Abstract
Carbon nanomaterials (CNMs) are a fascinating class of materials that have gained considerable interest in recent years. Their favourable biocompatibility, combined with unique chemical and mechanical properties, has attracted scientists from various disciplines. A significant hurdle in their deployment in biomedical applications is their hydrophobicity in their pristine form. This review surveys and discusses existing non-covalent methods of functionalising CNMs with biocompatible dispersants to facilitate their incorporation into aqueous solutions. Different types of dispersants will be examined and compared as well as the factors affecting their efficiency. This work seeks to provide a compilation of the various methods employed in producing biocompatible CNM dispersions.
1. Introduction
Carbon nanomaterials (CNMs) are a unique class of materials that have received growing interest in the past three decades. They are the most widely studied class of nanomaterials due to their unique chemical, physical, mechanical, thermal, and electrical properties [1]. CNMs are typically classified depending on their structural dimensionality. Zero-dimensional (0D) CNMs such as fullerenes and carbon nano-onions (CNOs) have all their dimensions in the nanoscale (i.e., <100 nm) [2]. In contrast, one-dimensional (1D) CNMs such as carbon nanotubes (CNTs) and two-dimensional (2D) materials such as graphene have one and two dimensions exceeding the nanoscale, respectively.
CNMs are unique in terms of their physical and electronic structures. Their impressive mechanical strength, extremely high surface area, and thermal properties have not escaped the interest of researchers [3]. With applications in engineering [4,5], biomedicine [6,7], and renewable energy [8,9], this unique class of materials, and indeed nanotechnology in general, is set to have an enormous impact on our future. In fact, nanomedicine has been recognised as a “key enabling technology” by the EU [10]; by 2016, the FDA had approved 51 nanomedicines [11].
It is widely known that hydrophobicity is a major problem for pristine CNMs, particularly when using them in biomedical applications, where aqueous dispersions are often needed. This is where biocompatible dispersants have a role to play; these molecules can be attached to a CNM’s surface and impart hydrophilicity to the nanomaterial. This can occur covalently; however, this process is known to damage the nanomaterial’s structure [12]. Therefore, non-covalent methods of CNM functionalisation are required to preserve their unique properties.
A range of biocompatible dispersants exist that are capable of non-covalently binding to a CNM’s surface. Many of these are biomolecules (such as bile acid salts) or naturally derived (for example, nanocrystalline cellulose), but there also exist many synthetic surfactants, such as sodium dodecyl sulphate (SDS). The different classes of dispersants will be detailed and compared in this review.
Many of these dispersants are toxic in high concentrations [13]; therefore, efficient dispersants are needed to ensure biocompatibility. Many factors affect the efficiency and efficacy of a surfactant. For example, aromatic groups on a surfactant can partake in π-π stacking interactions with a CNM, increasing the binding strength [14]. These factors will be detailed in this review, and metrics for comparing different dispersants will also be discussed.
2. The Problem of Hydrophobicity
A major bottleneck for the biomedical and nanomedical applications of carbon nanomaterials (CNMs) is their hydrophobicity. Forming stable aqueous suspensions of these materials is essential for their use in biological systems. One method of doing this is by covalently attaching hydrophilic groups onto the material’s surface—this functionalisation can result in enhanced solubility—but the covalent bonding process is known to damage the hybridisation of the sp2 lattice, resulting in the partial loss of the CNM’s unique properties [12]. Non-covalent methods, whilst resulting in weaker bonds, preserve the intrinsic structure and properties of the CNMs. Therefore, this review will focus on non-covalent methods of functionalising CNMs, to facilitate their dispersion in aqueous solutions.
A variety of non-covalent forces exist to be utilised in functionalising a CNM. The adsorption of surfactants to CNMs is dominated by π-electron, van der Waals, and electrostatic interactions [15]. Hydrophobic interactions explain the thermodynamically driven clumping of unmodified CNMs in aqueous solutions [16]. Interactions between hydrophobic materials in water are spontaneous, as they do not rely on the breaking and re-forming of hydrogen bonds. Therefore hydrophobic materials stick together in solution to minimise contact with water molecules and decrease the system’s entropy [17]. In the case of carbon nanotubes (CNTs), van der Waals (vdW) interactions such as dipole-dipole and London forces also contribute, causing them to form tightly packed bundles in solution. Their high aspect ratio and surface area lead to a vdW binding force of 500 eV/µm of tube-tube contact [16]. Pristine graphene and fullerenes are also insoluble due to their extensive agglomeration in solution; this is caused by vdW and hydrophobic interactions. Graphene can re-agglomerate to form graphite [14], whilst the agglomeration of fullerenes and carbon nano-onions (CNOs) is not as well studied.
3. Methods of Dispersing Carbon Nanomaterials
Dispersants are a class of molecules that can be added to suspensions of solid particles to prevent agglomeration and settling by increasing repulsion between particles through either steric or electrostatic mechanisms. Surfactants are often used as dispersants; these compounds work by lowering the surface tension between nanoparticles and liquid media. Dispersants are often toxic to humans, as they can denature proteins and destroy lipid membranes [13]. Non-ionic surfactants, in particular, have been found to damage the skin [18]. Therefore, biocompatible alternatives are needed for human use. Fortunately, in nature, there exist many non-toxic and effective surfactant molecules such as bile acid salts (BAS) [19], deoxyribonucleic acid (DNA) [20], and cellulose [21]. A range of synthetic biocompatible dispersants also exists, such as Triton X-100 [22] and Pluronic F127 [23]. Many of these dispersants are either not commercially available or quite expensive, and therefore they may be difficult to obtain. It is also known that the structure and charge of many biomolecules such as DNA are sensitive to temperature, pH, and ionic strength of the aqueous phase, among other factors. These variables have to be considered when using them as dispersants. An ideal surfactant should disperse a carbon nanomaterial (CNM) with a minimal concentration of surfactant needed. This dispersant should also have a strong binding affinity for the CNM surface, which is usually facilitated by aromatic groups or long aliphatic chains.
It should be noted that any physical methods used to aid dispersion may significantly affect the final CNM suspension. Excessive sonication of CNMs may lead to structural damage, loss of sp2 lattice, or unwanted functionalisation [24,25]. These undesired effects occur due to the shockwaves formed from ultrasonic cavitation. Although sonication helps in dispersing CNMs, it needs to be applied in a controlled manner. Centrifugation is also widely employed in the study of nanomaterials as it can be used to assess dispersion mechanics [3]. Furthermore, analytical ultracentrifugation can be used to separate CNMs based on size and shape [12]. This technique is also employed in purifying CNM dispersions by removing amorphous carbon, metallic residues and agglomerated CNMs [26]. However, it can also lead to a loss of dispersion in the resulting supernatant. Due to these effects, details involving physical techniques used to aid in the dispersion of CNMs must be rigorously reported to ensure reproducibility.
4. The η and η* Metrics
The efficiency of a surfactant can be described in terms of , which is the surfactant concentration needed to reach maximum nanoparticle dispersion. Dispersion effectiveness (η; Equation (1)) is a measure of the ability of a surfactant to disperse a given amount of hydrophobic material, where is the initial undispersed carbon nanomaterial (CNM) concentration and is the highest concentration of a dispersed CNM. The η and η* metrics:
Dispersion efficiency (η*; Equation (2)) is a more thorough representation of dispersants’ abilities, as an efficient dispersant may still have a high .
Table 1 displays a range of η and η* values, obtained from the supplementary information section of a paper written by Fernandes et al. [27]. The authors of this study utilised a range of surfactants, including sodium dodecylbenzene sulphonate (SDBS), sodium dodecyl sulphate (SDS), and hexadecyltrimethylammonium bromide (CTAB), to disperse single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) in water.

Table 1.
η and η* values for a range of CNT dispersions [27]. Table adapted with permission from [27] Copyright © 2015 American Chemical Society.
A high η* value suggests that either (a) the repulsion forces between surfactant functionalised CNMs is high, (b) a large portion of the CNM surface area is covered in surfactant, (c) a high percentage of surfactant is adsorbed onto the surface of the CNM, or (d) some synergistic combination of these factors. It should be noted that the η* metric is not a comprehensive description of a dispersant’s abilities, as there are many factors it does not account for. For example, the stability of the final dispersion is not considered; neither is the molecular mass of the surfactant molecule itself. Different CNM types will yield different values due to the varying mass-to-surface area ratios of different nanomaterial forms. As seen in Table 1, it was found by Fernandes et al. that MWCNT systems displayed higher η* values than their SWCNT counterparts due to the different mass-to-surface area ratios of the two CNMs [27].
5. Factors Affecting Dispersion Efficiency
The formation of micelles is a critical factor in the dispersion of carbon nanomaterials (CNMs) at high surfactant concentrations. The role of micelles depends heavily on the surfactant used and its concentration relative to the CNMs. It is worth noting that, while not very well studied, the adsorption of surfactant molecules onto the surface of graphene is believed to result in the formation of novel 2D micelles [28,29]. At low surfactant concentrations, individual dispersant molecules randomly adsorb onto the CNM surface. They then coalesce to form hemimicelles as their concentration increases. Finally, they encapsulate the carbon nanoparticle in a blanket micelle. The critical micelle concentration (CMC)—the concentration of surfactant above which micelles form in solution—of surfactants in CNM dispersions is higher than their CMC in water due to the adsorption of surfactant molecules onto the CNMs.
