. Pharmacol. 2014, 735, 52–58. [Google Scholar] [CrossRef]
- Ran, X.; Yan, Z.; Yang, Y.; Hu, G.; Liu, J.; Hou, S.; Guo, W.; Kan, X.; Fu, S. Dioscin Improves Pyroptosis in LPS-Induced Mice Mastitis by Activating AMPK/Nrf2 and Inhibiting the NF-κB Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8845521. [Google Scholar] [CrossRef]
- Zhiyu, W.; Yue, C.; Neng, W.; Mei, W.D.; Wei, L.Y.; Feng, H.; Gang, S.J.; De Po, Y.; Yuan, G.X.; Jian-Ping, C. Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biol. Ther. 2012, 13, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, R.-Y.; Zhang, J.; Zhang, W.-X.; Huang, Z.-H.; Hu, H.-F.; Li, Y.-L.; Li, B.; He, Q.-Y. Inhibition of Nrf2 enhances the anticancer effect of 6-O-angeloylenolin in lung adenocarcinoma. Biochem. Pharmacol. 2017, 129, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, B.; Hou, L.; Huang, L.; Cui, Y.; Xu, D.; Shen, X.; Li, S. Dioscin inhibits colon cancer cells’ growth by reactive oxygen species-mediated mitochondrial dysfunction and p38 and JNK pathways. Anti-Cancer Drugs 2018, 29, 234–242. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Yang, S.-F.; Hsieh, Y.-S.; Chen, T.-Y.; Chiou, H.-L. Autophagy inhibition enhances apoptosis induced by dioscin in huh7 cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 134512. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.-C.; Kim, H.; Kim, Y.-J.; Choi, K.-C.; Lee, I.H.; Lee, K.H.; Kim, M.K.; Ko, H. Dioscin suppresses TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration and invasion. Bioorg. Med. Chem. Lett. 2017, 27, 3342–3348. [Google Scholar] [CrossRef]
- Gu, L.; Tao, X.; Xu, Y.; Han, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Dioscin alleviates BDL-and DMN-induced hepatic fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway. Toxicol. Appl. Pharmacol. 2016, 292, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chu, L.; Liang, H.; Chen, J.; Liang, J.; Huang, Z.; Zhang, B.; Chen, X. Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of Sirt1/FOXO1/NF-κb signal. Front. Pharmacol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Wang, J. Dioscin attenuates Bleomycin-Induced acute lung injury via inhibiting the inflammatory response in mice. Exp. Lung Res. 2019, 45, 236–244. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Li, T. Dioscin alleviates lipopolysaccharide-induced acute lung injury through suppression of TLR4 signaling pathways. Exp. Lung Res. 2020, 46, 11–22. