Featured Application
Rowing on an air seat cushion with pressure set to 140 mmHg and using a low rowing cadence is suitable for beginners to increase the muscle oxygenation of the low back. If the purpose is to increase the cardiopulmonary function and muscle oxygenation, fast rowing on an air seat cushion with pressure set to 80 mmHg is recommended.
Abstract
This study investigated the effects of rowing with different seat cushion and cadence conditions on oxyhemoglobin (O2Hb) and total hemoglobin (tHb) levels of the erector spinae (ES) as well as the effects on heart rate (HR) and ratings of perceived exertion (RPE). Thirty healthy adults completed tests under three unstable air seat cushion pressure levels (0, 80, and 140 mmHg) and three rowing cadences (slow: 18 bpm, medium: 30 bpm, and fast: 36 bpm) on a rowing machine, for a total of nine test conditions. During the exercise period, rowing on cushions set to 80 mmHg resulted in greater O2Hb and tHb changes than did rowing at 0 mmHg (p < 0.05). When rowing cadence increased, the O2Hb and tHb decreased during the exercise period, whereas HR and RPE increased (p < 0.05). During the recovery period, O2Hb and tHb on cushions set to 140 mmHg during slow rowing were higher than those at 0 mmHg during slow rowing and 140 mmHg during fast rowing (p < 0.05). Rowing on an appropriate pressure of seat cushion and using a slow cadence contribute to increasing muscle oxygenation of low back during exercise.
1. Introduction
Sedentary behavior is defined as “any waking behavior characterized by an energy expenditure ≤1.5 METs (metabolic equivalent of task) while in a sitting or reclining posture” [1]. According to statistics, adults engage in sedentary behavior for approximately 8.65 h per day [2], and sitting for more than 7 h per day increases the risk of low back pain (LBP) [3]. Additionally, an immobile or inactive lifestyle can cause abnormal metabolism and an increased risk of cardiovascular disease in young people [4], adults, and older adults [5], and it may also decrease cardiopulmonary aerobic capacity [6]. Poor cardiopulmonary function is an independent risk factor for cardiovascular-related mortality [7].
Core muscles help control the position and motion of the trunk over the pelvis and legs to allow optimal production, transfer, and control of force and motion to the terminal segment during integrated kinetic chain activities [8], and the erector spinae is responsible for the extension and lateral flexion of the spine. Research has indicated that trunk muscle endurance (Biering–Sorenson and prone plank test) decreases after the age of 30 years [9], and the core muscle strength and endurance of people with LBP are lower than those of people without LBP [10]. Adequate blood perfusion provides sufficient oxygen and nutrients to muscle tissue and facilitates the removal of metabolites. Studies have demonstrated that fatigue occurs later when the body maintains a higher muscle tissue oxygenation concentration [11]. In certain studies, during dynamic exercise tests, healthy individuals experienced a lower decline in oxygen concentration in the back muscles than did patients with LBP [12,13], which means that less oxygen was consumed by the healthy individuals [14]. Tissue oxygenation in the lumbar extensor musculature was reduced as a function of contraction intensity (2% to 30% of maximal voluntary contraction) [12]. When the amount of blood that reaches the muscles is insufficient and blood volume is reduced, the aerobic exercise capacity of muscle fibers may decrease [15]. Studies have confirmed that exercise training can partially improve oxygen transport in the erector spinae [15,16]. Increasing the capacity of trunk muscles can not only reduce the symptoms of back muscle fatigue [17] but also alleviate LBP during physical activity [18].
The mechanical rowing machine is a dynamic and non-weight-bearing piece of equipment that combines muscle strength and cardiopulmonary training. It not only reduces the joint load of the lower limbs but also involves 70% of the body’s muscles, including the thighs, upper arms, and core muscles [19]. Rowing exercises target the back muscles, especially in the drive phase. Therefore, rowing is considered an excellent means of training the core and thigh muscles [20], and its cardio training intensity can reach 7–12 METs [21]. As rowing movement velocity (i.e., cadence or stroke frequency) increases, the power output and the activity of the erector spinae increase accordingly [22]. However, individuals must pay attention to muscle fatigue and excessive load on the lower back caused by the spine curve being involved in repeated full and fast strokes [23]. Therefore, determining the appropriate rowing cadence that benefits lower back muscles is crucial. Moreover, many studies have confirmed that spine movement helps to improve fluid transport in intervertebral discs and has beneficial effects against the shrinkage of the spine and compression of intervertebral discs [24,25,26]. Sitting on an unstable seat cushion has been proven to increase lumbar spine activity without increasing trunk muscle activity [27]. A previous study found that adding an unstable seat cushion to the rowing machine increased lower back sway during rowing [28]. Thus, rowing on an unstable seat cushion may be a suitable exercise means to improve oxygen transport in the erector spinae through adding spine movement. A hemodynamic study related to rowing mainly investigated the legs and hands [29]. To the best of our knowledge, there is a lack of relevant research on the erector spinae. In the present study, it was hypothesized that the instability of seat cushions and different rowing cadences affect muscle tissue oxygenation and blood volume of the erector spinae as well as heart rate (HR) and ratings of perceived exertion (RPE). The results of the present study can provide recommendations for rowing exercises that increase blood volume and oxygen in back muscles and increase cardiopulmonary stimulation.
