Carbon Nano-Onion Peroxidase Composite Biosensor for Electrochemical Detection of 2,4-D and 2,4,5-T
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
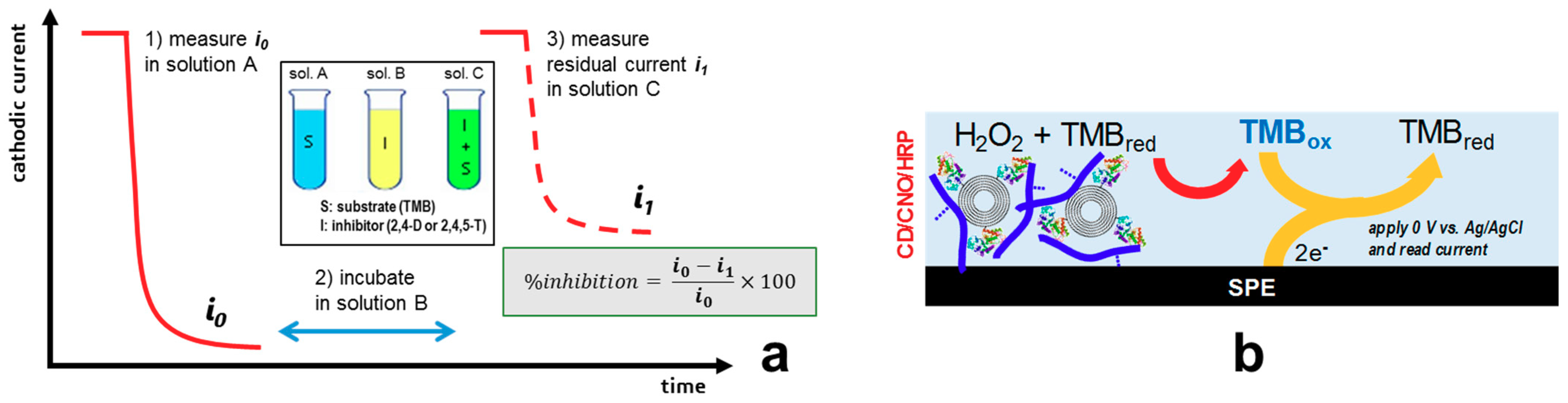
2.2. Immobilization of M-HRP and Inhibition Assays
2.3. Computational Docking Study
2.4. Synthesis of Thiolated Cyclodextrin Polymer (ACDPSH)
2.5. Biosensor Preparation and Herbicide Detection
3. Results and Discussion
3.1. Inhibition Mechanism of 2,4-D and 2,4,5-T on the Activity of HRP
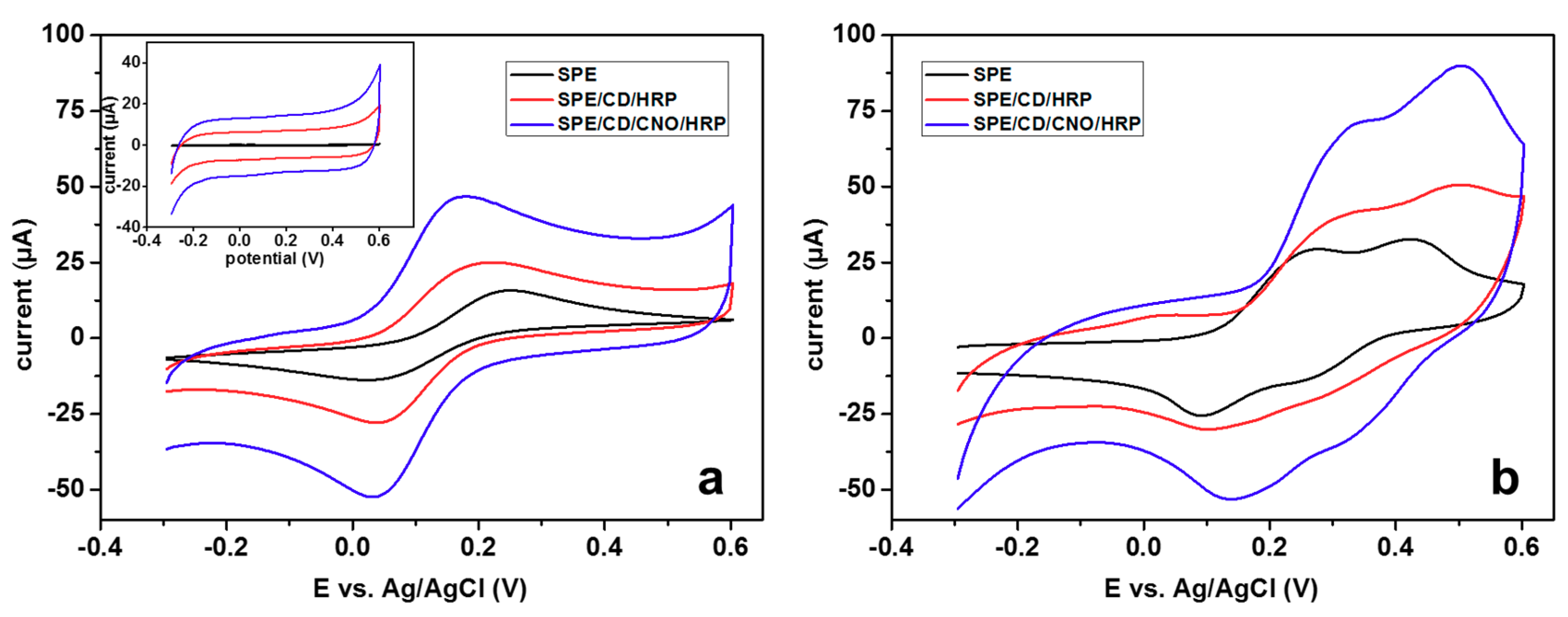
3.2. Characterization of Modified Electrodes
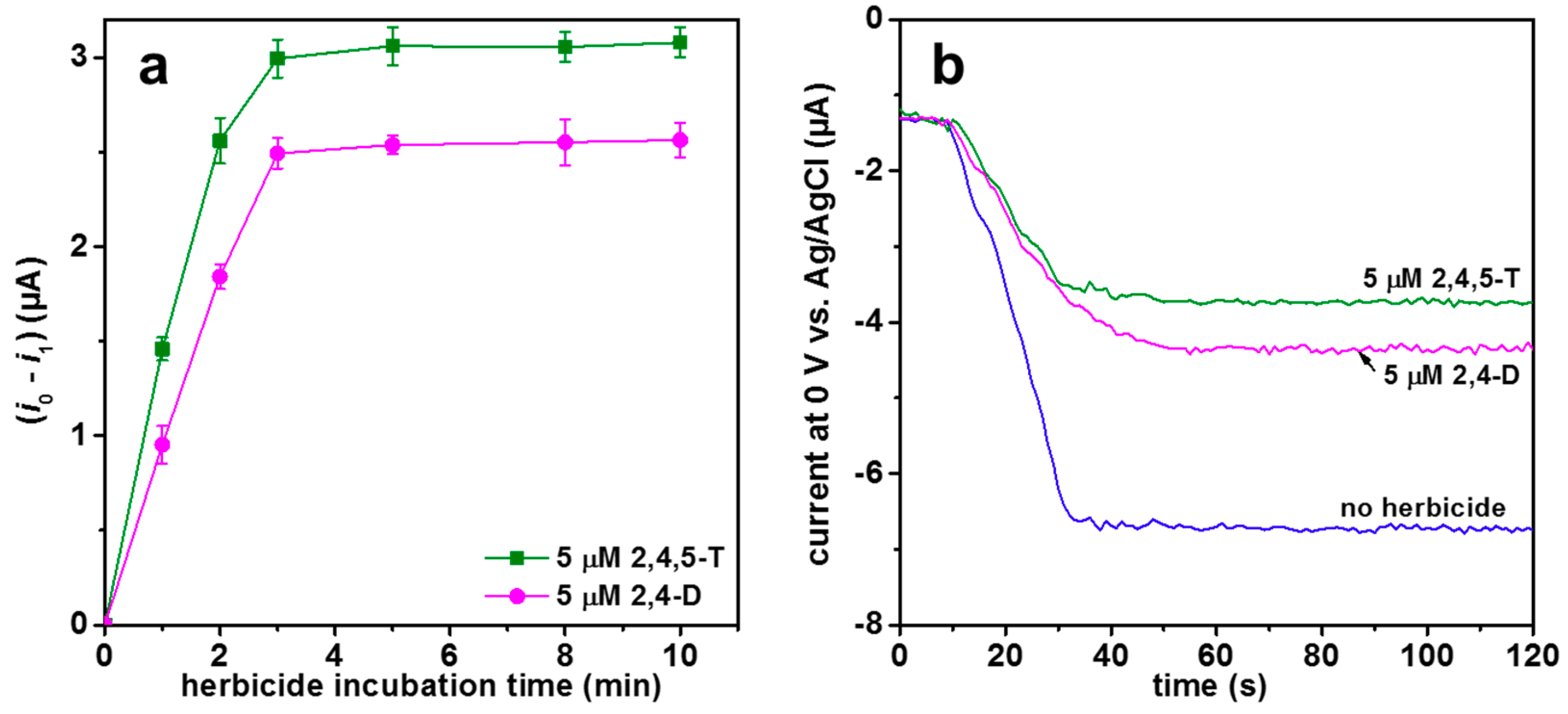
3.3. Amperometric Detection of 2,4-D and 2,4,5-T
3.4. Stability and Repeatability of the Developed Biosensors
3.5. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNO | carbon nano-onion |
| HRP | horseradish peroxidase |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| 2,4,5-T | 2,4,5-trichlorophenoxyacetic acid |
| CD | cyclodextrin |
| SPE | screen-printed carbon electrode |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| ELISA | enzyme-linked immunosorbent assay |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
References
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Bartolome, J.P.; Fragoso, A. Electrochemical detection of nitrite and ascorbic acid at glassy carbon electrodes modified with carbon nano-onions bearing electroactive moieties. Inorg. Chim. Acta 2017, 468, 223–231. [Google Scholar] [CrossRef]
- Sohouli, E.; Keihan, A.H.; Shahdost-Fard, F.; Naghian, E.; Plonska-Brzezinska, M.E.; Rahimi-Nasrabadi, M.; Ahmadi, F. A glassy carbon electrode modified with carbon nanoonions for electrochemical determination of fentanyl. Mat. Sci. Eng. C 2020, 110, 110684. [Google Scholar] [CrossRef] [PubMed]