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Yin, L.-H.; Xu, L.-N.; Qi, Y.; Sun, P.; Peng, J.-Y. Dioscin ameliorates methotrexate-induced liver and kidney damages via adjusting miRNA-145-5p-mediated oxidative stress. Free Radic. Biol. Med. 2021, 169, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yin, L.; Xu, L.; Qi, Y.; Li, H.; Xu, Y.; Han, X.; Liu, K.; Peng, J. Protective effect of dioscin against thioacetamide-induced acute liver injury via FXR/AMPK signaling pathway in vivo. Biomed. Pharmacother. 2018, 97, 481–488. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, L.; Tao, X.; Xu, L.; Zheng, L.; Han, X.; Xu, Y.; Wang, C.; Peng, J. Dioscin alleviates dimethylnitrosamine-induced acute liver injury through regulating apoptosis, oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2016, 45, 193–201. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Qi, Y.; Han, X.; Yin, L.; Xu, L.; Liu, K.; Peng, J. Potent effects of dioscin against thioacetamide-induced liver fibrosis through attenuating oxidative stress in turn inhibiting inflammation, TGF-β/Smad and MAPK signaling pathways. J. Funct. Foods 2015, 16, 436–447. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Yin, L.; Xu, L.; Qi, Y.; Xu, Y.; Sun, H.; Lin, Y.; Liu, K.; Peng, J. Potent effects of dioscin against liver fibrosis. Sci. Rep. 2015, 5, 9713. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Sun, Y.; Song, S.; Qi, Y.; Tao, X.; Xu, L.; Yin, L.; Han, X.; Xu, Y.; Li, H. Protective effects of dioscin against lipopolysaccharide-induced acute lung injury through inhibition of oxidative stress and inflammation. Front. Pharmacol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-J.; Tsai, T.-L.; Hsieh, Y.-S.; Wang, C.-J.; Chiou, H.-L. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch. Toxicol. 2013, 87, 1927–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, A.; Li, X.; Ma, X.; Wang, X.; Liu, C.; Song, Z.; Pan, F.; Xia, Y.; Li, C. Transcriptome and Proteome Analysis Reveals Corosolic Acid Inhibiting Bladder Cancer via Targeting Cell Cycle and Inducing Mitophagy In Vitro and In Vivo; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Zhang, B.Y.; Zhang, L.; Chen, Y.M.; Qiao, X.; Zhao, S.L.; Li, P.; Liu, J.F.; Wen, X.; Yang, J. Corosolic acid inhibits colorectal cancer cells growth as a novel HER2/HER3 heterodimerization inhibitor. Br. J. Pharmacol. 2021, 178, 1475–1491. [Google Scholar] [CrossRef]