When a surfactant concentration is increased above its , a phenomenon known as depletion attraction occurs [26,30]. Depletion attraction causes the re-agglomeration of individual carbon nanomaterials (CNTs), which occurs due to the osmotic pressure exerted on the CNTs by the surfactant micelles. At a high enough micelle concentration, the depletion attraction forces eventually overcome the CNT repulsive forces and cause re-bundling of the fibres. Another mechanism of re-agglomeration known as surfactant induced flocculation was proposed by Rastogi et al. [22]. Surfactant induced flocculation involves ionic head/tail surfactants forming a bilayer around the CNM, with their hydrophilic tails pointing out into solution. These tails interact with each other to cause re-bundling of CNMs; they do not provide steric hindrance due to their packing and relatively short chain lengths. Therefore, to achieve the best η* value in biocompatible hybrid systems, the optimal surfactant concentration must be determined.
For CNMs with curvature—fullerenes/carbon nano-onions (CNOs)/CNTs—curved or flexible hydrophobic regions in surfactants can be utilised to increase the surface coverage on the CNM, therefore increasing binding forces. This concept is known as complementarity [31]. Complementarity can also be applied to interatomic distances in the surfactant and CNM surface [32]. By optimising the curvature and interatomic distances in the hydrophobic regions of the surfactant molecule to match those of the CNM surface, enhanced binding and packing can be achieved, therefore increasing dispersion efficiency.
Once bound to the dispersed CNT’s surface, it is important to note that small-molecule dispersants, such as head/tail surfactants, exist in dynamic equilibrium with the surrounding solvent (meaning the dispersant molecules are reversibly bound). This dynamic equilibrium could negatively affect the biocompatibility of the final dispersion due to its reversible nature. For example, suppose a dynamic dispersion of a CNM-based therapeutic was to be injected. In that case, the resulting dilution of the solution in the bloodstream could drive desorption of the surfactant molecules, resulting in the re-aggregation of CNMs in the blood. In contrast to small-molecule surfactants, large polymer dispersants tend to wrap around the CNT surface (Figure 1). Due to the thermodynamic stability of the polymer coating around the CNT, polymer wrapping tends to result in irreversible, non-covalent adsorption, with the added benefit of enabling the removal of excess free-polymer molecules from solution [33].
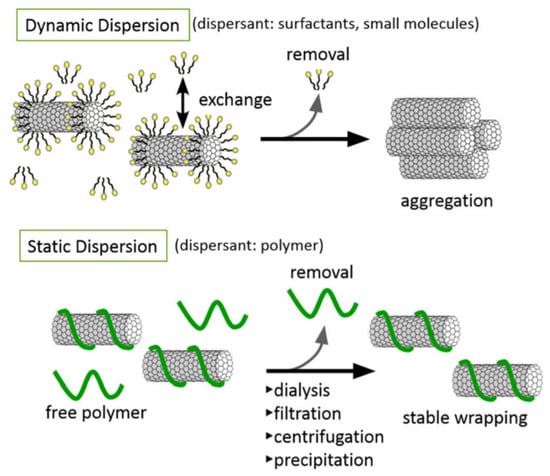
Figure 1.
Schematic of a dynamic and static CNT dispersion [33]. Figure reprinted with permission under CC 3.0.
The surfaces of CNMs are highly conjugated, meaning π-π interactions with aromatic groups are fundamental in stabilising hybrid systems [34]. It is known that polycyclic aromatic hydrocarbons can share their π-orbitals with graphene’s sp2 lattice, lowering the free energy of the surface and forming a strong non-covalent bond in the process [14]. Hydrogen bonding also plays a vital role, particularly in dispersant-solvent interactions [35].
Biocompatible dispersants are non-toxic molecules capable of solubilising materials in aqueous solutions. These are crucial in the deployment of carbon nanomaterials in biomedical applications. The different biocompatible dispersants classes are described in this review, along with relevant examples of their use as CNM dispersants.
6. Biocompatible Dispersants
6.1. Cellulose
Cellulose is an environmentally friendly, naturally occurring biopolymer. It is very cheap and ubiquitous, with an estimated 1011–1012 tons naturally produced each year [21]. This renewable material is very hydrophilic due to its hydroxyl-rich surface. Cellulose consists of repeating β-1,4-linked anhydro-D-glucose units (Figure 2); these can form inter and intramolecular hydrogen bonds. Different polymorphs arise from distinct, hydrogen-bonding-induced packing and orientations of the glucose units [36].
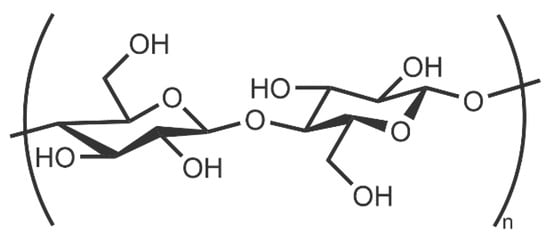
Figure 2.
Monomer unit of cellulose.
Nanocrystalline cellulose (NCC), also known as cellulose nanocrystals, are rod-shaped cellulose crystals typically 5–15 nm wide and 100–300 nm long [21]. NCC was found to bind to CNM surfaces by dipole-induced dipole interactions, and the NCC/CNM complex is stabilised electrostatically [37]. There are four reported cellulose polymorphs, with type I and type II being the most well studied [38]. The attraction between CNMs and NCC can also be varied by changing polymorph composition. The type II polymorph has a higher surface charge due to residual sulphate groups present from acid treatment. Type I NCC exhibits a parallel arrangement of individual cellulose polymer chains, whereas the chains in type II NCC are arranged in an antiparallel fashion. While type I is the naturally occurring form of NCC, many methods exist to convert it to type II, the most industrially viable of which is sulphuric acid treatment [39]. This process is both time and cost-friendly. This method also allows for the production of type I and II NCC mixtures—the ratios of which may be varied by careful control of experimental parameters. Both types have different properties; for example, type II NCC exists in a shorter and wider crystal form than type I. Cellulose can be a highly effective surfactant, up to 20 times more effective than conventional dispersants such as sodium dodecyl sulphate (SDS) or hexadecyltrimethylammonium bromide (CTAB) in some cases [40]. The main drawback of cellulose, and particularly nano-cellulose materials, is that they are hygroscopic. Upon absorbing water, these materials swell, which affects their surface interactions [41]. This is particularly problematic for nano-cellulose materials such as NCC due to their high surface area, which can cause reproducibility issues [41].
In 2019, Domínguez et al. demonstrated that NCC could be used to disperse single-walled carbon nanotubes (SWCNTs) in aqueous solutions at concentrations of approximately 0.1 mg/mL, with long-term dispersion stability (1.5 years) [38]. Details of this dispersion can be seen in Table 2 (1). This study revealed no significant difference between types I and II NCC dispersing ability for SWCNTs. However, TEM analysis (Figure 3) showed different morphologies of the hybrid systems. Type I NCC/SWCNT systems consisted of individual NCC particles binding to SWCNTs along their hydrophobic planes. In contrast, type II systems consisted of carbon nanotubes (CNTs) wrapped in a tangled mass of NCC [38].
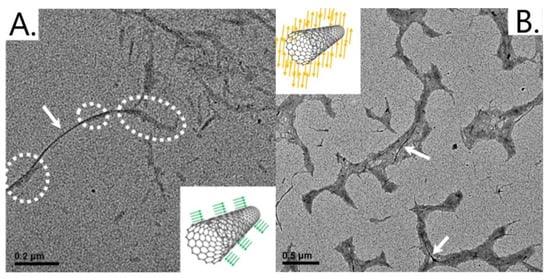
Figure 3.
TEM micrograph of SWCNT/NCC hybrids. (A) Type I NCC; (B) Type II NCC. Arrows show SWCNTs, and dotted circles show cellulose nanocrystals. Insets: Parallel and antiparallel NCC fibers [38]. Figure adapted with permission from [38] Copyright © 2019 American Chemical Society.
Whilst very little information exists on the use of NCC for dispersing other CNMs, colloidal probe AFM studies with graphene suggest a synergetic NCC/CNM adsorption mechanism, which could potentially be applied to other sp2 hybridised CNMs [37]. This study also proved that hydrogel and nano-paper preparations could be produced using SWCNTs and cellulose (Table 2 (2)). As is the case, Wang et al. used methylcellulose to disperse graphene nanoplatelets [42] (Table 2 (3)). This study also revealed that NCC with a higher surface charge binds more efficiently to CNMs. The tuning of NCC mixtures could also prove advantageous. For example, type II dominant mixtures could be used to disperse carbon nano-onions (CNOs) due to their smaller size. The better SWCNT surface coverage of NCC type II over type I seen by Domínguez et al. may apply to other CNMs [38]. The attraction between CNMs and NCC can also be varied by changing polymorph composition.
Nanocelluloses can be functionalised to add another dimension of capabilities to this class of dispersant, as demonstrated by Durairaj et al. earlier this year [43]. The study focussed on functionalising NCC and cellulose nanofibrils (CNFs)—with sulphate, carboxylate, and amino-silane moieties—to produce nanocellulose/multi-walled carbon nanotube (MWCNT) dispersions for electrochemical applications (Table 2 (4)). The functionalisation of the nanocellulose material tailored the final product’s physiochemical properties by changing its crystal structure, density, and electrochemical behaviour [43].