- Zuaznabar-Gardona, J.C.; Fragoso, A. Development of highly sensitive IgA immunosensors based on co-electropolymerized L-DOPA/dopamine carbon nano-onion modified electrodes. Biosens. Bioelectron. 2019, 141, 111357. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Amperometric biosensor for glyphosate based on the inhibition of tyrosinase conjugated to carbon nano-onions in a chitosan matrix on a screen-printed electrode. Microchim. Acta 2019, 186, 569. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E. Carbon nano-onions: A review of recent progress in synthesis and applications. ChemNanoMat 2019, 5, 568–580. [Google Scholar] [CrossRef]
- Bartkowski, M.; Giordani, S. Carbon nano-onions as potential nanocarriers for drug delivery. Dalton Trans. 2021, 50, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, K.; Carranza, C.S.; Aluffi, M.E. Herbicides based on 2,4-D: Its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ. Sci. Pollut. Res. 2020, 27, 38501–38512. [Google Scholar] [CrossRef]
- Boivin, A.; Amellal, S.; Schiavon, M.; van Genuchten, M.T. 2,4-Dichlorophenoxyacetic acid (2,4-D) sorption and degradation dynamics in three agricultural soils. Environ. Pollut. 2005, 138, 92–99. [Google Scholar] [CrossRef]
- Mei, X.Y.; Hong, Y.Q.; Chen, G.H. Review on analysis methodology of phenoxy acid herbicide residues. Food Anal. Methods 2016, 9, 1532–1561. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kaya, S.I.; Ozkan, S.A. Recent advances of enzyme biosensors for pesticide detection in foods. J. Food Meas. Charact. 2021, in press. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Medyantseva, E.; Vertlib, M.; Kutyreva, M.; Khaldeeva, E.; Budnikov, G.; Eremin, S. The specific immunochemical detection of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid pesticides by amperometric cholinesterase biosensors. Anal. Chim. Acta 1997, 347, 71–78. [Google Scholar] [CrossRef]