- Jia, M.; Xiong, Y.; Li, M.; Mao, Q. Corosolic acid inhibits cancer progress through inactivating YAP in hepatocellular carcinoma. Oncol. Res. 2020, 28, 371. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Hwang, J.-H.; Kim, D.-H.; Cho, Y.-E. Effect of corosolic acid on apoptosis and angiogenesis in MDA-MB-231 human breast cancer cells. J. Nutr. Health 2020, 53, 111–120. [Google Scholar] [CrossRef]
- Jin, M.; Wu, Y.; Lou, Y.; Liu, X.; Dai, Y.; Yang, W.; Liu, C.; Huang, G. Corosolic Acid Reduces NSCLC Cell Proliferation, Invasion, and Chemoresistance via Inducing Mitochondrial and Liposomal Oxidative Stress; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Yang, J.; Wu, R.; Li, W.; Gao, L.; Yang, Y.; Li, P.; Kong, A.N. The triterpenoid corosolic acid blocks transformation and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells. Mol. Carcinog. 2018, 57, 512–521. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Z.-Y.; Kong, A.-N.T. Current perspectives on epigenetic modifications by dietary chemopreventive and herbal phytochemicals. Curr. Pharmacol. Rep. 2015, 1, 245–257. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Wang, Q.; Li, W. Histone modifications and chromatin organization in prostate cancer. Epigenomics 2010, 2, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horlad, H.; Fujiwara, Y.; Takemura, K.; Ohnishi, K.; Ikeda, T.; Tsukamoto, H.; Mizuta, H.; Nishimura, Y.; Takeya, M.; Komohara, Y. Corosolic acid impairs tumor development and lung metastasis by inhibiting the immunosuppressive activity of myeloid-derived suppressor cells. Mol. Nutr. Food Res. 2013, 57, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, J.H.; Yang, X.S. Corosolic acid induces potent anti-cancer effects in CaSki cervical cancer cells through the induction of apoptosis, cell cycle arrest and PI3K/Akt signalling pathway. Bangladesh J. Pharmacol. 2016, 11, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhou, H.; An, Y.; Shen, K.; Yu, L. Biological effects of corosolic acid as an anti-inflammatory, anti-metabolic syndrome and anti-neoplasic natural compound. Oncol. Lett. 2021, 21, 84. [Google Scholar] [CrossRef]