2. Materials and Methods
2.1. Study Design
We used a randomized repeated measurements design. All participants completed tests under 3 unstable air seat cushion pressure levels (0, 80, and 140 mmHg), and 3 rowing cadences characterized by different stroke rates (slow: 18 bpm, medium: 30 bpm, and fast: 36 bpm), for a total of 9 test conditions. The order of the test conditions was randomly determined. All participants performed 10 strokes under each condition and followed the cadence of the metronome. Each condition test had at least a 2 min interval, and all 9 tests were completed on the same day.
2.2. Participants
Thirty healthy adults participated in this study. For inclusion, participants were required to have (1) no injuries, fractures, dislocations, tendinitis, or neuromuscular disease within 1 year prior to the experiment; (2) no LBP symptoms (their score on the Chinese version of the Oswestry LBP disability questionnaire was 0 points). Participant’s mass, body fat (%), and skeletal muscle (%) were measured by a body composition monitor (HBF-702T; Omron Healthcare Co. Ltd., Kyoto, Japan). The valid data of 5 participants could not be obtained because near-infrared spectroscopy (NIRS) data were lost for one or more tests. Therefore, only 25 participants’ data (age: 22.4 ± 5.8 years; Table 1) were included in the analysis for the physiological parameter. A total of 12 of the 30 participants (age: 24.6 ± 11.5 years, age range: 18–24 years) were randomly selected to participate in an additional session to record their pulling force during rowing with the same experimental conditions.

Table 1.
Participant characteristics.
2.3. Procedures
Before experiments, participants attended an orientation session to familiarize themselves with the rowing technique and the different rowing stroke rates. Participants practiced rowing until they could maintain the indicated rowing cadence and perform the correct rowing movement. A professional rowing instructor taught each participant the rowing technique and confirmed the correction of movements. The rowing strokes can be divided by a drive phase and a recovery phase. Participants were taught to follow several principles: (1) the drive phase is initiated with a push from the legs, followed by the hips beginning to open, and finally the arms begin to draw the handle to the body once the legs are fully extended and the back is vertical. (2) The handle finishes about half way up the body and the elbows follow the line of the handle and the wrists stay in line with the forearm. (3) The recovery is initiated with the arms straightening, followed by the body pivoting from the hips before the legs bend, and finally the legs flex until the shins are vertical. (4) Throughout the co-ordination of the drive and recovery phases, the back should remain in a neutral position.
In the orientation session, the rowing instructor observed that some participants could not keep up with the fast cadence (36 bpm) after 10 consecutive strokes, resulting in incorrect and inconsistent movements, even though they had been trained. Thus, this study selected the number of strokes in each rowing test as 10 strokes to ensure that all the participants’ movements during the rowing were cadenced, correct, and consistent.
On the day of the experiment, participants first performed a 7-min warm-up, including 2 min of shoulder, ankle, and wrist activities and 5 min of full body stretches. After participants finished warming up, the wireless NIRS device was attached to the L4 part of the right erector spinae and the HR monitor was worn (Forerunner 920XT GPS; Garmin, Taiwan). The order of the rowing tests was then randomly determined. Before each test, the air seat cushion pressure was adjusted in accordance with the test condition. The pre-exercise period began with the rowing test; during this period, the participant sat static on the rowing machine (Oxford II, Horizon Fitness, Inc., Taiwan) for 1 min. Next, in the exercise period, the participant performed 10 strokes in accordance with the cadence of the metronome and then rested for 1 min (recovery period). NIRS, HR, and RPE data were collected during the tests. During the experiment, the resistance of the rowing machine was set to the lightest setting.
2.4. Pressure Biofeedback Unit
A pressure biofeedback unit (PBU; Stabilizer, Chattanooga, United Kingdom; Figure 1) was used in the present study as an unstable air seat cushion. The PBU is a simple pressure transducer that consists of a 3-chamber air-filled pressure bag, a catheter, and a sphygmomanometer. The pressure bag was 16.7 × 24 cm2 in size and composed of inelastic material. The sphygmomanometer had a range of 0–200 mmHg (2-mmHg scale intervals). A previous study indicated that sitting on an air seat cushion (PBU) with high air pressure (140 mmHg) to perform rowing machine exercises increased lower back sway during rowing [28]. As in the previous setting [28], we set the air pressure of the PUB while the participant was sitting on it to 0% (0 mmHg, stable surface), 40% (80 mmHg), and 70% (140 mmHg) of the sphygmomanometer measurable range, i.e., the pressure testing conditions.

Figure 1.
Pressure biofeedback unit.
2.5. NIRS Measurement
We used a wireless PortaMon continuous near-infrared spectrometer to determine muscle blood volume and tissue oxygenation (PortaMon, Artinis Medical System BV, The Netherlands). The device was secured to the right erector spinae muscle corresponding to the right side of the fourth section of the lumbar spine, and it was fixed using nonwoven fabric tape. NIRS wavelengths were set to 760 and 850 nm, and the sampling rate was 10 Hz. The data acquisition systems and stopwatches were activated synchronistically by an experienced researcher to record the signals. The parameters of NIRS responses are displayed in Figure 2a,b.