Before this study, Hamedi et al. experimented with carboxymethylated CNFs to produce high-quality SWCNT dispersions [44]. The cellulose material was obtained by treating softwood pulp with monochloroacetic acid, followed by sonication, shear mixing, and centrifugation steps to purify the dispersant. SWCNTs were added to the resulting solution and sonicated to produce the dispersion. This solution was then used to produce aerogels, conductive films, and anisotropic microscale fibres. The authors chose this strategy to lower the cost and increase the conductivity and strength of the final material and facilitate colloidal self-assembly of the composite material. However, it is important to note that only low concentrations of SWCNTs were dispersed, and no stability data was present [44] (Table 2 (5)).
NCC also increases the electrical and thermal conductivity of CNM dispersions, as demonstrated by Dovjuu et al. in 2020 [5]. The study focused on using NCC to create stable, aqueous dispersions of MWCNTs and graphene for use as a heat transfer fluid—this was achieved by adding CNMs to solutions of varying concentrations of NCC and ultrasonication for 1 h. It was found that this strategy was able to form stable aqueous dispersions of both CNMs, whilst enhancing the thermal and electrical conductivity of the nanomaterials. Although the intended use of this dispersion was engineering-based, using a biocompatible dispersant in an aqueous solution meant that it was non-toxic and could be used for biomedical applications, such as drug delivery or cellular imaging. The details of this dispersion can be seen in Table 2 (6).

Table 2.
Cellulose-based CNM dispersions.
Table 2.
Cellulose-based CNM dispersions.
| Num. | Dispersant | CNM | Description | Application | Ref. |
|---|---|---|---|---|---|
| 1 | Type I/II NCC | SWCNT | SWCNTs dispersed with varying ratios of types I and II NCC | Stability testing; in-vitro bioactivity, biocompatibility studies | [38] |
| 2 | CNFs, NCC | SWCNT | SWCNTs functionalised with CNFs and NCC | Hydrogel and nano paper preparations; investigating the mechanisms of cellulose dispersants | [37] |
| 3 | Methylcellulose | Graphene nanoplatelets | Graphene nanoplatelets functionalised with methylcellulose | Aqueous graphene dispersions for reinforcing cement | [42] |
| 4 | Functionalised NCC and CNFs | MWCNT | Sulfate, carboxylate, amino-silane functionalised NCC and CNF materials used to disperse MWCNTs | Dispersions used to produce highly stable composite electrodes | [43] |
| 5 | Carboxymethylated CNFs | SWCNT | Carboxymethylated CNFs from softwood pulp used to disperse SWCNTs | This dispersion was utilised to produce conductive films, anisotropic microscale fibres, and aerogels | [44] |
| 6 | NCC | MWCNT and graphene | Non-covalent functionalization with NCC | Heat transfer fluids | [5] |
In conclusion, cellulose-based dispersants are cheap, biocompatible, environmentally friendly and available in large quantities [21], making them ideal for industrial and biomedical applications. They are effective at dispersing a range of CNMs [39,41]. However, no work has been done on using them to disperse 0D CNMs, such as carbon nano-onions (CNOs), fullerenes, and carbon quantum dots (CQDs). The benefits of these dispersants could very well translate to these materials. Functionalisation of cellulose dispersants allows for customisation of the material to suit a range of applications by modifying the dispersants physiochemical characteristics. This modifiability is particularly relevant when the dispersant will be used to produce a solid—aerogel, film, or coating—as factors such as porosity can be controlled [43]. However, it is important to note that the effects of covalent modification on celluloses structure, biocompatibility, and dispersing abilities are not well studied.
6.2. Bile Acid Salts
Bile acid salts (BASs) are another class of efficient biomolecular dispersants that are found in a range of vertebrates; including humans [45]. They are used to solubilise a range of hydrophobic biomolecules such as lipids, proteins, vitamins, and fatty acids [45]. BASs are easy to obtain and environmentally friendly [46]. The general structure of these anionic biosurfactants is shown in Figure 4. Groups R1–R4 are hydrophilic groups—typically OH or COOH—and the hydrophobic region is comprised of four rings. The steroid backbone provides a unique rigidity to the molecule [19]. The structure and charge distribution of these amphiphiles result in one face of the molecule being hydrophobic whilst the other is hydrophilic, thus maximising the surface coverage efficiency when adsorbed onto CNMs. The main contribution to BAS/CNM adsorption is hydrophobic interactions between the steroid backbone of the BAS and the CNM surface. The disadvantages of BAS are that they are known to denature proteins, damage DNA, chelate ions, and interfere with bacterial membranes in high concentrations [47]. This could limit their in-vivo usage and affect in-vitro studies.
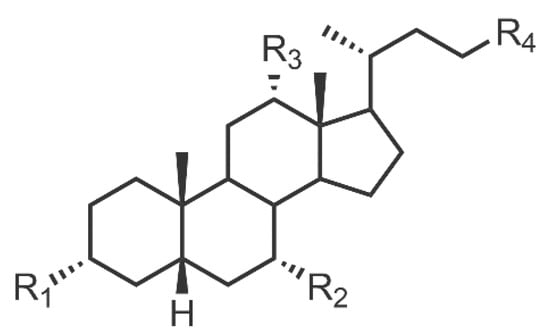
Figure 4.
The general structure of a bile acid salt (BAS); R groups are hydrophilic.
Unlike cellulose-based surfactants, BAS has been tested with 0D CNMs. In 2020, Camisasca and Giordani non-covalently functionalised CNOs with sodium deoxycholate (SDC) [48] (Table 3 (1)). The dispersion was produced through a simple process involving sonication and mixing. The aqueous dispersion was found to be stable for six weeks, with a CNO: SDC mass ratio of 2:1 found to be ideal for surfactant efficiency. This CNO functionalisation was driven by hydrophobic interactions [48].
BAS can also disperse carbon quantum dots (CQDs), as demonstrated by Zhao et al. in 2020 [49]. SDC was used to functionalise CQDs via hydrophobic interactions. The resulting dispersions were used to produce films via electrophoretic deposition (EPD). In solution, the deoxycholate (DC) anion is water-soluble. However, as protons are generated at the anode, they combine with the deoxycholate moiety to form an insoluble protonated adduct. As BAS act as dual dispersants and EPD agents, they are ideal for producing CNM-containing films [49] (Table 3 (2)).
Sun et al. used a similar approach to produce a range of stable graphene dispersions [46] (Table 3 (3)). SDC, taurodeoxycholic acid sodium salt hydrate, and sodium cholate (SC) were all employed to non-covalently functionalise graphene through the chemical reduction of graphene oxide in the presence of BAS solutions. These all displayed fantastic stability, remaining in solution for over 80 days. More recently, SC has also been used to disperse SWCNTs to facilitate chirality purification [50] (Table 3 (4)).
In another study, Sun et al. proved that SC could exfoliate CNTs to produce stable aqueous dispersions, with no noticeable agglomeration or sedimentation after 30 days [51]. This was achieved using high-power sonication and centrifugation steps. The different dispersions were compared through a range of quality metrics such as CNM concentration, aggregate size, and mean CNT length. It was found that SC-based dispersions were not as stable as those produced with other common dispersants—such as sodium dodecyl sulphate (SDS) or sodium dodecylbenzene sulphonate (SDBS). This was found to be due to the low charge density of the BAS molecule, which was not as high compared to an alkyl chain surfactant [51] (Table 3 (5)).
In an attempt to improve the dispersion efficiency of BAS, Gubitosi et al. functionalised a range of BASs with aromatic substituents at varying positions [52] (Table 3 (6)). It was postulated that adding aromatic groups to the BAS structure would increase the molecules’ affinity SWCNTs by inducing π-π stacking interactions. However, these modifications result in a lower charge density and increased hydrophobicity. Despite these concerns, it was found that some of the derivatives displayed better dispersion efficiencies than their BAS counterparts.

Table 3.
BAS-based CNM dispersions.
Table 3.
BAS-based CNM dispersions.
| Num. | Dispersant | CNM | Description | Application | Ref. |
|---|---|---|---|---|---|
| 1 | SDC | CNO | CNOs non-covalently functionalised with SDC | Stability studies; analysis of surfactant efficiency | [48] |
| 2 | SDC | CQD | SDC as both a dispersant and EPD agent | Formation of CQD containing composite films via EPD for corrosion protection of steel | [49] |
| 3 | SDC, SC, sodium taurodeoxycholate | Graphene | Graphene functionalised with a range of BAS via graphene oxide reduction | Production of Palladium nanoparticle-based catalysts for the electrooxidation of formic acid | [46] |
| 4 | SC | SWCNT | SWCNTs non-covalently functionalised with SC | Chiral purification of SWCNTs via two-phase aqueous extraction | [50] |
| 5 | SDC | SWCNT | SDC is used to exfoliate and disperse SWCNTs to form stable dispersions | Determination of dispersion quality and its correlation with Zeta potential | [51] |
| 6 | SC, SDC, sodium taurocholate | SWCNT | SWCNTs non-covalently functionalised with BAS; aromatic derivatives of these salts were also used | Investigating the effects of attaching aromatic groups to BAS on dispersant efficiency | [52] |
Although not quite as common as cellulose, BAS offer a combination of excellent dispersing abilities, biocompatibility, and low cost. What sets these molecules apart from other dispersants are their unique rigid and amphiphilic steroid backbone—which can be fully utilised to bind to CNM surfaces strongly whilst packing very well. This binding allows them to be much more efficient dispersants than traditional alkyl chain molecules [53]. However, there are some doubts about the quality of these dispersions [51].