- Peng, M.; Qiang, L.; Xu, Y.; Li, C.; Li, T.; Wang, J. Inhibition of JNK and activation of the AMPK-Nrf2 axis by corosolic acid suppress osteolysis and oxidative stress. Nitric Oxide 2019, 82, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Cha, J.-Y.; Kang, H.S.; Lee, J.-H.; Lee, J.Y.; Park, J.-H.; Bae, J.-H.; Song, D.-K.; Im, S.-S. Corosolic acid ameliorates acute inflammation through inhibition of IRAK-1 phosphorylation in macrophages. BMB Rep. 2016, 49, 276. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim. Pol. 2012, 59. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Marisiddiah, R.; Wiener, D.; Rubin, L.P. Regulation of Antioxidant Responses in Prostate Cancer: Nrf2-Dependent and Independent Effects of Lycopene; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Marisiddaiah, R.; Gong, X.; Wiener, D.; Rubin, L.P. Lycopene Alters Intracellular Glutathione Status and Antioxidant/Phase II Detoxifying Enzymes in Human Prostate Cancer Cells; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar]
- Li, D.; Chen, L.; Zhao, W.; Hao, J.; An, R. MicroRNA-let-7f-1 is induced by lycopene and inhibits cell proliferation and triggers apoptosis in prostate cancer. Mol. Med. Rep. 2016, 13, 2708–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assar, E.A.; Vidalle, M.C.; Chopra, M.; Hafizi, S. Lycopene acts through inhibition of IκB kinase to suppress NF-κB signaling in human prostate and breast cancer cells. Tumor Biol. 2016, 37, 9375–9385. [Google Scholar] [CrossRef] [Green Version]
- Aktepe, O.H.; Şahin, T.K.; Güner, G.; Arik, Z.; Yalçin, Ş. Lycopene sensitizes the cervical cancer cells to cisplatin via targeting nuclear factorkappa B (NF-κB) pathway. Turk. J. Med. Sci. 2021, 51, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Wang, F.-Y.; Kuo, Y.-H.; Tang, F.-Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014, 105, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.-M.; Chen, H.-Z.; Huang, Y.-T.; Hsieh, C.-W.; Wung, B.-S. Lycopene inhibits NF-κB activation and adhesion molecule expression through Nrf2-mediated heme oxygenase-1 in endothelial cells. Int. J. Mol. Med. 2017, 39, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.-N.; Liu, Y.-B.; Li, B.-H. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J. Androl. 2019, 21, 80. [Google Scholar]
- Yang, P.-M.; Wu, Z.-Z.; Zhang, Y.-Q.; Wung, B.-S. Lycopene inhibits ICAM-1 expression and NF-κB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sci. 2016, 155, 94–101. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J. Cancer Prev. 2018, 23, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796. [Google Scholar] [CrossRef] [Green Version]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ali, S.; Bahcecioglu, I.H.; Guler, O.; Ozercan, I.; Ilhan, N.; Kucuk, O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr. Cancer 2014, 66, 590–598. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.-Y.; Wang, X.; Shen, P.; Jia, Q.; Yu, S.; Wang, Y.; Li, X.; Chen, W.; Wang, A. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through p62-triggered autophagic Keap1 degradation. Aging 2020, 12, 8167. [Google Scholar] [CrossRef]
- Shen, C.; Wang, S.; Shan, Y.; Liu, Z.; Fan, F.; Tao, L.; Liu, Y.; Zhou, L.; Pei, C.; Wu, H. Chemomodulatory efficacy of lycopene on antioxidant enzymes and carcinogen-induced cutaneum carcinoma in mice. Food Funct. 2014, 5, 1422–1431. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Puah, B.-P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-κB, MAPKs, and Nrf2 signaling pathways in rats. Andrology 2020, 8, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Dong, J.; Li, W.; Cheng, L.-M.; Wang, G.-G. Lycopene attenuates LPS-induced liver injury by inactivation of NF-κB/COX-2 signaling. Int. J. Clin. Exp. Pathol. 2019, 12, 817. [Google Scholar] [PubMed]
- Akdemir, B.; Bahcecioglu, I.H.; Tuzcu, M.; Orhan, C.; Ispiroglu, M.; Ozercan, I.H.; Ilhan, N.; Celik, N.C.; Sahin, K. Effect of lycopene and genistein on hepatic inflammation and fibrosis in thioacetamide induced liver injury in rats. J. Adv. Med. Med. Res. 2016, 1–11. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Lee, J.Y. Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1–1003. [Google Scholar]
- Farruggia, C.; Kim, M.-B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.-K.; Lee, J.-Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Eroglu, A. The Promising Effects of Astaxanthin on Lung Diseases. Adv. Nutr. 2021, 12, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.-E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPARγ) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2− production. PLoS ONE 2014, 9, e88359. [Google Scholar]
- Zhou, Y.; Baker, J.S.; Chen, X.; Wang, Y.; Chen, H.; Davison, G.W.; Yan, X. High-dose astaxanthin supplementation suppresses antioxidant enzyme activity during moderate-intensity swimming training in mice. Nutrients 2019, 11, 1244. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Fuentes, F.; Shu, L.; Wang, C.; Pung, D.; Li, W.; Zhang, C.; Guo, Y.; Kong, A.