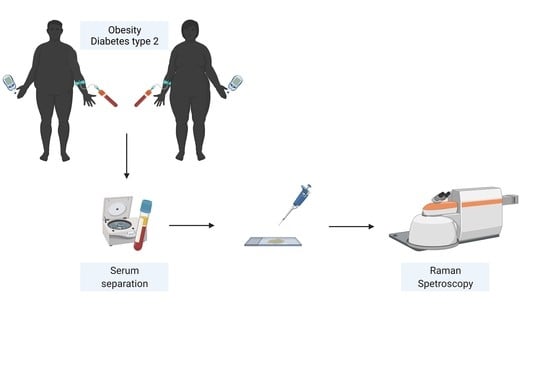
Metabolic Profiling of Type 2 Diabetes Patients after Bariatric Surgery by Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
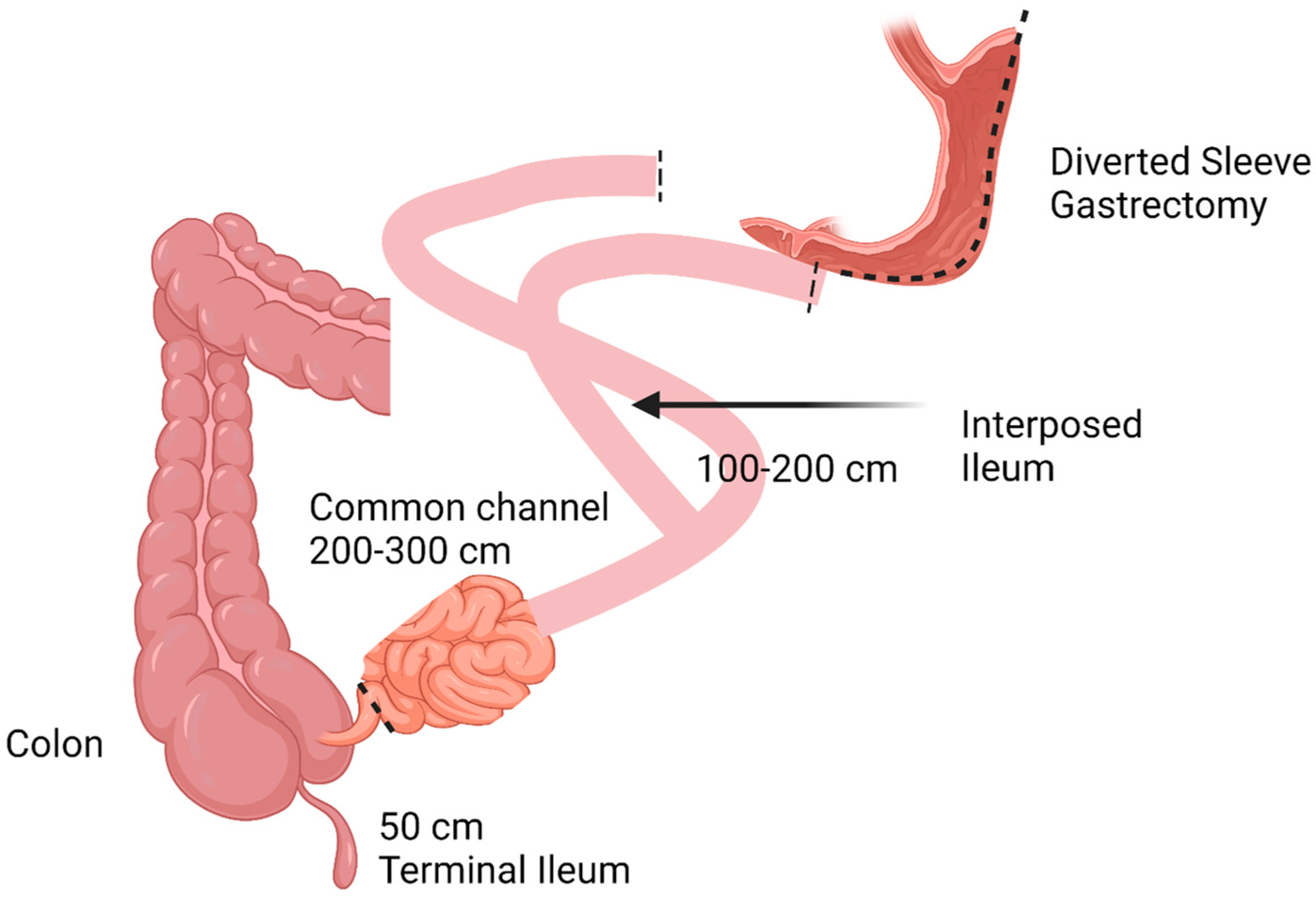
2.2. Brief Description of Surgical Procedures
2.3. Serum Biochemistry
2.4. Raman Spectroscopy
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Study Groups
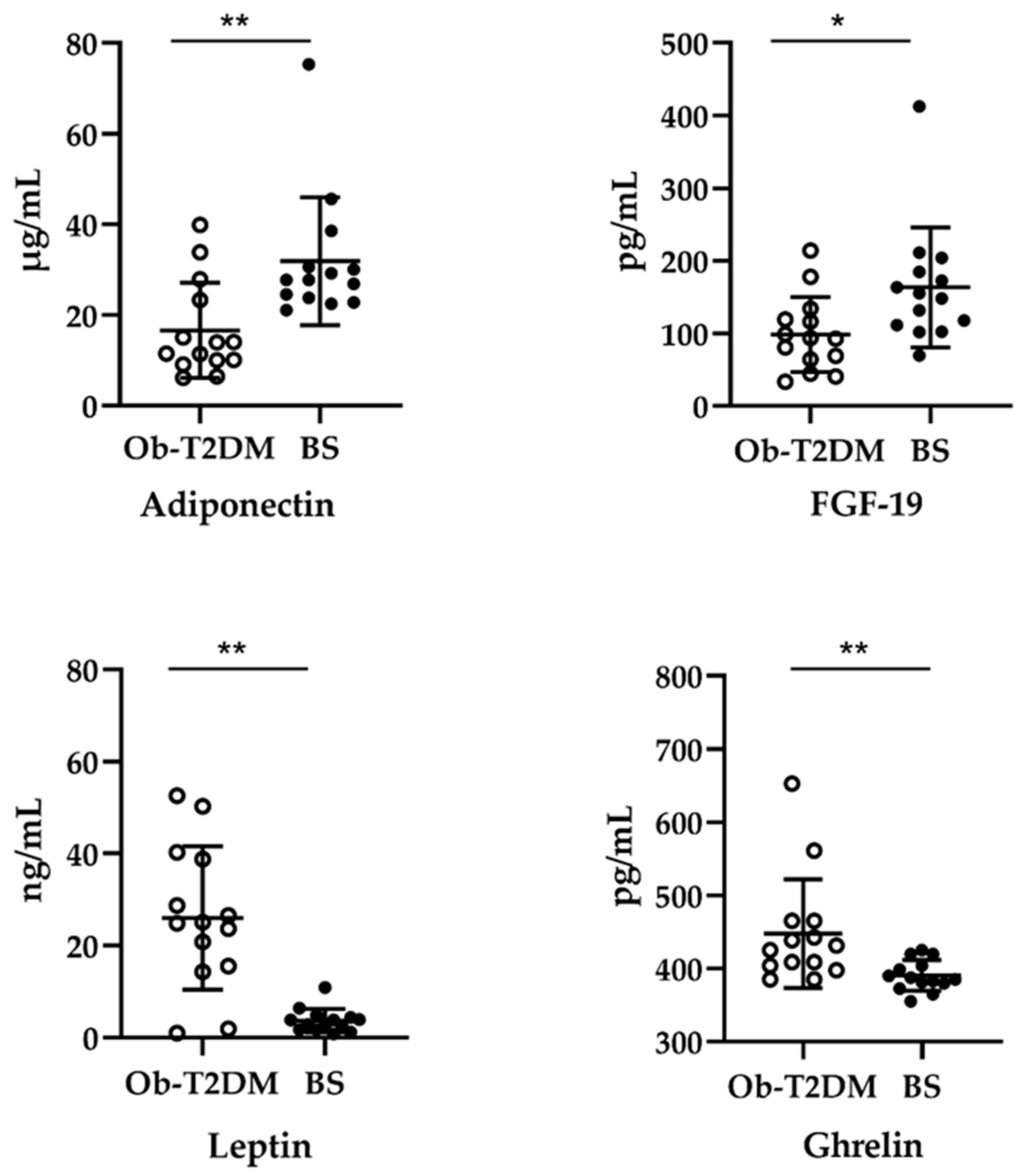
3.2. Effect of Bariatric Surgery on Serum Adipokines, Ghrelin, and FGF-19
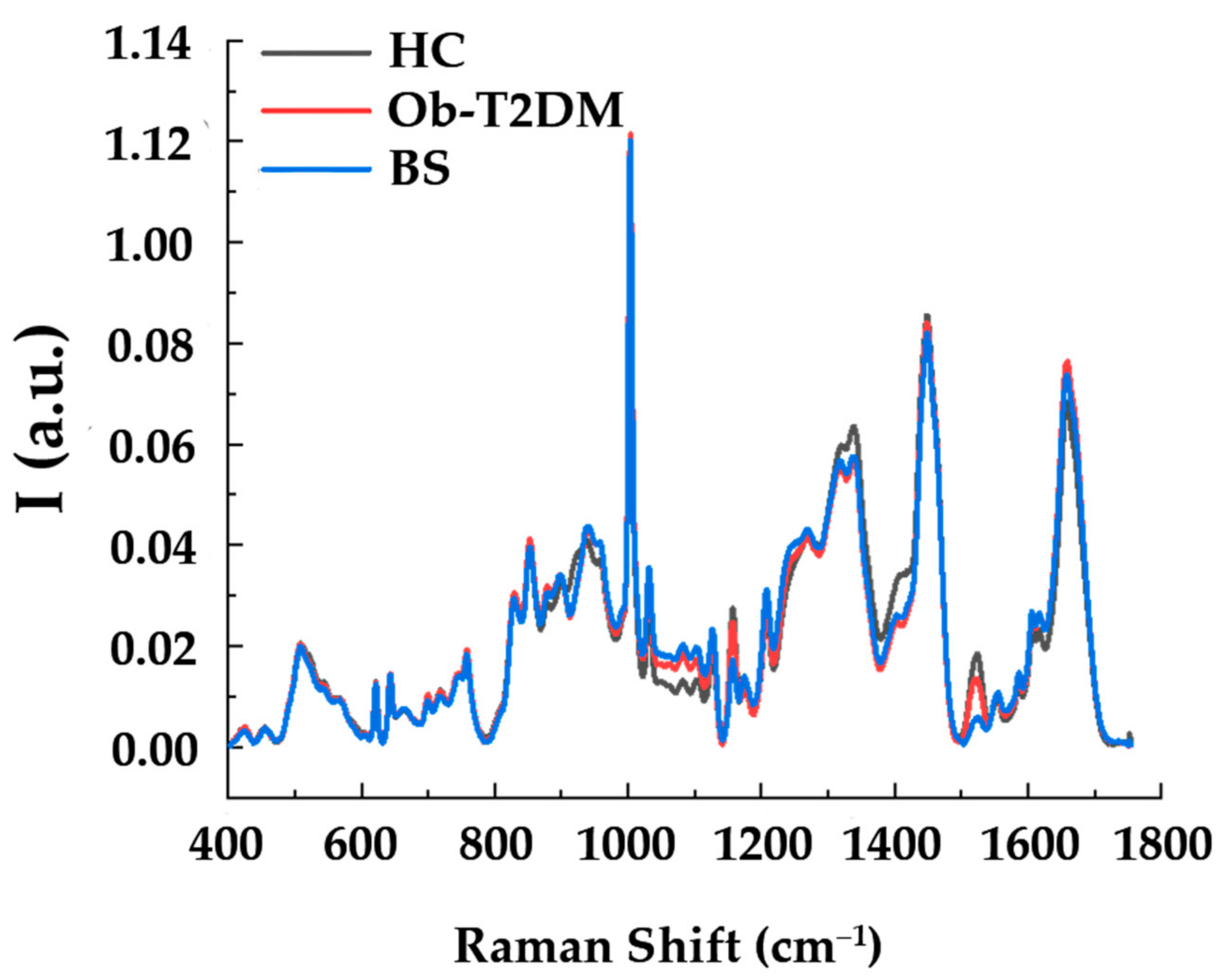
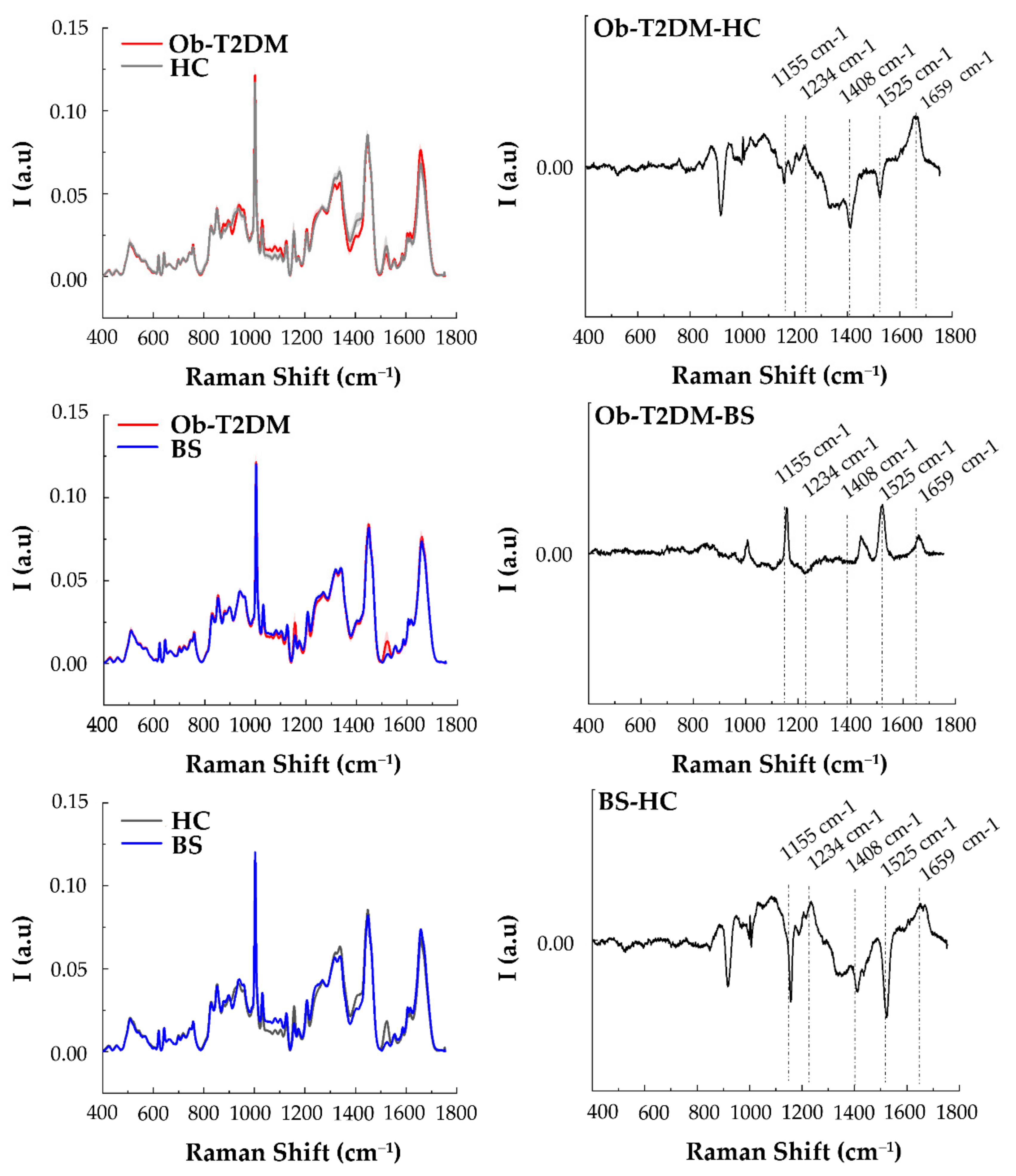
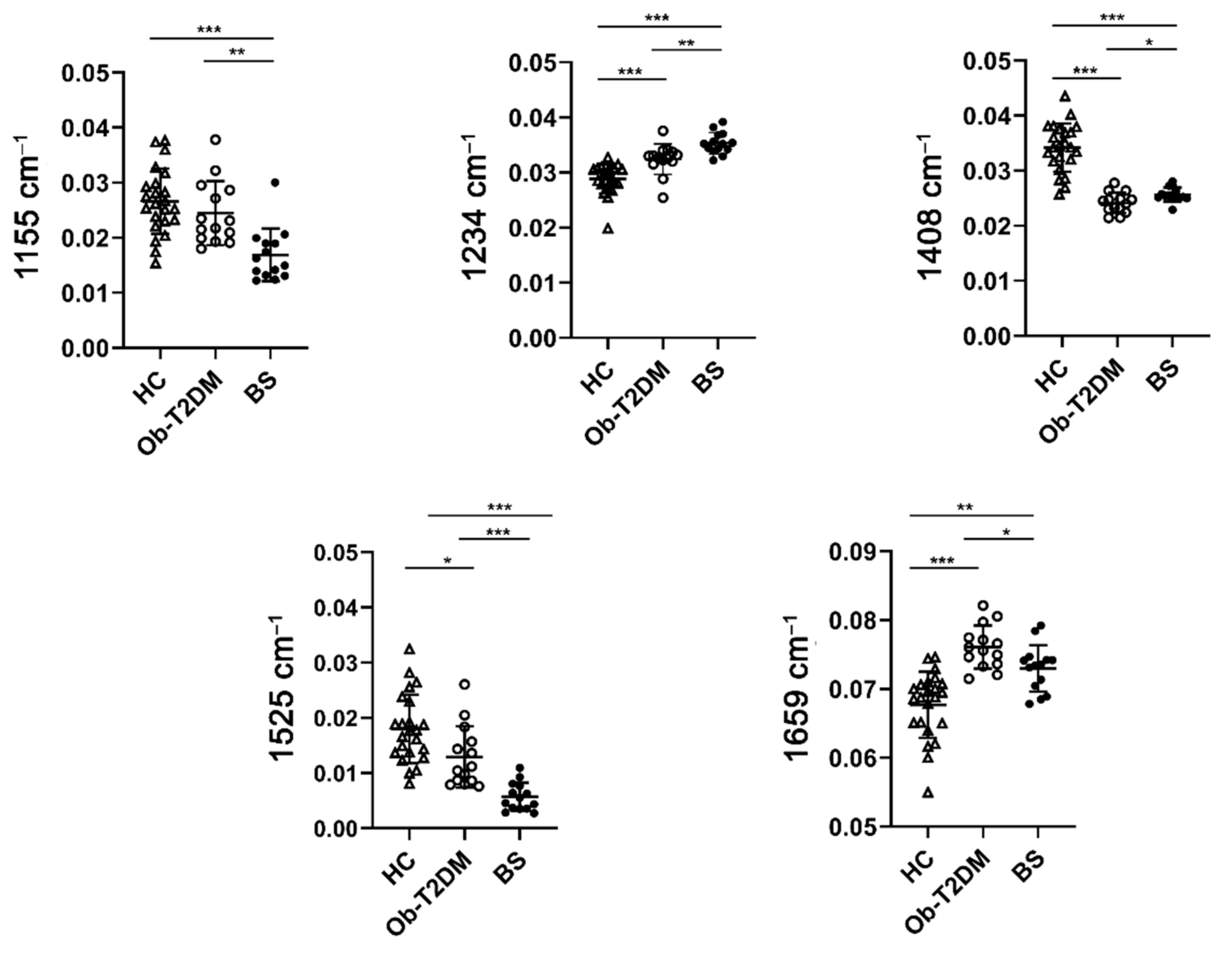
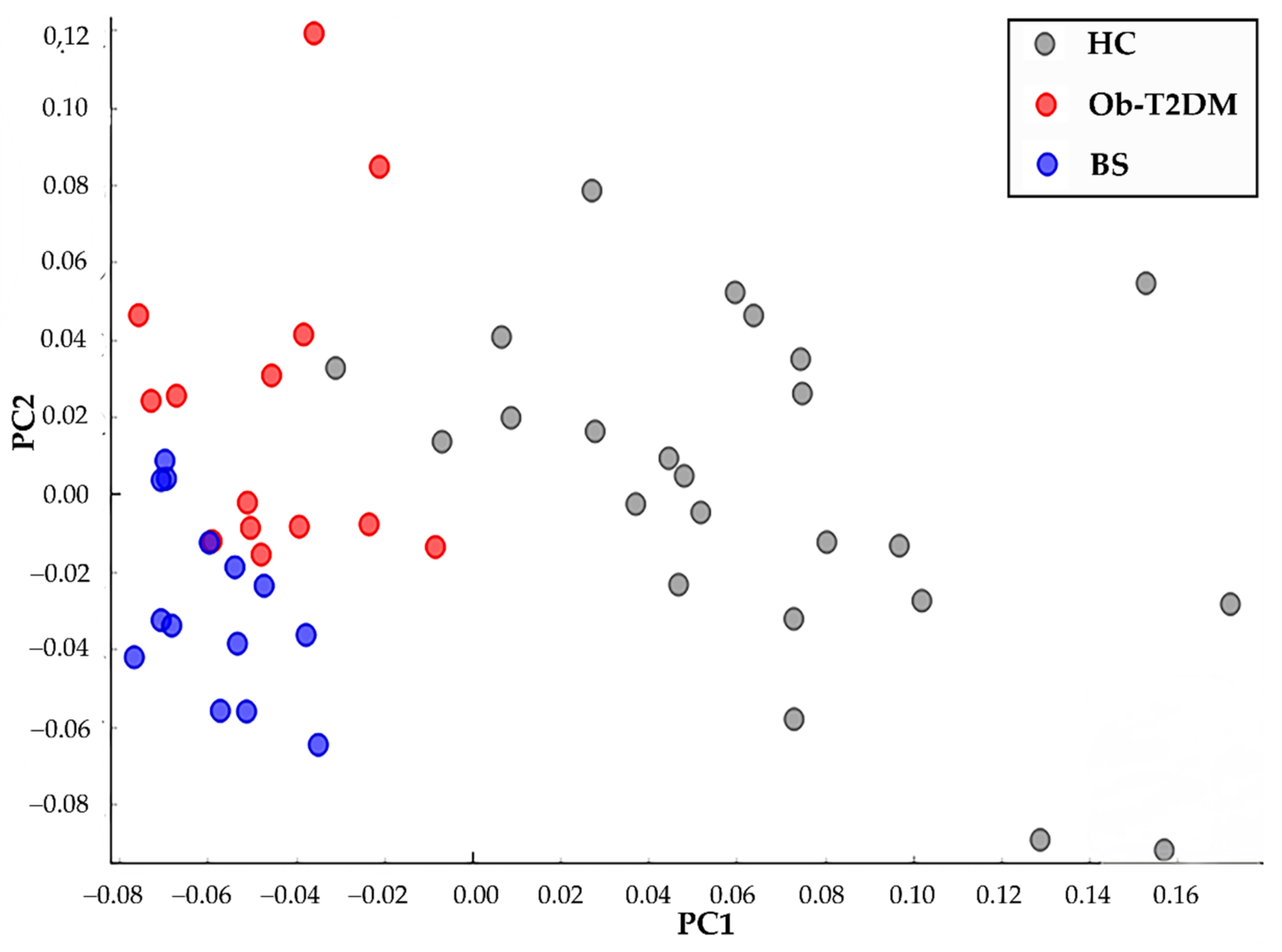
3.3. Raman Analysis of Serum of Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global Estimates of the Prevalence of Diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, B. The Burden of Cardiovascular Disease in Patients with Diabetes. Am. J. Manag. Care 2018, 24, S268–S272. [Google Scholar] [PubMed]
- Wu, G.; Cai, B.; Yu, F.; Fang, Z.; Fu, X.; Zhou, H.; Zhang, W.; Tian, Z. Meta-Analysis of Bariatric Surgery versus Non-Surgical Treatment for Type 2 Diabetes Mellitus. Oncotarget 2016, 7, 87511–87522. [Google Scholar] [CrossRef]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric Surgery versus Intensive Medical Therapy in Obese Patients with Diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Meyvis, K.; De Block, C.; Van Gaal, L.F. Can Bariatric Surgery Be Considered Standard Therapy to Treat Type 2 Diabetes? Eur. Endocrinol. 