6.3. Alkyl Chain Surfactants
Perhaps the most well-studied class of dispersants is alkyl chain surfactants (ACSs). As seen in Figure 5, they may be anionic or cationic. They consist of a hydrophilic ionic head group attached to an alkyl chain tail, which binds to the CNMs surface. The ionic head group can be either cationic, such as in the case of CTAB or anionic, as SDS is. Gemini surfactants, also known as di-cationic surfactants, consist of two cationic groups linked together with a spacer, whilst each group also has an alkyl chain attached to it. These head groups are typically quaternary ammonium [54] or imidazolium [55] in nature. Although they may not be as efficient as other classes of dispersants, these molecules have a variety of uses. Once bound to a CNM’s surface, ACSs cause nanoparticles to repel each other through electrostatic interactions. ACSs with small, highly charged head groups excel at de-bundling CNTs due to their ability to penetrate the dense fibres and instigate the unzipping process. The main disadvantage of ACSs is that they are toxic in high doses [13], which is not optimal for in-vivo studies.
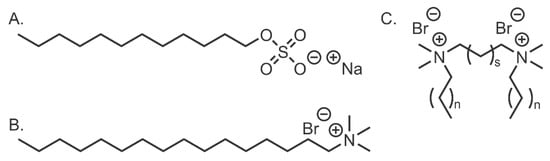
Figure 5.
Examples of (A), anionic, (B), cationic and (C), Gemini ACS.
ACSs are capable of dispersing CNOs, as demonstrated by our group using hexadecyltrimethylammonium bromide (CTAB) and SDBS [48] (Table 4 (1)). This study also found that ACSs with negatively charged head groups are more effective at dispersing CNOs than their cationic counterparts. This effectiveness is due to the electrostatic repulsion between the negatively charged CNM surface and the head group of the surfactant, forcing anionic ACSs to take a tail-first approach when binding to the CNO surface—thus resulting in more efficient packing of surfactant molecules and better coverage of the CNM’s surface.
Bobrowska et al. have also found success dispersing CNOs with ACSs, producing stable dispersions using CTAB, sodium dodecyl sulfate (SDS), and SDBS [56]. A wide range of CNO: surfactant mass ratios were investigated, which is beneficial as the surfactants optimal efficiency can be determined. Unlike many other studies, in vitro antimicrobial assays were performed. It was found that only the CNO/CTAB dispersion negatively affected cell viability. This was likely due to the dissociation of CTAB from the CNO surface. As discussed earlier, this is an unavoidable problem with ACSs as they form dynamic dispersions [56] (Table 4 (2)).
Dovjuu et al. further demonstrated that ACSs could be utilised to disperse MWCNTs and graphene; SDS, SDBS, and lauryl betaine were used [5] (Table 4 (3)). These dispersions were tested for use as heat transfer fluids due to the CNM’s conductivity. Unfortunately, many of these dispersions suffered from poor stability, likely because the CNM concentration was too high. These dispersions were mainly used as a comparison to cellulose-based dispersions, which were found to be more effective.
Sun et al. have also demonstrated the ability of ACSs to disperse SWCNTs, using SDBS, SDS, lithium dodecyl sulfate (LDS), and tetradecyl trimethyl ammonium bromide (TTAB) to form aqueous dispersions [51]. The quality of these dispersions was investigated using a range of metrics determined from physiochemical characterisation, such as mean nanotube length and bundle diameter. The quality of these dispersions ranked as follows: SDS > LDS > SDBS > TTAB. The position of TTAB as the molecule with the lowest quality dispersions is due to its cationic hydrophilic group, as discussed above. SDS, LDS, and SDBS all have anionic head groups. However, it is important to note that the dispersant efficiency was not considered in this study and, therefore, the concentration-dependent dispersing abilities of these surfactants need to be examined (Table 4 (4)).
A perhaps under-utilised use for this class of surfactants is the solubilisation of CNMs by a surfactant substitution mechanism. As mentioned above, these small molecules are ideal for de-bundling CNTs yet suffer from poor dispersion stability due to poor adhesion. Larger surfactants with better dispersion stability, particularly steric and polymer surfactants, often have poor de-bundling abilities due to steric repulsion at the bundle surface [16]. One way around this is to use a surfactant substitution solubilisation method, where the de-bundled CNT fibres are stabilised by replacing the head/tail surfactant molecules bound to its surface with larger surfactant. Datsyuk et al. successfully leveraged this method, using a combination of SDS/Surfhope 1216 on MWCNTs, to produce a dispersion with a high proportion of individual CNTs [16] (Table 4 (5)).
One avenue of increasing the efficiency of ACSs is through Gemini surfactants. These biocompatible, di-cationic molecules are proven to be more efficient than their single-chained counterparts [55,57], even at very low concentrations and even though they are cationic. This is due to two main factors (i) their double-tailed structure allows for stronger adsorption to CNMs due to increased non-polar surface area for hydrophobic interactions and (ii) enhanced packing of surfactant molecules due to the two anionic head groups being confined to a small area by the spacer groups.
For CNT systems, the structures of Gemini surfactants allow for more efficient exfoliation of CNT bundles and enhanced stability of the resulting individual CNT dispersions, as demonstrated by Dobies et al. in 2017 [55]. A range of imidazolium-based n-s-n Gemini surfactants was synthesised and used to disperse and exfoliate MWCNTs. These were produced by first synthesising a 1,1′-(1,6-hexanediol) bis (1H-imidazole) head group then reacting it with the appropriate chloromethyl alkyl ether to form a collection of n-6-n Gemini surfactants, where n = 8, 10, 12, and 14 (denoted IMIC6C8, IMIC6C10, IMIC6C12, and IMIC6C14, respectively). IMIC6C12 was found to be the most efficient surfactant, as its tail length was long enough to promote adequate CNM binding yet short enough to allow efficient packing of surfactants on the CNM surface (Table 4 (6)).
Abreu et al. found that by increasing the s value of a Gemini surfactant, more efficient MWCNT dispersants could be obtained [54]. This improvement was noticed after testing a range of n-s-n bis(quaternary ammonium) surfactants. It was found that the 16-2-16 compound (the one with the longest tail/shortest spacer) was the most effective due to its tight packing and CNT de-bundling efficiency. Therefore, it produced the most concentrated CNM dispersion. Conversely, the 12-12-12 compound (shortest tail/longest spacer) was the most efficient, as it had the highest η* value. Scanning electron microscopy data also show that these surfactants produce high-quality dispersions consisting of individual nanotubes [54] (Table 4 (7)).
Gemini surfactants with cyclic linkers containing both ionic groups have also been reported [57]. Cyclic linkers could be more efficient than straight-chain linkers due to better packing of head groups resulting from their decreased mobility. However, this has yet to be investigated using CNMs. Little is known about the biocompatibility of these molecules. There is also a lack of literature on the use of Gemini surfactants to solubilise CNOs. However, the versatility and superior properties of these molecules may prove advantageous in producing stable, biocompatible CNO dispersions. Whilst anionic Gemini surfactants exist [58], there is no literature comparing them directly to their cationic counterparts in dispersing CNMs. Anionic surfactants may be more efficient than anionic Gemini dispersants because of the effects discussed earlier. However, more work will have to be done to investigate this.

Table 4.
Alkyl chain surfactant-based CNM dispersions.
Table 4.
Alkyl chain surfactant-based CNM dispersions.
| Num. | Dispersant | CNM | Description | Application | Ref. |
|---|---|---|---|---|---|
| 1 | CTAB, SDBS | CNO | CNOs non-covalently functionalised with two different surfactants to form two stable dispersions. | Dispersion efficiency and long-term stability studies | [48] |
| 2 | SDS, SDBS, CTAB | CNO | A collection of ACS/CNO dispersions that includes a wide range of CNO: surfactant mass ratios | Physiochemical characterisation; in vitro antimicrobial assays | [56] |
| 3 | SDBS, SDS, lauryl betaine | MWCNT, graphene | MWCNTs and graphene solubilised with a range of ACSs | Physiochemical characterisation, electrical and thermal conductivity studies. Heat transfer fluids | [5] |
| 4 | SDS, LDS, SDBS, TTAB | SWCNT | A range of SWCNT/surfactant dispersions | Quality of dispersions determined, compared, and correlated with zeta potential measurements | [51] |
| 5 | SDS/Surfhope 1216 | MWCNT | MWCNTs dispersed via a surfactant substitution strategy, using SDS to de-bundle | Physiochemical characterisation, stability studies | [16] |
| 6 | IMIC6C8, IMIC6C10, IMIC6C12, and IMIC6C14 | MWCNT | A series of Alkyloxy methylimidazolium surfactants used to produce MWCNT dispersions | Investigating the effectiveness and mechanisms of Gemini surfactants | [55] |
| 7 | 16-2-16 12-12-12 | MWCNT | A series of bis(quaternary ammonium) Gemini surfactants used to solubilise MWCNTs | Determining the effects of tail and spacer length on dispersing efficiency and effectiveness | [54] |
Whilst they may not be the most efficient surfactants, ACSs are ubiquitous in the literature and have found many uses in solubilising many different types of CNMs. They are often used as benchmarks for comparison against other dispersants. ACSs excel at de-bundling CNTs and can produce high-quality dispersions of single CNTs. Surfactant substitution methods enable the exploitation of these properties whilst combating ACSs poor efficiency. Gemini surfactants are an exciting new class of ACS that seek to improve their dispersing abilities, yet not much is known about them.