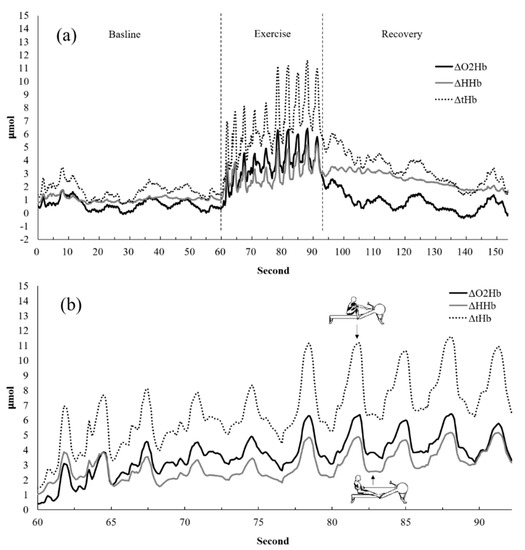
Figure 2.
Representative participant’s erector spinae muscle oxygenation response (a) during the rowing exercise test and (b) during the drive and recovery phase in the rowing exercise test. ΔO2Hb: oxyhemoglobin change. ΔHHb: deoxyhemoglobin change. ΔtHb: total hemoglobin change.
OxySoft analysis software was used to download and export NIRS data and analyze oxyhemoglobin (O2Hb) and total hemoglobin (tHb) information. Changes to O2Hb and tHb during each cadence and pressure condition in the seated participants were used as indicators in the present study. The resting data were at least 10 s of data obtained when participants were seated and stationary in the initial pre-exercise period. The average of peak high values of each rowing during the exercise period presented the exercise phase response. The average of the peak high values of each rowing during 1 min recovery presented the recovery phase response. The response of the exercise phase was the average of the 10 peak high value during exercise period minus the average of the resting data. The response of the recovery phase was the average of the peak high value during recovery period minus the average of the resting data.
2.6. Heart Rate Measurement
We used the heart rate monitors (Forerunner 920XT GPS; Garmin, Taiwan) during the experiment to monitor HR. HR measurements were conducted 10 s before the exercise (resting), immediately after exercise (post exercise), and at the end of the recovery period (recovery). Changes in HR were represented as heart rate response (HRR) and calculated using the following formulas:
HRRexe = (HR post exercise) − (HR resting)
HRR recovery = (HR recovery) − (HR post exercise)
2.7. Rate of PER
The category-ratio (0–10) RPE scale (BORG-CR10) developed by Borg [30] was used to evaluate participants’ effort during the exercise and recovery periods. RPE was measured at resting, post exercise, and recovery. Changes to the two RPE measures were presented as the rate of PER (RPER), which was calculated using the following formulas:
RPERexe = (RPE post exercise) − (RPE resting)
RPER recovery = (RPE recovery) − (RPE post exercise)
2.8. Pull Force Measurement
An additional session was held in this study to record the pulling force during rowing with the same experimental conditions (3 unstable air seat cushion pressure levels and 3 rowing cadences). The pull force was measured by a load cell (MLP-300, Transducer Techniques, CA, USA) mounted between the handle and the cable and recorded at 1000 Hz by a data acquisition system (Biopac MP150, Biopac Systems, Inc., Goleta, CA, USA). A 10-Hz low-pass filter was used to eliminate high-frequency noises. The maximal pull forces for each stroke were analyzed and the average of 10 strokes was taken for statistical analysis.
2.9. Statistical Analyses
IBM SPSS statistics 20 software was used for analysis of the effects of seated pressure and rowing cadence. A two-factor (cushion pressure × rowing cadence) repeated measures analysis of variance (ANOVA) was used to assess the effects of different cadences and pressure as well as their interaction effects on NIRS parameters, HR, RPE, and pull force. An LSD post hoc analysis was conducted. The alpha level for declaring statistical significance was set at p ≤ 0.05. Data are presented as mean ± standard error. We performed G Power software (Heinrich–Heine–Univer sität Düsseldorf, Germany) to calculate the effect size and data power. In the exercise period, the effect size were from 0.21 to 1.53 and the power were from 0.60 to 0.99 with O2Hb, RPE, tHb, and HR. During the recovery period, the HR effect size of cadences main effect was 1.47, and power was 0.99. The cadences effect size of RPE were from 0.24 to 0.25, and power were from 0.70 to 0.72. The cadences effect size of O2Hb under 140 mmHg PBU was 0.52, and power was 0.99. The pressure effect size of O2Hb under fast and low cadence were 0.3 and 0.2, respectively. The power were 0.88 and 0.53, respectively. The cadences effect size of tHb under 140 mmHg PBU was 0.54, and power was 0.99. The pressure effect size of tHb under low cadence was 0.3 and power was 0.87. The pull force effect size of cadences main effect was 1.79 and power was 0.99.