2010, 9, 86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric Surgery versus Non-Surgical Treatment for Obesity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Szoka, B.; Chudziak, D.; Batóg, P.; Macioch, T.; Niewada, M.; Belarbi, S.; Wcisło, J. Bariatric Surgery Versus Non-Surgical Treatment of Morbid Obesity: A Systematic Review. Value Health 2016, 19, A575–A576. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric–Metabolic Surgery versus Conventional Medical Treatment in Obese Patients with Type 2 Diabetes: 5 Year Follow-up of an Open-Label, Single-Centre, Randomised Controlled Trial. Lancet 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Papamargaritis, D.; le Roux, C.W. Do Gut Hormones Contribute to Weight Loss and Glycaemic Outcomes after Bariatric Surgery? Nutrients 2021, 13, 762. [Google Scholar] [CrossRef]
- Scopinaro, N.; Adami, G.F.; Bruzzi, P.; Cordera, R. Prediction of Diabetes Remission at Long Term Following Biliopancreatic Diversion. Obes. Surg. 2017, 27, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Ionut, V.; Burch, M.; Youdim, A.; Bergman, R.N. Gastrointestinal Hormones and Bariatric Surgery-Induced Weight Loss: Gut Hormones and Bariatric Surgery. Obesity 2013, 21, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Hutch, C.R.; Sandoval, D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology 2017, 158, 4139–4151. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y. Prediction of Type 2 Diabetes Remission after Bariatric or Metabolic Surgery. J. Obes. Metab. Syndr. 