6.4. Polymer Dispersants
Unlike ACSs, polymer dispersants can be used to functionalise CNMs through a polymer wrapping mechanism, producing a static dispersion. This wrapping results in favourable CNM surface coverage, dispersion stability, and homogeneity [35]. Furthermore, by applying this thermodynamically stable CNM coating, free surfactant molecules can be removed from the final solution without the re-agglomeration of CNMs. This removal can be achieved through centrifugation [59], dialysis [60,61], and filtration [62] methods. A range of synthetic and natural biocompatible polymers can be utilised as surfactants (Figure 6).
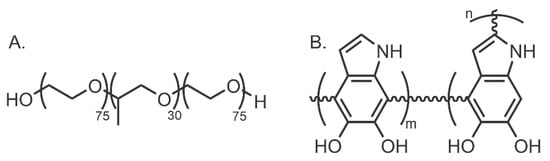
Figure 6.
Structures of two polymer surfactants: (A) Pluronic F127 (synthetic); and (B) a proposed structure of polydopamine (natural).
Biocompatible block copolymers with hydrophobic and hydrophilic components such as Pluronic F127 (F127) offer excellent, tunable properties that can be investigated experimentally to develop dispersant systems for different CNMs. This was demonstrated by Fernandes et al. in 2018, where SWCNTs were exfoliated and dispersed with F127 [25] (Table 5 (1)). The hydrophobicity of F127 comes from poly(propylene oxide) (PPO), the middle block of 30 units in Figure 6, whereas the poly(ethylene oxide) (PEO) component, the two edge blocks of 75 units in Figure 6, provides the polymer with hydrophilicity. The hydrophobic PPO units wrap around the CNMs surface via hydrophobic interactions. The hydrophilic PEO tails then interact with the surrounding solvent, causing them to orient away from the CNM surface, thereby providing steric repulsion between nanoparticles. A previous study found that F127 had excellent surface coverage of SWCNTs, with nearly full saturation achieved [63]. The polydispersity index and copolymer composition were critical factors in dispersant effectiveness and efficiency. However, these will likely have to be optimised for different nanomaterials.
Xin et al. improved upon the structure of F127, developing a PPO/PEO block copolymer with a starlike structure, a hydrophilic backbone, and hydrophilic arms (AP432) [64]. This interesting surfactant could exfoliate and disperse both SWCNTs and MWCNTs (Table 5 (2)). It was a better dispersant than F127 due to the increased steric repulsion between functionalised CNTs due to the hydrophilic arms repelling each other.
The copolymer dispersant approach was also applied by Max et al. in 2019, who successfully developed a polydehydroalanine (PDha)-based copolymer capable of dispersing MWCNTs [65]. This polyzwitterion dispersant has exceptional charge density, and, by extension, great hydrophilicity. When combined with hydrophobic elements to anchor it to the CNMs surface, this material makes for an ideal dispersant. One downside of polyzwitterions is their sensitivity to pH and temperature, which may affect their hydrophilicity. Comprehensive biocompatibility data is not yet available on this material either (Table 5 (3)).
The main drawback of polymer wrapping is that the final polymer coating is quite hard to remove from the CNMs as it is so strongly bound. Therefore, it makes sense for biomedical applications to develop a range of functional dispersants that also act as targeting/imaging/therapeutic agents whilst utilising the CNM as a stable scaffold. Our group has recently investigated this approach using CNOs wrapped in a hyaluronic acid-1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (HA-DMPE) conjugate polymer [61] (Table 5 (4)). The HA portion of the coating is hydrophilic and interacts with the solvent. It also acts as a targeting ligand for the CD44+ receptor, which is overexpressed in a range of cancer cells [66]. The phospholipid (DMPE) component of the conjugate adsorbs onto the surface of the CNOs through hydrophobic interactions. Conjugate systems such as these have a range of potential applications; we integrated fluoresceinamine (a fluorophore) into the polymer conjugate to track in vitro and in vivo biodistribution. The resulting system displayed excellent biocompatibility. However, the use of HA resulted in the nanocarriers concentrating in the digestive tract of zebrafish, an effect that could translate to other organisms during further in vivo usage [61].
More recently, Arpicco et al. applied this approach to SWCNTs. Through the addition of HA-DMPE and doxorubicin (DOX), they produced a DOX/SWCNT/HA-DMPE hybrid system for intelligent, targeted drug delivery [6] (Table 5 (5)). This innovative system showed excellent biocompatibility, targeting of CD44+ overexpressing cells and pH-triggered drug release. A range of different HA molecular masses was tested, and it was found that 200 kDa HA was the best for facilitating cellular uptake.
Biomolecules may also act as polymer dispersants. For example, polydopamine (PD) has been reported as a dispersant for CNMs [67]. However, the biomedical use of PD dispersions has not been widely investigated. PD is a biocompatible and cost-effective coating for nanomaterials [68], and the plentiful catechol groups give it great hydrophilicity. The main problem with PD is that it tends to self-aggregate in solution [69]. One way around that is to utilise O2 purging during the coating process [70] to produce a uniform, thin coating. Lee et al. developed a PD/polyether amine surface coating for MWCNTs that allowed for tunable solubility by varying chain length and amine groups in the final coating [70] (Table 5 (6)).
Deoxyribonucleic acid (DNA) is another amphiphilic biopolymer with surfactant properties. It can be cheaply obtained from animal/plant waste material [71]. There have been many examples of using DNA as a biocompatible dispersant to produce stable CNM dispersions. For instance, Patil et al. produced graphene dispersions with concentrations of up to 2.5 mg/mL that were stable for several months [72]. They used single-strand DNA (ssDNA) as a dispersant. However, the method they employed required the use of hydrazine, which may negatively affect the biocompatibility of the final product [72] (Table 5 (7)). Since then, innovative ways have been developed to solubilise graphene using environmentally friendly techniques. Zabihi et al. developed a method of producing non-covalently DNA-functionalised graphene using a ball-milling technique with graphite and DNA extracted from fish waste [71] (Table 5 (8)). The downside of this method is that ball milling is very rough on the CNMs. It also results in a small degree of covalent graphene modification [71].
Plenty of literature exists on using DNA to solubilise CNTs. Ostojic et al. successfully functionalised SWCNT with DNA to produce stable aqueous dispersions. Cathcart et al. have also achieved this using natural salmon testes DNA to disperse SWCNTs [73] (Table 5 (9)). They incorporated cisplatin and potassium tetra-chloroplatinate into these hybrid systems by covalently attaching them to the immobilised DNA. They also reduced the cisplatin molecules on the SWCNT surface to form platinum nanocrystals, successfully retaining the optical and electronic properties of the CNTs, the biological properties of the DNA and the chemical functionality of the platinum [74] (Table 5 (10)). In more recent studies, Xhyliu and Ao demonstrated that ssDNA could be utilised to recognise and purify different SWCNT enantiomers by a surfactant substitution mechanism [20]. This discovery lays the foundations for using enantiomerically pure CNTs in various applications, such as bio-imaging and sensor technology (Table 5 (11)).
Using DNA as a dispersant for CNMs has many advantages, as the DNA itself can additionally be used as a therapeutic, imaging, or targeting agent. For example, DNA can be utilised as a non-viral vector for animal gene therapy and plant genetic engineering [75]. These materials have many advantages over traditional viral vectors, such as increased payload capacity, lower toxicity concerns, high transfection efficiency, and increased targeting capabilities [75]. Another benefit of DNA is that tremendous work has been done on its chemistry to date—allowing for many options in terms of functionalisation and use in hybrid systems. DNA exhibits strong binding to CNMs due to π-π stacking interactions between the aromatic nucleobases and the surface of the CNM. This causes the hydrophobic inner DNA surface to orientate itself towards the CNM surface whilst the hydrophilic sugar-phosphate backbone orientates itself towards the aqueous solution, forming a hydrophilic layer around the CNM. CNTs can fit inside the spiral structure of the double helix, causing synergistic binding due to complementarity. For graphene surfaces, ssDNA forms globular aggregates on its surface [72], perhaps leading to less efficient dispersion due to unfavourable surface coverage, although this is yet to be investigated.
A range of commercially available polymers that can disperse CNMs also exists. One example is Triton X-100, a phenolated polyethylene glycol derivative. This non-ionic surfactant is biocompatible, cheap, and widely studied as a dispersant for CNMs. Rastogi et al. demonstrated the impressive efficiency and effectiveness of Triton X-100 as a dispersant for MWCNTs [22]—these dispersions consisted of individual MWCNTs (Table 5 (12)). There are examples of Triton X-100:CNT dispersions that are stable for up to two months [3]. The high efficiency of this surfactant stems from the presence of an aromatic ring in its hydrophobic region. This ring takes part in π-π interactions, leading to enhanced binding to the CNM surface. The hydrophilic polyethylene glycol chain orientates away from the CNM surface, into the solvent. This orientation provides steric repulsion with other dispersed CNMs and, to a degree, electrostatic repulsion between the polar oxygen atoms. Stable, biocompatible dispersions of CNOs using Triton X-100 have been described by Bobrowska et al. [56], and graphene dispersions using this surfactant have also been produced [3]. Overall, this dispersant is cheap, versatile, and biocompatible, making it an excellent choice in producing CNM dispersions for biomedical uses (Table 5 (13)).