3. Results
3.1. Exercise Period
All outcomes in the exercise period were related to the main effects of cushion pressure or rowing cadence (Table 2). For different cushion pressure levels, increments of O2Hb and tHb in the exercise period under 80 mmHg were significantly higher than those at 0 mmHg (p = 0.039). No main effects of different cushion pressures on HR were observed. The increments of RPE in the exercise period under 140 mmHg tended to be higher than those under 0 mmHg (p = 0.083). As for different rowing cadences, the increments of O2Hb and tHb at high rowing cadences during the exercise period tended to be lower than those at low rowing cadences (p = 0.067; p = 0.097). HR and RPE increased with rowing cadence; they were significantly higher at high rowing cadences than at low (p < 0.001; p = 0.001) and medium rowing cadences (p = 0.004; p = 0.021), and HR and RPE increments were higher at medium cadences than at low cadences (p < 0.001; p = 0.026).

Table 2.
Main effects of exercise under different seat pressure and rowing cadence conditions (Mean ± SE).
3.2. Recovery Period
The results of O2Hb and tHb in the recovery period indicated the presence of interaction effects (Figure 3). The increments of O2Hb under 80 mmHg during fast rowing were significantly higher than those under 140 mmHg during fast rowing (p = 0.005). The increments of O2Hb under 140 mmHg during slow rowing were also significantly higher than those under 140 mmHg during fast rowing (p = 0.045) and under 0 mmHg during slow rowing (p = 0.039). The increments of tHb under 140 mmHg during slow rowing were significantly higher than those under 140 mmHg during fast rowing (p = 0.041) and under 0 mmHg during slow rowing (p = 0.045, Figure 4). Moreover, the increments of tHb under 80 mmHg at medium and high rowing cadences tended to be higher than those under 140 mmHg at the same cadences (p = 0.091). Decreases in HR indicated the main effects of rowing cadence (Figure 5); decreases in HR during medium and fast rowing were significantly larger than those during slow rowing (p < 0.001). The decreases in RPE indicated interaction effects as follows (Figure 6): decreases in RPE under 0 mmHg during medium and fast rowing were significantly larger than those during slow rowing under the same pressure (p = 0.018; p = 0.002), RPE decreases under 80 and 140 mmHg during fast rowing were significantly larger than those during slow rowing under the same pressure (p = 0.015; p = 0.006), and RPE decreases under 140 mmHg during medium rowing were significantly larger than those under 0 and 80 mmHg at the same rowing cadence (p < 0.001; p = 0.004).
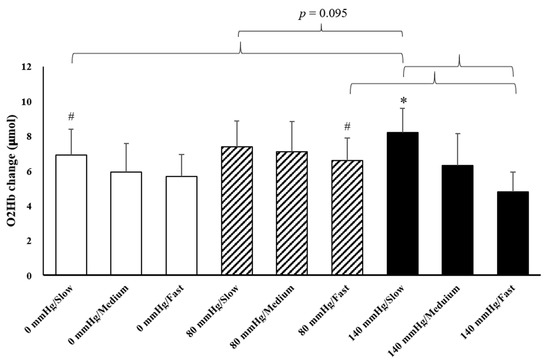
Figure 3.
Changes in O2Hb to various pressure and rowing cadence conditions during the recovery period (mean ± SE). * Significantly different from a high cadence under 140 mmHg. # Significantly different from 140 mmHg at the same cadence.
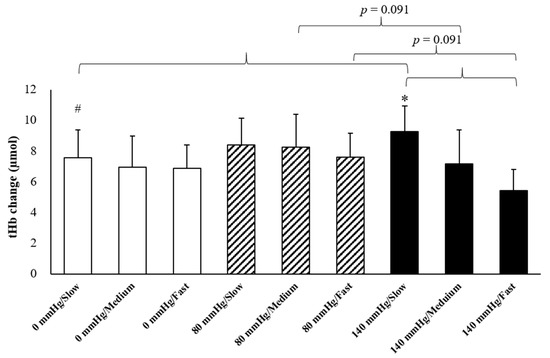
Figure 4.
Changes in tHb under various pressure and rowing cadence conditions during the recovery period (mean ± SE). * Significantly different from a high cadence under 140 mmHg. # Significantly different from 140 mmHg under the same cadence.
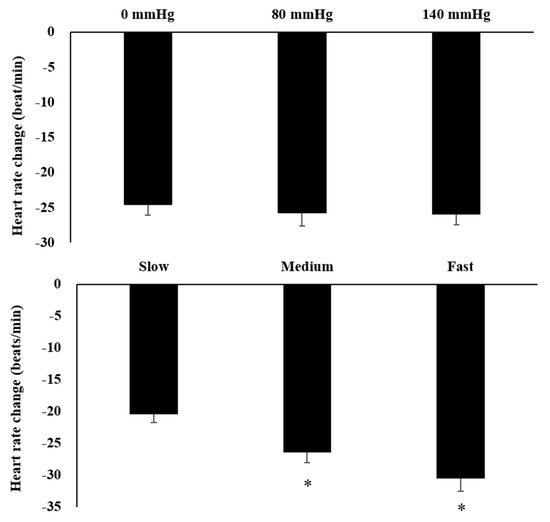
Figure 5.
Main effects of heart rate on various pressure and rowing cadence conditions during the recovery period (mean ± SE). * Significantly different from a low cadence.
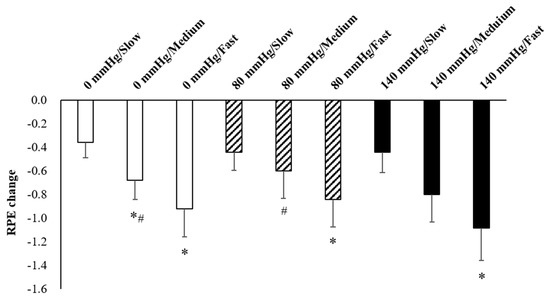
Figure 6.