2018, 27, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, R.; Bunt, J.C.; Cortés, M.C.; Sugerman, H.J. Type 2 Diabetes after Gastric Bypass: Remission in Five Models Using HbA1c, Fasting Blood Glucose, and Medication Status. Surg. Obes. Relat. Dis. 2012, 8, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Belligoli, A.; Bettini, S.; Segato, G.; Busetto, L. Predicting Responses to Bariatric and Metabolic Surgery. Curr. Obes. Rep. 2020, 9, 373–379. [Google Scholar] [CrossRef]
- Shah, A.; Laferrère, B. Diabetes after Bariatric Surgery. Can. J. Diabetes 2017, 41, 401–406. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Bogart, A.; Sherwood, N.E.; Sidney, S.; Coleman, K.J.; Haneuse, S.; O’Connor, P.J.; Theis, M.K.; Campos, G.M.; McCulloch, D.; et al. A Multisite Study of Long-Term Remission and Relapse of Type 2 Diabetes Mellitus Following Gastric Bypass. Obes. Surg. 2013, 23, 93–102. [Google Scholar] [CrossRef]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Aminian, A.; Romero-Talamás, H.; Batayyah, E.; Mackey, J.; Kennedy, L.; Kashyap, S.R.; Kirwan, J.P.; Rogula, T.; Kroh, M.; et al. Can Diabetes Be Surgically Cured? Long-Term Metabolic Effects of Bariatric Surgery in Obese Patients with Type 2 Diabetes Mellitus. Ann. Surg. 2013, 258, 628–637. [Google Scholar] [CrossRef]
- Elshaer, A.M.; Almerie, M.Q.; Pellen, M.; Jain, P. Relapse of Diabetes After Roux-En-Y Gastric Bypass for Patients With Obesity: 12 Years Follow-up Study. Obes. Surg. 2020, 30, 4834–4839. [Google Scholar] [CrossRef] [PubMed]