- Shyuan, L.; Heng, L.; Ahmad, M.; Aziz, S.; Ishak, Z. Evaluation of Pesticide and Heavy Metal Toxicity Using Immobilized Enzyme Alkaline Phosphatase with an Electrochemical Biosensor. Asian J. Biochem. 2008, 3, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhong, A.; Xu, Q.; Cao, H.; Hu, X. Inhibition of 2,4-Dichlorophenoxyacetic Acid to Catalase Immobilized on Hierarchical Porous Calcium Phosphate: Kinetic Aspect and Electrochemical Biosensor Construction. J. Phys. Chem. C 2016, 120, 15966–15975. [Google Scholar] [CrossRef]
- Veitch, N. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Songa, E.; Somerset, V.; Waryo, T.; Baker, P.; Iwuoha, E. Amperometric nanobiosensor for quantitative determination of glyphosate and glufosinate residues in corn samples. Pure Appl. Chem. 2009, 81, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xu, G.; Gong, L.; Dai, H.; Zhang, S.; Li, Y.; Lin, Y. An enzyme-assisted electrochemiluminescent biosensor developed on order mesoporous carbons substrate for ultrasensitive glyphosate sensing. Electrochim. Acta 2015, 186, 624–630. [Google Scholar] [CrossRef]
- Moccelini, S.; Vieira, I.; de Lima, F.; Lucca, B.; Barbosa, A.; Ferreira, V. Determination of thiodicarb using a biosensor based on alfalfa sprout peroxidase immobilized in self-assembled monolayers. Talanta 2010, 82, 164–170. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Preparation and characterization of alkaline phosphatase, horseradish peroxidase, and glucose oxidase conjugates with carboxylated carbon nano-onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Torréns, M.; Fragoso, A.; O’Sullivan, C. Highly sensitive colorimetric enzyme-linked oligonucleotide assay based on cyclodextrin-modified polymeric surfaces. Anal. Bioanal. Chem. 2012, 403, 195–202. [Google Scholar] [CrossRef]