Responses of RPE to various pressure and rowing cadence conditions during the recovery period (mean ± SE). * Significantly different from low cadence under the same pressure. # Significantly different from 140 mmHg under the same cadence.
3.3. Pull Force
The result showed the main effects of rowing cadence on the pull force (p < 0.001, Table 2). The average of maximal pull force all significantly increase with rowing cadence (p < 0.01).
4. Discussion
We determined the best rowing seat cushion pressure and rowing cadence conditions that increased tissue oxygenation and blood volume of the erector spinae muscle, increased cardiopulmonary stimulation, and reduced effort during exercise and recovery periods. The present study confirmed that rowing on cushion pressure under 80 mmHg can increase O2Hb and tHb during the exercise period, whereas fast rowing can result in a higher HR. Moreover, we also determined that a cushion pressure of 140 mmHg is suitable for slow rowing and can increase O2Hb and tHb in the recovery period and reduce effort.
In the present study, we determined that the tissue oxygenation and blood volume of the erector spinae under different seat cushion pressure levels increased during the exercise period. The increments of O2Hb and tHb at 80 mmHg were higher than those at 0 mmHg. This may have been caused by a slight increase in the sway of the lower back in the sagittal plane under 80 mmHg pressure, but the sway did not cause instability. However, it increased the flexion–extension activity of the lumbar spine and even reduced the contraction of the erector spinae. In a previous study, which used the same rowing conditions (i.e., same rowing machine, seat cushion, and cadence) as the present study, researchers determined that the seat cushion pressure of 80 mmHg increased sagittal sway in the lower back (about + 4%) during rowing but slightly reduced erector spinae activation (reduced from 10.89% to 10.11% maximum voluntary isometric contraction (MVIC)) compared with stable surface erector spinae activation [28]. Movement of the spine helps improve fluid transport in intervertebral discs and has beneficial effects against shrinkage of the spine and compression of intervertebral discs [24,25,26]. However, if isometric contraction intensity is greater than 20% MVIC, tissue oxygen levels and blood volume decrease with increases in contraction time and intensity [31]. Therefore, blood and oxygen reperfusion during the rowing recovery phase (which occurred as the participant returned to the catch position for the next stroke) could be increased by increasing lumbar activity (including muscle contraction and relaxation), but the intensity of muscle contractions should be maintained under low activation. For example, in the present study, rowing on the seat cushion under 80 mmHg with light resistance increased the tissue oxygenation and blood volume of the erector spinae by slightly increasing potential lumbar activity. However, the tissue oxygenation and of the erector spinae under 140 mmHg were not greater than those under 0 mmHg. The RPE results indicated a significant increase compared with 0 mmHg (p = 0.083; Table 2). The increased difficulty related to the unstable surface may have caused greater stress, thereby increasing RPE and obstructing tissue oxygenation and blood volume. The previous study also determined that a seat cushion with 140 mmHg of pressure significantly increased lower back sway in the frontal and horizontal planes during rowing but also had a trend intended to increase erector spinae activation (an increase from 10.89% to 12.02% MVC) compared with that under stable surface conditions [28]. Therefore, according to the results of the present study, higher cushion pressure does not always lead to increased muscle oxygenation and blood volume. A proper cushion pressure range is required to increase tissue oxygenation and blood volume in the back muscles.
In the present study, the increases in O2Hb and tHb during fast rowing tended to be lower than those during slow rowing (p = 0.067). This indicated that faster stroke rates are not conducive to tissue oxygenation and of the erector spinae muscle. Muscle contraction type and exercise intensity affected the oxygen concentration of muscle tissue and blood volume responses. A previous study revealed that regarding isometric contraction, whether the contraction was 20%, 40%, 60% or 80% of MVIC, tissue oxygenation and blood volume decreased with contraction time and intensity increases [31]. During the dynamic exercise mode test, oxygen saturation decreased as action cadence increased when participants performed dynamic knee extension exercises with added weights of 2.4 kg. Blood volume was higher at knee extension frequencies of 30 and 50 bpm than blood volume before exercise, but blood volume was lower than before exercise at action frequencies of >50 bpm [32]. That previous study showed that the oxygen saturation of muscle tissue decreased with an increase in treadmill load, but when speed was increased and load decreased, the oxygen saturation in the muscles increased [33]. According to the aforementioned research, exercise load has marked effects on the oxygen saturation of muscle tissue. Oxygen consumption increases as exercise load increases, and oxygen consumption decreases as exercise load decreases. If exercise load is reduced, blood volume increases even though speed increases, but blood volume decreases if the load is increased alongside a speed increase. In the present study, the pull force during rowing increased with stroke rate (cadence). Therefore, in the present study, the increases in tissue oxygenation and blood volume of the erector spinae during exercise were lower during fast rowing than that during slow rowing because rowing load increased with speed (cadence).