Biocompatible sugar-based polymer surfactants such as Montanov 82 (an alkyl polyglucoside), and Surfhope 1216 (a sucrose based dispersant) also show promise [16]. These compounds are cheap and already approved for use in pharmaceutical, beauty, and food products [16]. Datsyuk et al. were able to produce stable aqueous MWCNT dispersions via a surfactant substitution method using Montanov 82, Surfhope 1216, gum Arabic (a natural gum consisting of polysaccharides and glycoproteins), and Tween 20 (a commercially available polymer surfactant) [16] (Table 5 (14)).
Polymer surfactants are perhaps the most promising class of non-covalent CNM dispersants due to their versatility, effectiveness, biocompatibility, and stability of dispersions produced by polymer wrapping mechanisms. Block copolymers can be tuned to the specific CNMs to ensure high dispersing efficiency. Biopolymers such as DNA and PD offer negligible toxicity, low cost, and biorecognition/therapeutic properties. The addition of drug molecules, targeting ligands, and fluorophores to form composite polymers is a potential game-changer in the field of non-covalent CNM functionalisation. This approach will have many future applications; for example, these polymers could be used to produce targeted drug delivery systems, and the incorporation of fluorophores also allows for cellular tracking experiments to be performed. The main downside to polymer dispersants (particularly composite polymers), compared to small-molecule surfactants, is that they are difficult to prepare and characterise. They are not always commercially available either.

Table 5.
Polymer dispersant based CNM dispersions.
Table 5.
Polymer dispersant based CNM dispersions.
| Num. | Dispersant | CNM | Description | Application | Ref. |
|---|---|---|---|---|---|
| 1 | F127 | SWCNT | A PEO/PPO block copolymer used to disperse SWCNTs | Physiochemical characterisation, NMR studies | [23] |
| 2 | AP432 | MWCNT SWCNT | An amphiphilic PEO/PPO block copolymer with a starlike structure used to disperse CNTs | Optimisation of surfactant structure | [64] |
| 3 | PDha | MWCNT | MWCNTs functionalised with a polydehydroalanine based copolymer | Stability studies; physiochemical characterisation. | [65] |
| 4 | HA-DMPE f-HA-DMPE | CNO | Supramolecular functionalisation of CNOs with a dispersant polymer conjugated with a fluorophore and targeting ligand | In-vitro biosafety and cell tracking studies. In vivo toxicity studies | [61] |
| 5 | HA-DMPE-DOX | SWCNT | A HA-DMPE polymer was used to coat SWCNTs and encapsulate DOX | pH-responsive drug delivery targeting CD44+ cancer cells | [6] |
| 6 | PD-PEA | MWCNT | A PD-PEA copolymer used to disperse MWCNTs | Polymer nanofillers | [70] |
| 7 | ssDNA | Graphene | A soluble Bio-Nanocomposite of DNA and graphene | Multifunctional lamellar nanocomposite production | [72] |
| 8 | DNA | Graphene | DNA functionalised graphene formed via ball milling of graphite | Environmentally friendly flame retardant | [71] |
| 10 | DNA | SWCNT | Aqueous dispersion produced with DNA wrapped SWCNTs | Developing a cheap, simple, and efficient method of producing high-quality dispersions of individual SWNTs | [73] |
| 9 | poly(GT) DNA | SWCNT | DNA wrapped SWCNTs functionalised with platinum nanocrystals | Thin-film preparation | [74] |
| 11 | DNA | SWCNT | DNA functionalised SWCNTs produced via surfactant substitution | Production and sorting of pure chirality CNTs | [22] |
| 12 | Triton X-100 | MWCNT | MWCNTs functionalised with Triton X-100 | Investigation of Triton X-100 dispersing mechanism, comparison to other surfactants | [22] |
| 13 | Triton X-100 | CNO | CNOs non-covalently functionalised with Triton X-100 | Stability studies; in vitro antimicrobial assay | [56] |
| 14 | Montanov 82, Surfhope 1216, Gum Arabic, Tween 20 | MWCNT | MWCNTs dispersed with a range of polymer surfactants via a surfactant substitution mechanism | Preparation of stable aqueous MWCNT dispersions | [16] |
7. Conclusions
In this review, problems facing the production of stable, aqueous dispersions of carbon nanomaterials (CNMs) were introduced, and selected methods of overcoming their inherent hydrophobicity using non-covalent methods were reviewed and compared. The importance of mechanical dispersion methods was discussed, and the binding forces utilised in functionalisation were detailed. The benefits of the η* metric (a thorough measure of a surfactants abilities, taking into account its efficiency and effectiveness) were described; however, this value is not used widely in the literature. The morphology and stability of different dispersions were also discussed. Many synthetic biocompatible dispersants were found. For example, Gemini surfactants were established as being very effective. Bio-inspired materials such as polydopamine (PD), deoxyribonucleic acid (DNA), bile acid salts (BASs), and nanocrystalline cellulose (NCC) proved to be efficient, biocompatible, cheap, and environmentally friendly dispersants. However, much work must be done on these promising materials for graphene and fullerene systems. Some of these molecules show favourable dispersion properties and superior biocompatibility over synthetic dispersants. Many of these are also more accessible and cheaper to obtain than manufactured surfactants due to their abundance in nature. Polymer wrapping was also introduced, although it is yet to be extensively studied for materials such as graphene and carbon nano-onions (CNOs). However, examples do exist for carbon nanotubes (CNTs) [35]. Polymer dispersants have many advantages over small molecule surfactants, as they can be designed to incorporate fluorophores, targeting ligands, and drug molecules. While not the most effective, alkyl chain surfactants were ubiquitous in the literature and excelled at de-bundling CNMs. BAS and cellulose were found to be excellent bioinspired dispersants. Overall, there are many different options for non-covalently dispersing CNMs for use in biomedical applications, as highlighted in this review.
There exist many methods of dispersing CNMs in aqueous solutions; however, bioinspired dispersants such as those mentioned above will likely be vital in the future biomedical uses of CNMs. Cellulose-based dispersants combine strong dispersing abilities with biocompatibility, widespread availability, and cheap cost. This makes them perfect for dispersing CNMs on an industrial scale. BASs are also powerful surfactants that are both cheap and naturally occurring; this makes them ideal for biomedical applications when used in small amounts. Alkyl chain surfactants (ACSs) are ubiquitous in the literature and their chemistry is well studied. The future of this class of incredibly useful dispersants will likely involve the development of gemini surfactants to improve their dispersion efficiency and effectiveness. Polymer wrapping will likely prove to be indispensable, especially when the combined drug carrying, imaging, and targeting properties of conjugate polymers are further explored. The mechanics of polymer wrapping are not well understood for many nanomaterials such as CNOs, hence more work is needed in this area. The adaptation of the η* metric in future studies will allow for easier comparison between different dispersants. The possibility of customising a dispersant for a particular nanomaterial by considering factors such as complementarity is also an exciting development that will likely result in the development of extremely efficient surfactants.