-N. Epigenetic CpG methylation of the promoter and reactivation of the expression of GSTP1 by Astaxanthin in human prostate LNCaP cells. AAPS J. 2017, 19, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, W.; Xia, Y.; Chen, K.; Li, S.; Liu, T.; Zhang, R.; Wang, J.; Lu, W.; Zhou, Y. Astaxanthin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells via Inhibition of NF-κB P65 and Wnt/β-catenin in vitro. Mar. Drugs 2015, 13, 6064–6081. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Palozza, P.; Torelli, C.; Boninsegna, A.; Simone, R.; Catalano, A.; Mele, M.C.; Picci, N. Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett. 2009, 283, 108–117. [Google Scholar] [CrossRef]
- Niu, T.; Xuan, R.; Jiang, L.; Wu, W.; Zhen, Z.; Song, Y.; Hong, L.; Zheng, K.; Zhang, J.; Xu, Q. Astaxanthin induces the Nrf2/HO-1 antioxidant pathway in human umbilical vein endothelial cells by generating trace amounts of ROS. J. Agric. Food Chem. 2018, 66, 1551–1559. [Google Scholar] [CrossRef]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative stress regulation on endothelial cells by hydrophilic astaxanthin complex: Chemical, biological, and molecular antioxidant activity evaluation. Oxid. Med. Cell. Longev. 2017, 2017, 8073798. [Google Scholar] [CrossRef] [Green Version]
- Saw, C.L.L.; Yang, A.Y.; Guo, Y.; Kong, A.-N.T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2–ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef]
- Su, X.-Z.; Chen, R.; Wang, C.-B.; Ouyang, X.-L.; Jiang, Y.; Zhu, M.-Y. Astaxanthin combine with human serum albumin to abrogate cell proliferation, migration, and drug-resistant in human ovarian carcinoma SKOV3 cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2019, 19, 792–801. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kim, Y.-J. Astaxanthin treatment induces maturation and functional change of myeloid-derived suppressor cells in tumor-bearing mice. Antioxidants 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, F.; Wang, M.; Li, L.; Qiao, T.; Cui, H.; Li, Z.; Sun, C. Dietary natural astaxanthin at an early stage inhibits N-nitrosomethylbenzylamine–induced esophageal cancer oxidative stress and inflammation via downregulation of NFκB and COX2 in F344 rats. OncoTargets Ther. 2019, 12, 5087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.; Jena, G. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 696, 69–80. [Google Scholar] [CrossRef]