Another finding in the present study was the critical influence of cadence on HR and RPE responses to exercise. HR and RPE increased as the rowing cadence increased, which meant that a stronger cardiopulmonary stimulation occurred. To our knowledge, only one study investigated HR responses to rowing on an unstable surface [34]. That study compared the 2000 m rowing performance and HRs measured by a traditional stable ergometer, a transversally compliant ergometer, and a frontally compliant ergometer (FCE) attached to a tilt-board underneath. The study indicated that the FCE resulted in a higher HR response and poorer participant performance in 2000 m rowing racing, and this was potentially due to issues with balance maintenance [34]. In the present study, HR increased by approximately 30 bpm during fast rowing, and the average intensity of exercise (65–67% HRmax) was moderate (64–76% HRmax), as classified by the ACSM [35]. Because rowing exercises involve approximately 70% of the body’s muscles [19], the fast rowing demonstrated in the present study can be a favorable means of performing cardiopulmonary exercise when paired with the movement of the whole body’s large muscle groups. However, the different seat cushion pressure levels in the present study had no effect on HR and RPE, which suggested that the difficulty related to the use of unstable surfaces did not change the exercise intensity.
In the present study, increases in O2Hb in the recovery period under 80 mmHg during fast rowing were higher than those under 140 mmHg at the same cadence (Figure 3). The increases in O2Hb during the recovery period were calculated through the subtraction of O2Hb before exercise, which reflects increases in O2Hb during the recovery period compared with the rest period. We determined that fast rowing could prevent large increases in tissue oxygenation and blood volume of the erector spinae. Moreover, sitting on cushions with pressure 140 mmHg increased instability and RPE; these increases were obstructive to increases in tissue oxygenation and blood volume of the erector spinae. Therefore, increases in O2Hb and tHb in the recovery period under 140 mmHg during fast rowing were significantly lower than under 80 mmHg with fast rowing. We also determined that increases in O2Hb during slow rowing under 140 mmHg were significantly higher than those during fast rowing under the same cushion pressure, and increases in O2Hb tended to be higher under 140 mmHg than under 0 mmHg and 80 mmHg during slow rowing (p = 0.095). As mentioned, cushion pressure of 140 mmHg resulted in a relatively unstable surface, and fast rowing was not conducive to tissue oxygenation and blood volume of the erector spinae. Therefore, as displayed in Figure 3 and Figure 4, the increases in tissue oxygenation and blood volume were lowest under 140 mmHg during fast rowing. Additionally, Figure 3 indicates that slow rowing under three different seat cushion pressure levels better maintained blood volume and tissue oxygenation than medium and high rowing cadences did. Therefore, slow rowing was more conducive to tissue oxygenation and blood volume of the erector spinae during the recovery period because it helped maintain oxygen concentration and increase blood volume. The different pressure levels were also important factors.
In the present study, the magnitude of HR decrease during fast and medium rowing were significantly larger than those during slow rowing. The previous study demonstrated that larger decreases in HR during the recovery period after exercise were associated with higher exercise intensity [36]. Increased HR during the exercise period was significantly larger during fast and medium rowing than during slow rowing; therefore, decreases in HR during the recovery period were also greater. RPE had similar trends; decreases in RPE during the recovery period under all pressure levels were significantly higher during fast rowing than during slow rowing. At a medium rowing cadence, decreases in RPE during the recovery period were significantly greater under 140 mmHg than under 0 mmHg or 80 mmHg. This could be because at the same rowing cadence, the RPE under 140 mmHg tended to increase more during exercise, thus leading to a greater decrease in recovery.
The results of the present study can be used to provide exercise suggestions related to nonresistance rowing exercises to enhance blood volume to the back muscles and improve cardiopulmonary workouts. Because a beginner’s movement familiarity, muscle strength, muscular endurance, and cardiorespiratory fitness are poor, we suggest that beginners use slow rowing to develop movement skills and physical fitness. If the purpose of the exercise is to effectively increase the tissue oxygenation and blood volume of back muscles, we recommend using seat cushion pressure under 140 mmHg with a low rowing cadence (stroke rate: 18 bpm). If an individual is familiar with the related movements, has an adequate level of physical fitness, and seeks to strengthen their cardiopulmonary function and increase their tissue oxygenation and blood volume of the back muscles, we recommend using seat cushion pressure under 80 mmHg with a high rowing cadence (stroke rate: 36 bpm). Recreational boating is a popular leisure activity in western countries (ex: USA) Recreation boats include several types of power boats (ex: pontoon boats, PWCs, open power boats), canoe, kayaks, paddleboards, row boats, and so on [37]. The present study demonstrated that rowing cadence affects the tissue oxygenation and blood volume of the erector spinal muscle, HR, and RPE during exercise and recovery period. Therefore, the present study results do not apply to recreation boating except rowed boats with controlled rowing cadences.
The present study had three limitations. First, it only analyzed changes in tissue oxygenation and blood volume of the erector spinae and several physiological parameters during 10 strokes, which did not meet the requirement of at least 10 min of cardiopulmonary stimulation [38]. The second limitation is that the present study did not investigate rowing ergometer resistance load. In the present study, HR increased by 20–30 bpm during exercise and RPE increased by 0.38–1.17, which indicates that this was a relatively effortless rowing test. Therefore, the present study could not provide a reference for responses of tissue oxygenation, blood volume, and physiological parameters to rowing exercises with different resistance and exhaustion levels. Moreover, the different responses between sports rowers and untrained rowers was not well known. The present study did not compare the responses between sports rowers and uninitiated people. In the future, researchers can further investigate the three aforementioned conditions.