Author Contributions
H.M. wrote the initial draft of the manuscript and prepared the figures. M.B. proofread and edited the manuscript. S.G. conceived the original idea, supervised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Irish Research Council (IRC) is gratefully acknowledged for providing a Government of Ireland Postgraduate Scholarship (GOIPG) (grant number GOIPG/2021/210) to H.M.; and a Government of Ireland Postgraduate Scholarship (GOIPG) (grant number GOIPG/2019/1820) to M.B.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Adalberto Camisasca for his valuable proofreading and feedback.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACS | alkyl chain surfactant |
| BAS | bile acid salt |
| CMC | critical micelle concentration |
| CNF(s) | cellulose nano-fibril(s) |
| CNM(s) | carbon nanomaterial(s) |
| CNO(s) | carbon nano-onion(s) |
| CNTs | carbon nanotube(s) |
| CQD(s) | carbon quantum dot(s) |
| CTAB | hexadecyltrimethylammonium bromide |
| DC | deoxycholate |
| DMPE | 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine |
| DNA | deoxyribonucleic acid |
| DOX | doxorubicin |
| EPD | electrophoretic deposition |
| F127 | pluronic F127 |
| HA | hyaluronic acid |
| LDS | lithium dodecyl sulphate |
| MWCNT(s) | multi-walled carbon nanotube(s) |
| NCC | nanocrystalline cellulose |
| PD | polydopamine |
| PDha | polydehydroalanine |
| PEO | polyethylene oxide |
| PPO | polypropylene oxide |
| SC | sodium cholate |
| SDBS | sodium dodeclybenzene sulphonate |
| SDC | sodium deoxycholate |
| SDS | sodium dodecyl sulphate |
| ssDNA | single-stranded deoxyribonucleic acid |
| SWCNT(s) | single-walled carbon nanotubes(s) |
| TTAB | tetradecyl trimethyl ammonium bromide |
| vdW | van der Waal |
References
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Potočnik, J. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial Text with EEA Relevance. Off. J. Eur. Union 2011, 275, 38–40. [Google Scholar]
- Borode, A.O.; Ahmed, N.A.; Olubambi, P.A. Surfactant-Aided Dispersion of Carbon Nanomaterials in Aqueous Solution. Phys. Fluids 2019, 31, 071301. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Tolkou, A.K.; Efstathiou, S.; Rahdar, A.; Favvas, E.P.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials in Cementitious Composites: An Update. Molecules 2021, 26, 1430. [Google Scholar] [CrossRef]
- Dovjuu, O.; Kim, S.; Lee, A.; Kim, J.; Noh, J.; Huh, S.; Choi, B.; Jeong, H. A Simple Approach for Heat Transfer Enhancement of Carbon Nanofluids in Aqueous Media. J. Nanosci. Nanotechnol. 2020, 20, 2337–2343. [Google Scholar] [CrossRef]
- Arpicco, S.; Bartkowski, M.; Barge, A.; Zonari, D.; Serpe, L.; Milla, P.; Dosio, F.; Stella, B.; Giordani, S. Effects of the Molecular Weight of Hyaluronic Acid in a Carbon Nanotube Drug Delivery Conjugate. Front. Chem. 2020, 8, 578008. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, R.; Li, Y.; Wang, Z.; Li, L.; Tian, L. Folate-Conjugated Nanodiamond for Tumor-Targeted Drug Delivery. RSC Adv. 2015, 5, 82711–82716. [Google Scholar] [CrossRef]
- Camisasca, A.; Sacco, A.; Brescia, R.; Giordani, S. Boron/Nitrogen Co-Doped Carbon Nano-Onion Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2018, 1, 5763–5773. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Eredia, M.; Qiu, H.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Bonn, M.; Wang, H.I.; Samorì, P. Water-Dispersed High-Quality Graphene: A Green Solution for Efficient Energy Storage Applications. ACS Nano 2019, 13, 9431–9441. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.-K. Nanomedicines: Current Status and Future Perspectives in Aspect of Drug Delivery and Pharmacokinetics. J. Pharm. Investig. 2018, 49, 43–60. [Google Scholar] [CrossRef] [Green Version]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New Developments in Non-Covalent Surface Modification, Dispersion and Electrophoretic Deposition of Carbon Nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Chemistry, Toxicity and Remediation. In Pollutant Diseases, Remediation and Recycling; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2013; pp. 277–320. ISBN 978-3-319-02387-8. [Google Scholar]
- Ciesielski, A.; Samorì, P. Supramolecular Approaches to Graphene: From Self-Assembly to Molecule-Assisted Liquid-Phase Exfoliation. Adv. Mater. 2016, 28, 6030–6051. [Google Scholar] [CrossRef]
- Bartkowski, M.; Giordani, S. Supramolecular Chemistry of Carbon Nano-Onions. Nanoscale 2020, 12, 9352–9358. [Google Scholar] [CrossRef]
- Datsyuk, V.; Landois, P.; Fitremann, J.; Peigney, A.; Galibert, A.M.; Soula, B.; Flahaut, E. Double-Walled Carbon Nanotube Dispersion via Surfactant Substitution. J. Mater. Chem. 2009, 19, 2729–2736. [Google Scholar] [CrossRef] [Green Version]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2018; ISBN 978-0-19-876986-6. [Google Scholar]
- Lémery, E.; Briançon, S.; Chevalier, Y.; Bordes, C.; Oddos, T.; Gohier, A.; Bolzinger, M.-A. Skin Toxicity of Surfactants: Structure/Toxicity Relationships. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 166–179. [Google Scholar] [CrossRef]
- Galantini, L.; Di Gregorio, M.C.; Gubitosi, M.; Travaglini, L.; Vázquez Tato, J.; Jover, A.; Meijide, F.; Tellini, V.S.; Pavel, N. Bile Salts and Derivatives: Rigid Unconventional Amphiphiles as Dispersants, Carriers and Superstructure Building Blocks. Curr. Opin. Colloid Interface Sci. 2015, 20, 170–182. [Google Scholar] [CrossRef]
- Xhyliu, F.; Ao, G. Chirality-Pure Carbon Nanotubes Show Distinct Complexation with Recognition DNA Sequences. Carbon 2020, 167, 601–608. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative Study of Carbon Nanotube Dispersion Using Surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.M.F.; Dai, J.; Regev, O.; Marques, E.F.; Furó, I. Block Copolymers as Dispersants for Single-Walled Carbon Nanotubes: Modes of Surface Attachment and Role of Block Polydispersity. Langmuir 2018, 34, 13672–13679. [Google Scholar] [CrossRef]
- De Siqueira, J.E.L.; Gleize, P.J.P. Effect of Carbon Nanotubes Sonication on Mechanical Properties of Cement Pastes. IBRACON Struct. Mater. J. 2020, 13, 455–463. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X. Different Effects of Sonication Pretreatment on Carbon Nanomaterials under Low Hydrogen Peroxide Concentrations. Ultrason. Sonochem. 2017, 34, 19–26. [Google Scholar] [CrossRef]
- Blanch, A.J.; Lenehan, C.E.; Quinton, J.S. Optimizing Surfactant Concentrations for Dispersion of Single-Walled Carbon Nanotubes in Aqueous Solution. J. Phys. Chem. B 2010, 114, 9805–9811. [Google Scholar] [CrossRef]
- Fernandes, R.M.F.; Abreu, B.; Claro, B.; Buzaglo, M.; Regev, O.; Furó, I.; Marques, E.F. Dispersing Carbon Nanotubes with Ionic Surfactants under Controlled Conditions: Comparisons and Insight. Langmuir 2015, 31, 10955–10965. [Google Scholar] [CrossRef]
- Robinson, B.; Bailey, S.; O’Driscoll, L.; Visontai, D.; Welsh, D.; Mostert, A.; Mazzocco, R.; Rabot, C.; Jarvis, S.; Kolosov, O.; et al. Formation of Two-Dimensional Micelles on Graphene: Multi-Scale Theoretical and Experimental Study. ACS Nano 2017, 11, 3404–3412. [Google Scholar] [CrossRef]
- Patil, R.; Marathe, D.; Roy, S.P.; Ray, D.; Aswal, V.K.; Jha, P.K.; Bahadur, P.; Tiwari, S. Colloidal Stability of Graphene Oxide Nanosheets in Association with Triblock Copolymers: A Neutron Scattering Analysis. Mater. Sci. Eng. C 2020, 109, 110559. [Google Scholar] [CrossRef]
- Cui, H.; Xiantong, Y.; Monasterio, M.; Xing, F. Effects of Various Surfactants on the Dispersion of MWCNTs-OH in Aqueous Solution. Nanomater 2017, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Pérez, E.M.; Martín, N. π-π Interactions in Carbon Nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef]
- Bakshi, M.S. Engineered Nanomaterials Growth Control by Monomers and Micelles: From Surfactants to Surface Active Polymers. Adv. Colloid Interface Sci. 2018, 256, 101–110. [Google Scholar] [CrossRef]
- Fujigaya, T.; Nakashima, N. Non-Covalent Polymer Wrapping of Carbon Nanotubes and the Role of Wrapped Polymers as Functional Dispersants. Sci. Technol. Adv. Mater. 2015, 16, 024802. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of Pi.-.Pi. Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Bruce, C.; Senapati, S.; Berkowitz, M.; Perera, L.; Forbes, M. Molecular Dynamics Simulations of Sodium Dodecyl Sulfate Micelle in Water: The Behavior of Water. J. Phys. Chem. B 2002, 106, 10902–10907. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Ansón-Casaos, A.; Grasa, L.; Abenia, L.; Salvador, A.; Colom, E.; Mesonero, J.E.; García-Bordejé, J.E.; Benito, A.M.; Maser, W.K. Unique Properties and Behavior of Nonmercerized Type-II Cellulose Nanocrystals as Carbon Nanotube Biocompatible Dispersants. Biomacromolecules 2019, 20, 3147–3160. [Google Scholar] [CrossRef]