- Heng, N.; Gao, S.; Chen, Y.; Wang, L.; Li, Z.; Guo, Y.; Sheng, X.; Wang, X.; Xing, K.; Xiao, L. Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poult. Sci. 2021, 100, 101045. [Google Scholar] [CrossRef]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Xiong, H.; Wang, M.; Hang, W.; Zhu, X.; Zheng, Y.; Ge, B.; Li, R.; Cui, H. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. 2020, 11, 4659–4671. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S. Astaxanthin protects OTA-induced lung injury in mice through the Nrf2/NF-κB pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-B.; Kang, H.; Li, Y.; Park, Y.-K.; Lee, J.-Y. Fucoxanthin inhibits lipopolysaccharide-induced inflammation and oxidative stress by activating nuclear factor E2-related factor 2 via the phosphatidylinositol 3-kinase/AKT pathway in macrophages. Eur. J. Nutr. 2021, 60, 3315–3324. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.-y.; Wu, R.; Guo, Y.; Fang, M.; Kong, A.-N. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Chiu, Y.-T.; Hu, M.-L. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL. 2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J. Agric. Food Chem. 2011, 59, 11344–11351. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, Y.-J.; Qi, J.; Zhu, M.-M.; Zhang, T.-L.; Cheng, M.; Liu, S.-M.; Wang, G.-C. Fucoxanthin exerts cytoprotective effects against hydrogen peroxide-induced oxidative damage in L02 cells. BioMed Res. Int. 2018, 2018, 1085073. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.W.; Na, S.J.; Kim, W.K. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr. Res. Pract. 2013, 7, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-mediated antioxidant defense and inhibition of TLR4-mediated inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Lin, C.-H.; Lee, A.-S.; Liu, H.-J.; Li, C.-C.; Tseng, K.-W. Protective Effects of Fucoxanthin Dampen Pathogen-Associated Molecular Pattern (PAMP) Lipopolysaccharide-Induced Inflammatory Action and Elevated Intraocular Pressure by Activating Nrf2 Signaling and Generating Reactive Oxygen Species. Antioxidants 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef] [PubMed]
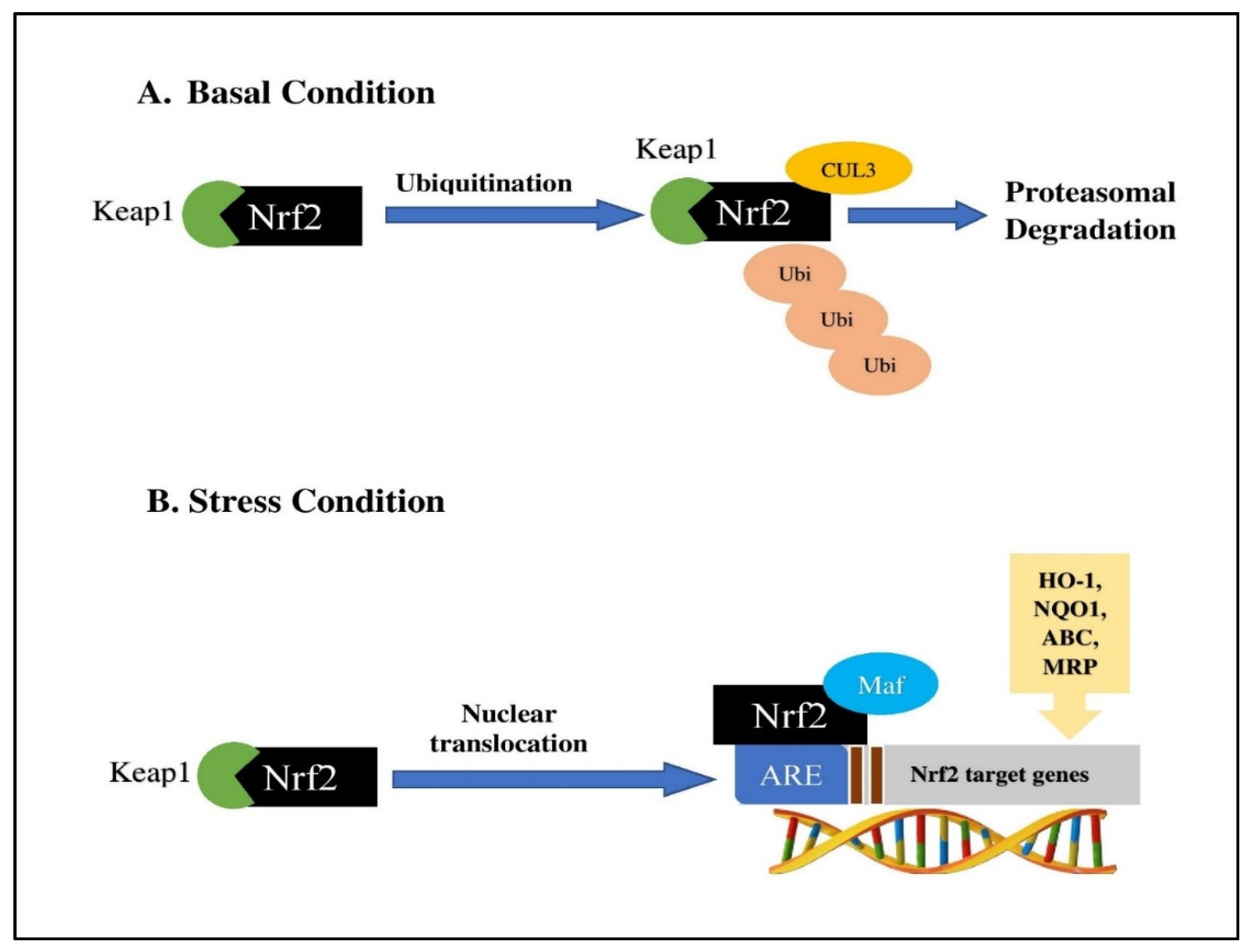
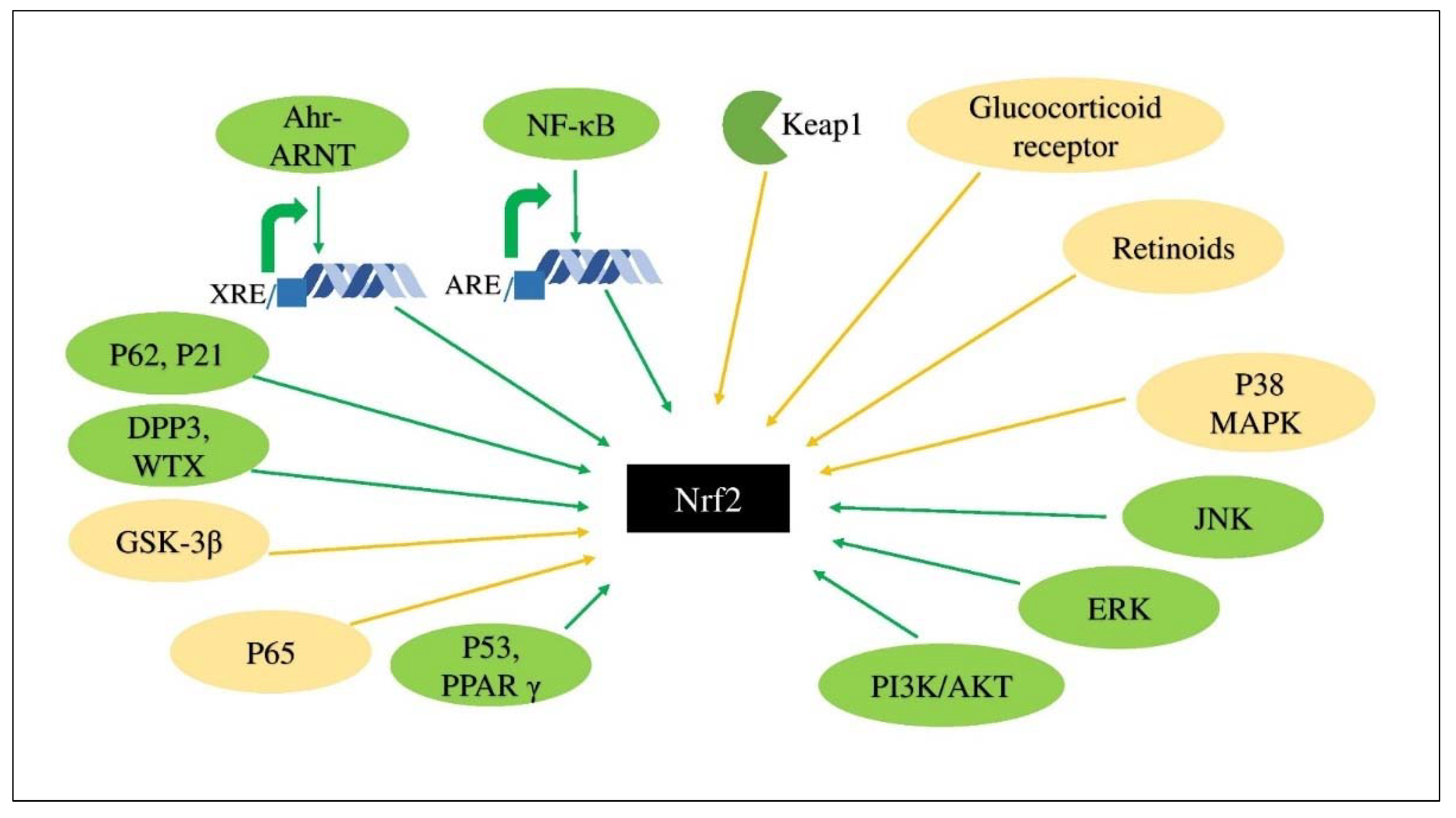
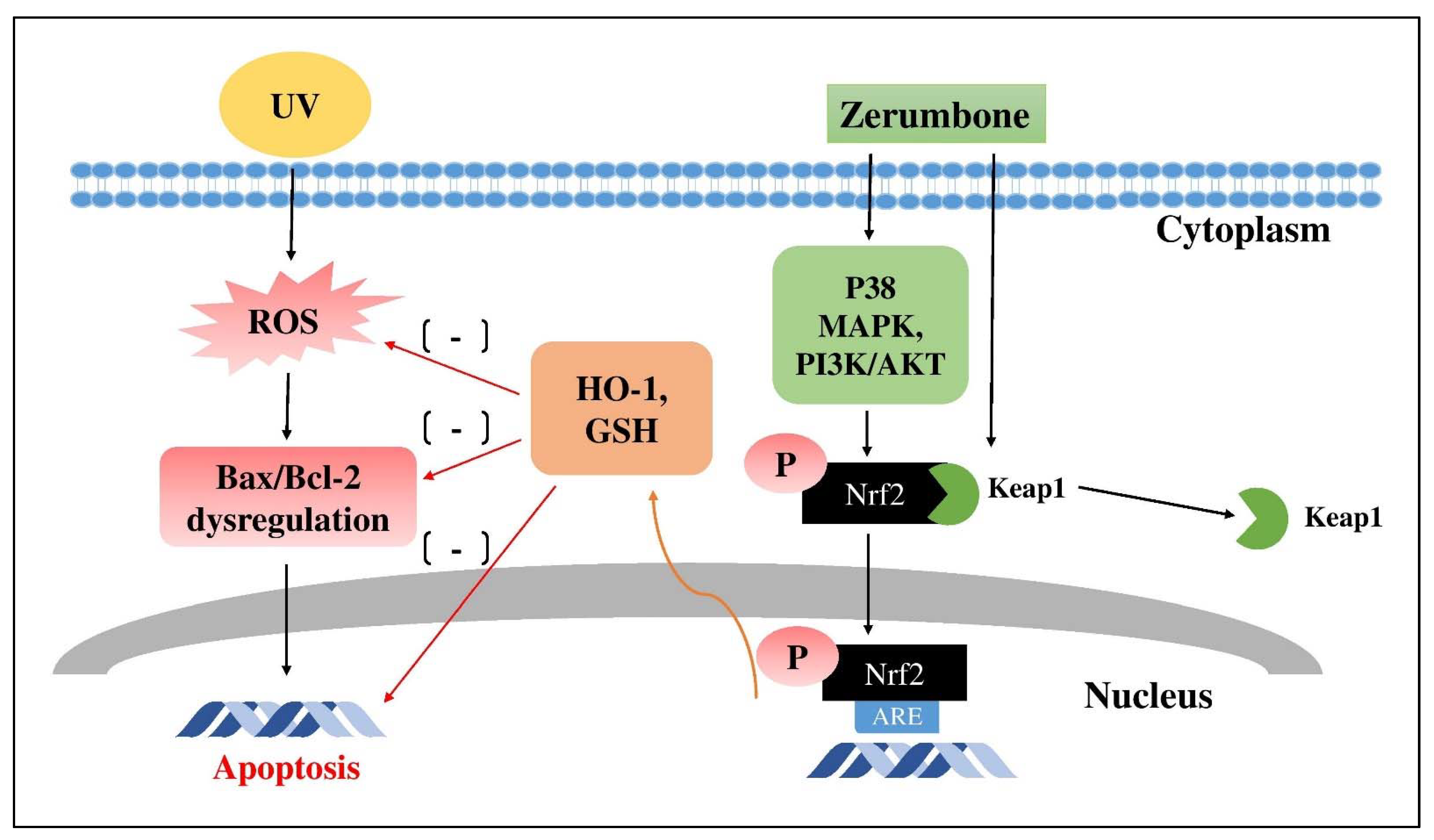
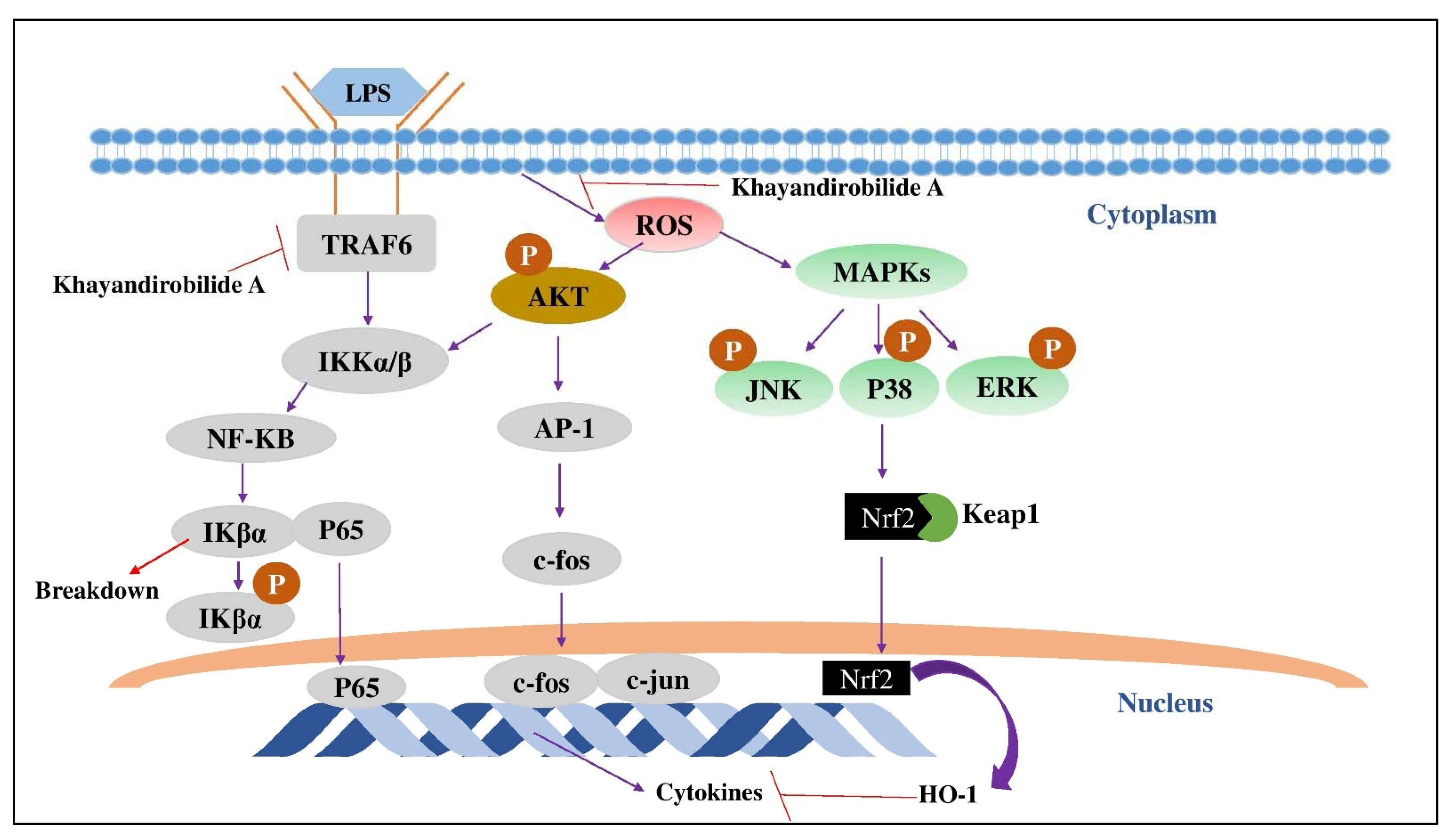
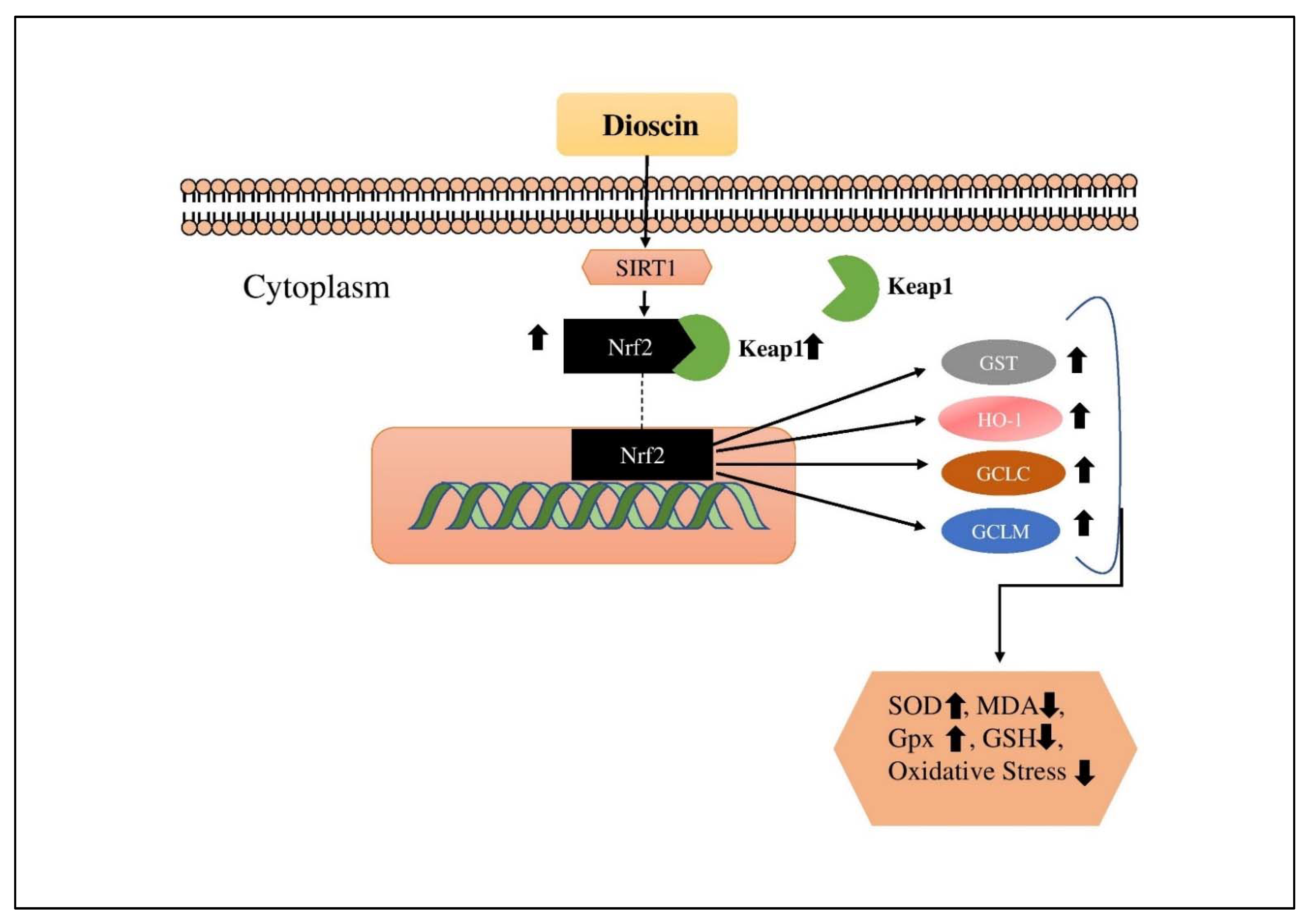
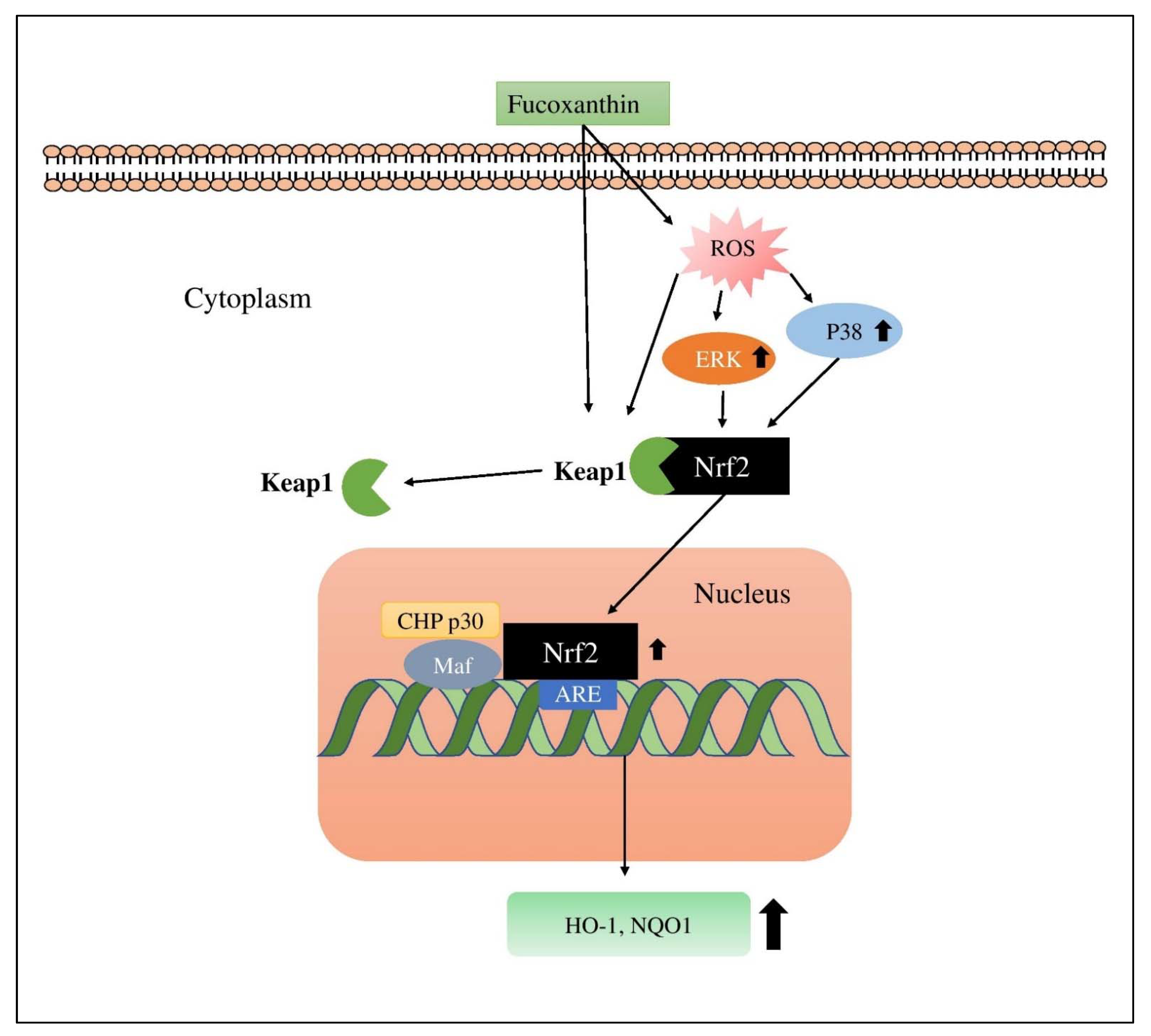
| Compound Number | Compound Name | Proteins Modulated Keap1-Nrf2-ARE System | Proteins Modulated after Nrf2 Activation/Inhibition | References |