5. Conclusions
Rowing cadence affects the tissue oxygenation and blood volume of the erector spinae muscle, HR, and RPE during exercise. When rowing cadence increased, blood volume decreased, whereas HR and subjective effort increased. During the recovery period, the increases in O2Hb and tHb were higher after rowing exercises under 80 mmHg during fast rowing (stroke rate: 36 bpm) and under 140 mmHg during slow rowing (stroke rate: 18 bpm). Therefore, slow rowing was suitable for seat cushion pressure under 140 mmHg, and fast rowing was suitable for seat cushion pressure under 80 mmHg.
Author Contributions
Conceptualization, F.-Y.C.; data curation, W.-H.C., Y.Y., H.-Y.C. and E.-T.W.; formal analysis, Y.Y. and E.-T.W.; funding acquisition, F.-Y.C.; investigation, K.-Y.C. and W.-H.C.; methodology, K.-Y.C., W.-H.C. and H.-Y.C.; project administration, F.-Y.C.; resources, F.-Y.C.; supervision, F.-Y.C.; writing—original draft, K.-Y.C. and W.-H.C.; writing—review & editing, K.-Y.C., W.-H.C., F.-Y.C. and W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Science and Technology, Taiwan, under grant MOST 109-2637-H-346-001.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee of National Cheng Kung University (No. 108–384).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bames, J.; Behrens, T.K.; Benden, M.E.; Biddle, S.; Bond, D.; Brassard, P.; Brown, H.; Carr, L.; Carson, V.; Chaput, J. Letter to the editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar]
- Van Dyck, D.; Cerin, E.; De Bourdeaudhuij, I.; Hinckson, E.; Reis, R.S.; Davey, R.; Sarmiento, O.L.; Mitáš, J.; Troelsen, J.; MacFarlane, D.; et al. International study of objectively measured physical activity and sedentary time with body mass index and obesity: IPEN adult study. Int. J. Obes. 2015, 39, 199–207. [Google Scholar] [CrossRef]
- Park, S.-M.; Kim, H.-J.; Jeong, H.; Kim, H.; Chang, B.-S.; Lee, C.-K.; Yeom, J.S. Longer sitting time and low physical activity are closely associated with chronic low back pain in population over 50 years of age: A cross-sectional study using the sixth Korea National Health and Nutrition Examination Survey. Spine J. 2018, 18, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- McGavock, J.M.; Anderson, T.J.; Lewanczuk, R.Z. Sedentary Lifestyle and Antecedents of Cardiovascular Disease in Young Adults. Am. J. Hypertens. 2006, 19, 701–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patterson, R.; McNamara, E.; Tainio, M.; De Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Lakka, T.A.; Laaksonen, D.E.; Lakka, H.-M.; Männikkö, N.; Niskanen, L.K.; Rauramaa, R.; Salonen, J.T. Sedentary Lifestyle, Poor Cardiorespiratory Fitness, and the Metabolic Syndrome. Med. Sci. Sports Exerc. 2003, 35, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P. Physical Activity, Sedentary Behaviours, and Cardiovascular Health: When Will Cardiorespiratory Fitness Become a Vital Sign? Can. J. Cardiol. 2016, 32, 505–513. [Google Scholar] [CrossRef]
- Ben Kibler, W.; Press, J.; Sciascia, A. The Role of Core Stability in Athletic Function. Sports Med. 2006, 36, 189–198. [Google Scholar] [CrossRef]
- Liu, F.; Jones, A.Y.; Evans, K.; Tsang, R.C.; Ao, L. Trunk muscle endurance in Chinese adults. J. Back Musculoskelet. Rehabil. 2018, 31, 593–602. [Google Scholar] [CrossRef]
- Hultman, G.; Nordin, M.; Saraste, H.; Ohlsèn, H. Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. J. Spinal Disord. 1993, 6, 114–123. [Google Scholar] [CrossRef]
- Jin, S.; An, J.; Lee, S.; Lee, I.; Kim, H.J. NIRS-based experimental evaluation of driver back fatigue during long-term driving. Biotechnol. Biotechnol. Equip. 2018, 32, 804–814. [Google Scholar] [CrossRef]
- McGill, S.M.; Hughson, R.L.; Parks, K. Lumbar erector spinae oxygenation during prolonged contractions: Implications for prolonged work. Ergonomics 2000, 43, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Ito, S.; Hida, T.; Ito, K.; Koshimizu, H.; Harada, A. Low Back Pain in Patients with Lumbar Spinal Stenosis-Hemodynamic and electrophysiological study of the lumbar multifidus muscles. Spine Surg. Relat. Res. 2017, 1, 82–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kovacs, K.M.; Marras, W.S.; Litsky, A.S.; Gupta, P.; Ferguson, S.A. Localized Oxygen Use of Healthy and Low Back Pain Individuals During Controlled Trunk Movements. J. Spinal Disord. 2001, 14, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, P.; Franchi, M.V.; Gerber, C.; Fluck, M. Does a Better Perfusion of Deconditioned Muscle Tissue Release Chronic Low Back Pain? Front. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Olivier, N.; Thevenon, A.; Berthoin, S.; Prieur, F. An Exercise Therapy Program Can Increase Oxygenation and Blood Volume of the Erector Spinae Muscle during Exercise in Chronic Low Back Pain Patients. Arch. Phys. Med. Rehabil. 2013, 94, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.K.; Wu, S.; Nie, J.; Baker, J.S.; Lin, H. The Occurrence of Core Muscle Fatigue During High-Intensity Running Exercise and its Limitation to Performance: The Role of Respiratory Work. J. Sports Sci. Med. 2014, 13, 244–251. [Google Scholar]