- Torquati, A.; Lutfi, R.; Abumrad, N.; Richards, W. Is Roux-En-Gastric Bypass Surgery the Most Effective Treatment for Type 2 Diabetes Mellitus in Morbidly Obese Patients? J. Gastrointest. Surg. 2005, 9, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Morasso, C.F.; Sproviero, D.; Mimmi, M.C.; Giannini, M.; Gagliardi, S.; Vanna, R.; Diamanti, L.; Bernuzzi, S.; Piccotti, F.; Truffi, M.; et al. Raman Spectroscopy Reveals Biochemical Differences in Plasma Derived Extracellular Vesicles from Sporadic Amyotrophic Lateral Sclerosis Patients. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102249. [Google Scholar] [CrossRef] [PubMed]
- Vanna, R.; Ronchi, P.; Lenferink, A.T.M.; Tresoldi, C.; Morasso, C.; Mehn, D.; Bedoni, M.; Picciolini, S.; Terstappen, L.W.M.M.; Ciceri, F.; et al. Label-Free Imaging and Identification of Typical Cells of Acute Myeloid Leukaemia and Myelodysplastic Syndrome by Raman Microspectroscopy. Analyst 2015, 140, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Donjuán-Loredo, G.; Espinosa-Tanguma, R.; Ramírez-Elías, M.G. Raman Spectroscopy in the Diagnosis of Metabolic Syndrome. Appl. Spectrosc. Rev. 2021, 1–21. [Google Scholar] [CrossRef]
- González-Solís, J.L.; Villafan-Bernal, J.R.; Martínez-Zérega, B.E.; Sánchez-Enríquez, S. Type 2 Diabetes Detection Based on Serum Sample Raman Spectroscopy. Lasers Med. Sci. 2018, 33, 1791–1797. [Google Scholar] [CrossRef]
- Silveira, L.; De Cássia, R.; Borges, F.; Navarro, R.S.; Giana, H.E.; Zângaro, R.A.; Tadeu, M.; Pacheco, T.; Fernandes, A.B. Quantifying Glucose and Lipid Components in Human Serum by Raman Spectroscopy and Multivariate Statistics. Lasers Med. Sci. 2017, 32, 787–795. [Google Scholar] [CrossRef]