- Khili, F.; Borges, J.; Almeida, P.L.; Boukherroub, R.; Omrani, A.D. Extraction of Cellulose Nanocrystals with Structure I and II and Their Applications for Reduction of Graphene Oxide and Nanocomposite Elaboration. Waste Biomass Valorization 2019, 10, 1913–1927. [Google Scholar] [CrossRef]
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 10, 186. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wu, Y.; Xie, X. Water Vapor Sorption Properties of Cellulose Nanocrystals and Nanofibers Using Dynamic Vapor Sorption Apparatus. Sci. Rep. 2017, 7, 14207. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, R.; Wu, Z. Investigation of the Mechanical Properties and Microstructure of Graphene Nanoplatelet-Cement Composite. Nanomaterials 2016, 6, 200. [Google Scholar] [CrossRef]
- Durairaj, V.; Li, P.; Liljeström, T.; Wester, N.; Etula, J.; Leppänen, I.; Ge, Y.; Kontturi, K.S.; Tammelin, T.; Laurila, T.; et al. Functionalized Nanocellulose/Multiwalled Carbon Nanotube Composites for Electrochemical Applications. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Håkansson, K.; Salajkova, M.; Lundell, F.; Wågberg, L.; Berglund, L.A. Highly Conducting, Strong Nanocomposites Based on Nanocellulose-Assisted Aqueous Dispersions of Single-Wall Carbon Nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- De Buy Wenniger, L.M.; Pusl, T.; Beuers, U. Bile Salts. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 167–171. ISBN 978-0-12-378631-9. [Google Scholar]
- Sun, Z.P.; Zhang, W.Q.; Lu, X.M. Reduced Graphene Oxide Nanosheets Functionalized with Bile Salts as Support for Electrochemical Catalysts. AMR 2012, 535–537, 1467–1477. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordani, S. Surfactant-Mediated Dispersions of Carbon Nano-Onions in Aqueous Solution. Nano Express 2020, 1, 010018. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Veldhuis, S.; Zhitomirsky, I. Sodium Deoxycholate as a Versatile Dispersing and Coating-Forming Agent: A New Facet of Electrophoretic Deposition Technology. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124382. [Google Scholar] [CrossRef]
- Podlesny, B.; Olszewska, B.; Yaari, Z.; Jena, P.V.; Ghahramani, G.; Feiner, R.; Heller, D.A.; Janas, D. En Route to Single-Step, Two-Phase Purification of Carbon Nanotubes Facilitated by High-Throughput Spectroscopy. Sci. Rep. 2021, 11, 10618. [Google Scholar] [CrossRef]
- Sun, Z.; Nicolosi, V.; Rickard, D.; Bergin, S.D.; Aherne, D.; Coleman, J.N. Quantitative Evaluation of Surfactant-Stabilized Single-Walled Carbon Nanotubes: Dispersion Quality and Its Correlation with Zeta Potential. J. Phys. Chem. C 2008, 112, 10692–10699. [Google Scholar] [CrossRef]
- Gubitosi, M.; Trillo, J.V.; Vargas, A.A.; Pavel, N.V.; Gazzoli, D.; Sennato, S.; Jover, A.; Meijide, F.; Galantini, L. Characterization of Carbon Nanotube Dispersions in Solutions of Bile Salts and Derivatives Containing Aromatic Substituents. J. Phys. Chem. B 2014, 118, 1012–1021. [Google Scholar] [CrossRef]
- Wenseleers, W.; Vlasov, I.I.; Goovaerts, E.; Obraztsova, E.D.; Lobach, A.S.; Bouwen, A. Efficient Isolation and Solubilization of Pristine Single-Walled Nanotubes in Bile Salt Micelles. Adv. Funct. Mater. 2004, 14, 1105–1112. [Google Scholar] [CrossRef]
- Abreu, B.; Rocha, J.; Fernandes, R.M.F.; Regev, O.; Furó, I.; Marques, E.F. Gemini Surfactants as Efficient Dispersants of Multiwalled Carbon Nanotubes: Interplay of Molecular Parameters on Nanotube Dispersibility and Debundling. J. Colloid Interface Sci. 2019, 547, 69–77. [Google Scholar] [CrossRef]
- Dobies, M.; Iżykowska, J.; Wilkowska, M.; Woźniak-Braszak, A.; Szutkowski, K.; Skrzypczak, A.; Jurga, S.; Kozak, M. Dispersion of Water Proton Spin–Lattice Relaxation Rates in Aqueous Solutions of Multiwall Carbon Nanotubes (MWCNTs) Stabilized via Alkyloxymethylimidazolium Surfactants. J. Phys. Chem. C 2017, 121, 11839–11850. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Czyrko, J.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon Nano-Onion Composites: Physicochemical Characteristics and Biological Activity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 185–192. [Google Scholar] [CrossRef]
- Nessim, M.I.; Osman, M.M.; Ismail, D.A. Surface-Active Properties of New Cationic Gemini Surfactants with Cyclic Spacer. J. Dispers. Sci. Technol. 2018, 39, 1047–1055. [Google Scholar] [CrossRef]
- Liu, X.-P.; Feng, J.; Zhang, L.; Gong, Q.-T.; Zhao, S.; Yu, J.-Y. Synthesis and Surface Activity of Anionic Gemini Surfactants with Alkyl Spacers. J. Dispers. Sci. Technol. 2011, 32, 568–575. [Google Scholar] [CrossRef]
- Stranks, S.D.; Habisreutinger, S.N.; Dirks, B.; Nicholas, R.J. Novel Carbon Nanotube-Conjugated Polymer Nanohybrids Produced by Multiple Polymer Processing. Adv. Mater. 2013, 25, 4365–4371. [Google Scholar] [CrossRef]
- Fujigaya, T.; Yamamoto, Y.; Kano, A.; Maruyama, A.; Nakashima, N. Enhanced Cell Uptake via Non-Covalent Decollation of a Single-Walled Carbon Nanotube-DNA Hybrid with Polyethylene Glycol-Grafted Poly(l-Lysine) Labeled with an Alexa-Dye and Its Efficient Uptake in a Cancer Cell. Nanoscale 2011, 3, 4352. [Google Scholar] [CrossRef]
- D’Amora, M.; Camisasca, A.; Boarino, A.; Arpicco, S.; Giordani, S. Supramolecular Functionalization of Carbon Nano-Onions with Hyaluronic Acid-Phospholipid Conjugates for Selective Targeting of Cancer Cells. Colloids Surf. B Biointerfaces 2020, 188, 110779. [Google Scholar] [CrossRef]
- Okamoto, M.; Fujigaya, T.; Nakashima, N. Design of an Assembly of Poly(Benzimidazole), Carbon Nanotubes, and Pt Nanoparticles for a Fuel-Cell Electrocatalyst with an Ideal Interfacial Nanostructure. Small 2009, 5, 735–740. [Google Scholar] [CrossRef]
- Fernandes, R.M.F.; Buzaglo, M.; Regev, O.; Marques, E.F.; Furo, I. Surface Coverage and Competitive Adsorption on Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 22190–22197. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.; Zhao, T.; Zhu, Y.; Shi, X.; Gong, H.; Zhang, Z. Dispersing Carbon Nanotubes in Aqueous Solutions by a Starlike Block Copolymer. J. Phys. Chem. C 2008, 112, 16377–16384. [Google Scholar] [CrossRef]
- Max, J.B.; Pergushov, D.V.; Sigolaeva, L.V.; Schacher, F.H. Polyampholytic Graft Copolymers Based on Polydehydroalanine (PDha)—Synthesis, Solution Behavior and Application as Dispersants for Carbon Nanotubes. Polym. Chem. 2019, 10, 3006–3019. [Google Scholar] [CrossRef]
- Matthaiolampakis, G.; Milane, L.; Singh, A.; Amiji, M. Hyaluronic Acid Targeting of CD44 for Cancer Therapy: From Receptor Biology to Nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Zhao, H.; Chao, Y.; Liu, J.; Huang, J.; Pan, J.; Guo, W.; Wu, J.; Sheng, M.; Yang, K.; Wang, J.; et al. Polydopamine Coated Single-Walled Carbon Nanotubes as a Versatile Platform with Radionuclide Labeling for Multimodal Tumor Imaging and Therapy. Theranostics 2016, 6, 1833–1843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Hu, W.; Wu, M.; Gong, L.; Tang, A.; Xiang, L.; Zhu, B.; Zhu, L.; Zeng, H. Cost-Effective Strategy for Surface Modification via Complexation of Disassembled Polydopamine with Fe(III) Ions. Langmuir 2019, 35, 4101–4109. [Google Scholar] [CrossRef]
- Ding, Y.; Weng, L.-T.; Yang, M.; Yang, Z.; Lu, X.; Huang, N.; Leng, Y. Insights into the Aggregation/Deposition and Structure of a Polydopamine Film. Langmuir 2014, 30, 12258–12269. [Google Scholar] [CrossRef]
- Lee, H.D.; Yoo, B.M.; Lee, T.H.; Park, H.B. Defect-Free Surface Modification Methods for Solubility-Tunable Carbon Nanotubes. J. Colloid Interface Sci. 2018, 509, 307–317. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Li, Q.; Ferdowsi, M.R.G.; Mahmoodi, R.; Kalali, E.N.; Wang, D.-Y.; Naebe, M. A Sustainable Approach to Scalable Production of a Graphene Based Flame Retardant Using Waste Fish Deoxyribonucleic Acid. J. Clean. Prod. 2020, 247, 119150. [Google Scholar] [CrossRef]
- Patil, A.J.; Vickery, J.L.; Scott, T.B.; Mann, S. Aqueous Stabilization and Self-Assembly of Graphene Sheets into Layered Bio-Nanocomposites Using DNA. Adv. Mater. 2009, 21, 3159–3164. [Google Scholar] [CrossRef]
- Cathcart, H.; Quinn, S.; Nicolosi, V.; Kelly, J.M.; Blau, W.J.; Coleman, J.N. Spontaneous Debundling of Single-Walled Carbon Nanotubes in DNA-Based Dispersions. J. Phys. Chem. C 2007, 111, 66–74. [Google Scholar] [CrossRef]
- Ostojic, G.N.; Ireland, J.R.; Hersam, M.C. Noncovalent Functionalization of DNA-Wrapped Single-Walled Carbon Nanotubes with Platinum-Based DNA Cross-Linkers. Langmuir 2008, 24, 9784–9789. [Google Scholar] [CrossRef]
- Singh, A.; Hsu, M.H.; Gupta, N.; Khanra, P.; Kumar, P.; Prakash Verma, V.; Kapoor, M. Derivatized Carbon Nanotubes for Gene Therapy in Mammalian and Plant Cells. ChemPlusChem 2020, 85, 466–475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).