- Hibbs, A.E.; Thompson, K.G.; French, D.N.; Wrigley, A.; Spears, I.R. Optimizing Performance by Improving Core Stability and Core Strength. Sports Med. 2008, 38, 995–1008. [Google Scholar] [CrossRef]
- Hase, K.; Kaya, M.; Zavatsky, A.B.; Halliday, S.E. Musculoskeletal Loads in Ergometer Rowing. J. Appl. Biomech. 2004, 20, 317–323. [Google Scholar] [CrossRef]
- Asaka, M.; Usui, C.; Ohta, M.; Takai, Y.; Fukunaga, T.; Higuchi, M. Elderly oarsmen have larger trunk and thigh muscles and greater strength than age-matched untrained men. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 108, 1239–1245. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Turpin, N.A.; Guével, A.; Durand, S.; Hug, F. Effect of power output on muscle coordination during rowing. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 111, 3017–3029. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.S.; McNair, P.J.; Williams, M. The effects of repetitive motion on lumbar flexion and erector spinae muscle activity in rowers. Clin. Biomech. 2003, 18, 704–711. [Google Scholar] [CrossRef]
- Van Dieën, J.H.; De Looze, M.P.; Hermans, V. Effects of dynamic office chairs on trunk kinematics, trunk extensor EMG and spinal shrinkage. Ergonomics 2001, 44, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Nachemson, A. Variations in the Nutrition of the Canine Intervertebral Disc Induced by Motion. Spine 1983, 8, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Belavý, D.L.; Albracht, K.; Bruggemann, G.-P.; Vergroesen, P.-P.A.; Van Dieën, J.H. Can Exercise Positively Influence the Intervertebral Disc? Sports Med. 2015, 46, 473–485. [Google Scholar] [CrossRef]
- O’Sullivan, P.; Dankaerts, W.; Burnett, A.; Straker, L.; Bargon, G.; Moloney, N.; Perry, M.; Tsang, S.M.H. Lumbopelvic Kinematics and Trunk Muscle Activity During Sitting on Stable and Unstable Surfaces. J. Orthop. Sports Phys. Ther. 2006, 36, 19–25. [Google Scholar] [CrossRef]
- Chang, F.Y.; Chien, K.Y.; Wang, E.T.; Chang, H.Y.; Chen, W.H. Effects on trunk sway and core muscle activity when using a rowing machine with an air seat cushion. Phys. Educ. J. 2021, 54, 147–160. [Google Scholar]
- Zhang, Z.; Wang, B.; Gong, H.; Xu, G.; Nioka, S.; Chance, B. Comparisons of muscle oxygenation changes between arm and leg muscles during incremental rowing exercise with near-infrared spectroscopy. J. Biomed. Opt. 2010, 15, 017007. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Felici, F.; Quaresima, V.; Fattorini, L.; Sbriccoli, P.; Filligoi, G.C.; Ferrari, M. Biceps brachii myoelectric and oxygenation changes during static and sinusoidal isometric exercises. J. Electromyogr. Kinesiol. 2009, 19, e1–e11. [Google Scholar] [CrossRef]
- Chien, K.; Chang, W.; Sanders, M.E.; Chen, C.; Wu, W.; Chen, W. Effects of land vs water jump exercise: Implications for exercise design targeting bone health. Scand. J. Med. Sci. Sports 2019, 29, 826–834. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Lutjemeier, B.J.; Townsend, D.K.; Barstow, T.J. Effects of pedal frequency on estimated muscle microvascular O2 extraction. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 96, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Šarabon, N.; Kozinc, Ž.; Babič, J.; Markovič, G. Effect of rowing ergometer compliance on biomechanical and physiological indicators during simulated 2000-metre race. J. Sports Sci. Med. 2019, 18, 264–270. [Google Scholar] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Mann, T.N.; Webster, C.; Lamberts, R.P.; Lambert, M. Effect of exercise intensity on post-exercise oxygen consumption and heart rate recovery. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Duffy, T.; Kilpatrick, G.; Krotki, K.; Lewis, T.; McMichael, J.; Palmer, D.; Ridenhour, J.; Ryder-Burge, A.; Thomas, I.; Willis, S. National Recreational Boating Safety Survy Exposrue Vurvery Final Report. Available online: https://boatingsurvey.org/data/NRBSS-Exposure-Survey-Final-Report-October-28-2020.pdf (accessed on 2 November 2021).
- Mendes, R.; Sousa, N.; Barata, J.L.T. Physical activity and public health: Recommendations for exercise prescription. Acta Med. Port. 2012, 24, 1025–1030. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).