- Guevara, E.; Torres-Galván, J.C.; Ramírez-Elías, M.G.; Luevano-Contreras, C.; González, F.J. Use of Raman Spectroscopy to Screen Diabetes Mellitus with Machine Learning Tools. Biomed. Opt. Express 2018, 9, 4998. [Google Scholar] [CrossRef]
- Ricciardi, A.; Piuri, G.; Porta, M.D.; Mazzucchelli, S.; Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Corsi, F.; Cazzola, R.; et al. Raman Spectroscopy Characterization of the Major Classes of Plasma Lipoproteins. Vib. Spectrosc. 2020, 109, 103073. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Koliaki, C.; Kokkinos, A. Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction? Int. J. Environ. Res. Public Health 2019, 16, 3171. [Google Scholar] [CrossRef] [PubMed]
- Captieux, M.; Prigge, R.; Wild, S.; Guthrie, B. Defining Remission of Type 2 Diabetes in Research Studies: A Systematic Scoping Review. PLoS Med. 2020, 17, e1003396. [Google Scholar] [CrossRef] [PubMed]
- Foschi, D.A.; Rizzi, A.; Tubazio, I.; Conti, L.; Vago, T.; Bevilacqua, M.; Magni, A.; Del Puppo, M. Duodenal Diverted Sleeve Gastrectomy with Ileal Interposition Does Not Cause Biliary Salt Malabsorption. Surg. Obes. Relat. Dis. 2015, 11, 372–376. [Google Scholar] [CrossRef]