|---|---|---|---|---|
| 1 | Zerumbone | p38 MAPK, AKT/PI3K, ERK, JNK, PKC, AMPK | HO-1, γ-GCLC, and Gpx | [78,86,90] |
| 2 | Khayandirobilide A | p38 MAPK | HO-1 | [40] |
| 3 | Brusatol | HER2/AKT/ERK1/2, PI3K/AKT/mTOR, | HO-1, NQO1, GSTm2, and GCLC | [102,122] |
| 4 | Withaferin A | ERK1/2, JNK, p38 MAPK, AKT/mTOR, JAK/STAT | HO-1, NQO1, GSR, CAT, SOD1, and TXN | [131,132,137] |
| 5 | Betulin | AMPK/AKT/GSK-3β, MAPK, AhR | NADPH, NQO1, HO-1, GCLC, GCLM, Gpx, CAT and SOD | [145,148] |
| 6 | Ganodermanondiol | ERK, JNK, p38 MAPK, AMPK | GSH, GCL, HO-1 | [45,151,158] |
| 7 | Oleanolic acid | p62, NF-κB, ERK, PI3K/AKT, MAPKs | SOD-1, NQO1, GCL, GCLC, HO-1 | [170,171,180,184] |
| 8 | Ursolic acid | NF-κB, AKT, JNK | HO-1, NQO1, GST, UDP-glucuronosyltransferase 1A1, GSTT | [192,194,198,202,205] |
| 9 | Dioscin | Sirt1/FOXO1/NF-κB, IκBα, FXR/AMPK, TGF-β/Smad, p38 MAPK | HO-1, GST, NQO1, GCLC, GCLM | [217,218,222,224] |
| 10 | Corosolic acid | JNK, AMPK | HO-1, NQO1, GCLC | [233,240] |
| 11 | Lycopene | NF-κB, JNK, STAT3, AKT/mTOR, p62 | HO-1, NQO1, GSH, CAT, SOD, GCL, GST-π, Gpx | [245,252,255,256,257,258] |
| 12 | Astaxanthin | NF-κB p65, Wnt/β-catenin, PI3K/AKT, ERK, JAK2/STAT3, p53, p38 | NQO1, HO-1, SOD1, SOD2, Gpx4 | [276,285,286,288] |
| 13 | Fucoxanthin | AKT1, ERK1/2, JNK, NF-κB, p38, PI3K/AKT | NQO1, HO-1, SOD1, SOD2, CAT | [293,295,299] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siraj, M.A.; Islam, M.A.; Al Fahad, M.A.; Kheya, H.R.; Xiao, J.; Simal-Gandara, J. Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update. Appl. Sci. 2021, 11, 10806. https://doi.org/10.3390/app112210806
Siraj MA, Islam MA, Al Fahad MA, Kheya HR, Xiao J, Simal-Gandara J. Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update. Applied Sciences. 2021; 11(22):10806. https://doi.org/10.3390/app112210806
Chicago/Turabian StyleSiraj, Md Afjalus, Md. Arman Islam, Md. Abdullah Al Fahad, Habiba Rahman Kheya, Jianbo Xiao, and Jesus Simal-Gandara. 2021. "Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update" Applied Sciences 11, no. 22: 10806. https://doi.org/10.3390/app112210806
APA StyleSiraj, M. A., Islam, M. A., Al Fahad, M. A., Kheya, H. R., Xiao, J., & Simal-Gandara, J. (2021). Cancer Chemopreventive Role of Dietary Terpenoids by Modulating Keap1-Nrf2-ARE Signaling System—A Comprehensive Update. Applied Sciences, 11(22), 10806. https://doi.org/10.3390/app112210806