- Morasso, C.; Truffi, M.; Vanna, R.; Albasini, S.; Mazzucchelli, S.; Colombo, F.; Sorrentino, L.; Sampietro, G.; Ardizzone, S.; Corsi, F. Raman Analysis Reveals Biochemical Differences in Plasma of Crohn’s Disease Patients. J. Crohn’s Colitis 2020, 14, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, C.; Hocevar, T.; Milutinovic, M.; Možina, M.; Polajnar, M.; Toplak, M.; Staric, A.; et al. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Toplak, M.; Read, S.T.; Sandt, C.; Borondics, F. Quasar: Easy Machine Learning for Biospectroscopy. Cells 2021, 10, 2300. [Google Scholar] [CrossRef]
- Hansen, D.; Dendale, P.; Beelen, M.; Jonkers, R.A.M.; Mullens, A.; Corluy, L.; Meeusen, R.; van Loon, L.J.C. Plasma Adipokine and Inflammatory Marker Concentrations Are Altered in Obese, as Opposed to Non-Obese, Type 2 Diabetes Patients. Eur. J. Appl. Physiol. 2010, 109, 397–404. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 Serum Concentrations in Human Obesity and Type 2 Diabetes Behave Differently after Diet- or Surgically-Induced Weight Loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef]
- Lin, E. The Effects of Gastric Surgery on Systemic Ghrelin Levels in the Morbidly Obese. Arch. Surg. 2004, 139, 780. [Google Scholar] [CrossRef]
- Morínigo, R.; Casamitjana, R.; Moizé, V.; Lacy, A.M.; Delgado, S.; Gomis, R.; Vidal, J. Short-Term Effects of Gastric Bypass Surgery on Circulating Ghrelin Levels. Obes. Res. 2004, 12, 1108–1116. [Google Scholar] [CrossRef]
- Tymitz, K.; Engel, A.; McDonough, S.; Hendy, M.P.; Kerlakian, G. Changes in Ghrelin Levels Following Bariatric Surgery: Review of the Literature. Obes. Surg. 2011, 21, 125–130. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A. Raman Spectroscopy for the Detection of Cancers and Precancers. J. Biomed. Opt. 1996, 1, 31. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Simal-Antón, A.; Blanco-Navarro, I.; González-Dominguez, T.; Pérez-Sacristán, B. Depletion of Serum Carotenoid and Other Fat-Soluble Vitamin Concentrations Following Obesity Surgery. Obes. Surg. 2011, 21, 1605–1611. [Google Scholar] [CrossRef]
- Sawaya, R.A.; Jaffe, J.; Friedenberg, L.; Friedenberg, F.K. Vitamin, Mineral, and Drug Absorption Following Bariatric Surgery. Curr. Drug Metab. 2012, 13, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference Database of Raman Spectra of Biological Molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Ferraz, Á.A.B.; Carvalho, M.R.C.; Siqueira, L.T.; Santa-Cruz, F.; Campos, J.M. Micronutrient deficiencies following bariatric surgery: A comparative analysis between sleeve gastrectomy and Roux-en-Y gastric bypass. Rev. Col. Bras. Cir. 2018, 45, e2016. [Google Scholar] [CrossRef]
- Verger, E.O.; Aron-Wisnewsky, J.; Dao, M.C.; Kayser, B.D.; Oppert, J.-M.; Bouillot, J.-L.; Torcivia, A.; Clément, K. Micronutrient and Protein Deficiencies After Gastric Bypass and Sleeve Gastrectomy: A 1-Year Follow-Up. Obes. Surg. 2016, 26, 785–796. [Google Scholar] [CrossRef]
| Parameters | Ob-T2DM | BS |
|---|---|---|
| Patients | 14 (5 M; 9 F) | 14 (5 M; 9 F) |
| Age, years | 48.5 ± 12.4 | 48.5 ± 12.4 |
| Weight, kg | 112.4 ± 21.7 | 83.9 ± 13.9 *** |
| BMI, kg/m2 | 41.4 ± 6.2 | 30.2 ± 5.3 *** |
| Waist circumference, cm | 125.2 ± 15.1 | 103.9 ± 15 ** |
| Hips circumference, cm | 128.4 ± 15.9 | 104.6 ± 13.4 *** |
| Insulin, mU/mL | 14.1 ± 8 | 4.9 ± 5.8 *** |
| Glycemia, mg/dL | 158.5 ± 41.7 | 82.2 ± 10 *** |
| Glycemia 120′, mg/dL | 223.9 ± 62.5 | 137.8 ± 41.2 *** |
| HAb1c % | 7.7 ± 1.2 | 5.3 ± 0.4 *** |
| Total cholesterol, mg/dL | 211.8 ± 20.3 | 142.8 ± 26 *** |
| HDL-C, mg/dL | 42.6 ±7.2 | 52.3 ± 4.8 *** |
| Triglycerides, mg/dL | 178.1 ± 58.6 | 117.1 ± 31 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonizzi, A.; Sevieri, M.; Sitia, L.; Rizzi, A.; Conti, L.; Corsi, F.; Morasso, C.; Mazzucchelli, S. Metabolic Profiling of Type 2 Diabetes Patients after Bariatric Surgery by Raman Spectroscopy. Appl. Sci. 2021, 11, 10710. https://doi.org/10.3390/app112210710
Bonizzi A, Sevieri M, Sitia L, Rizzi A, Conti L, Corsi F, Morasso C, Mazzucchelli S. Metabolic Profiling of Type 2 Diabetes Patients after Bariatric Surgery by Raman Spectroscopy. Applied Sciences. 2021; 11(22):10710. https://doi.org/10.3390/app112210710
Chicago/Turabian StyleBonizzi, Arianna, Marta Sevieri, Leopoldo Sitia, Andrea Rizzi, Lorenzo Conti, Fabio Corsi, Carlo Morasso, and Serena Mazzucchelli. 2021. "Metabolic Profiling of Type 2 Diabetes Patients after Bariatric Surgery by Raman Spectroscopy" Applied Sciences 11, no. 22: 10710. https://doi.org/10.3390/app112210710
APA StyleBonizzi, A., Sevieri, M., Sitia, L., Rizzi, A., Conti, L., Corsi, F., Morasso, C., & Mazzucchelli, S. (2021). Metabolic Profiling of Type 2 Diabetes Patients after Bariatric Surgery by Raman Spectroscopy. Applied Sciences, 11(22), 10710. https://doi.org/10.3390/app112210710