- Sok, V.; Fragoso, A. Kinetic, spectroscopic and computational docking study of the inhibitory effect of the pesticides 2,4,5-T, 2,4-D and glyphosate on the diphenolase activity of mushroom tyrosinase. Int. J. Biol. Macromol. 2018, 118, 427–434. [Google Scholar] [CrossRef]
- Fragoso, A.; Sanromà, B.; Ortiz, M.; O’Sullivan, C. Layer-by-layer self-assembly of peroxidase on gold electrodes based on complementary cyclodextrin–adamantane supramolecular interactions. Soft Matter 2009, 5, 400–406. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, P.; Pei, Y.; Xu, H.; Xin, X.; Pei, Z. Regioselective acetylation of carbohydrates and diols catalyzed by tetramethyl-ammonium hydroxide in water. Green Chem. 2014, 16, 4510–4514. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular Chemistry of Cyclodextrins in Enzyme Technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Fragoso, A.; O’Sullivan, C. Detection of Antigliadin Autoantibodies in Celiac Patient Samples Using a Cyclodextrin-Based Supramolecular Biosensor. Anal. Chem. 2011, 83, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Visser, C.; Stevanović, S.; Voorwinden, L.; Bloois, L.; Gaillard, P.; Danhof, M.; Crommelin, D.; Boer, A. Targeting liposomes with protein drugs to the blood–brain barrier in vitro. Eur. J. Pharm. Sci. 2005, 25, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Konopka, S.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Zuaznabar-Gardona, J.C.; Fragoso, A. Electrochemical characterisation of the adsorption of ferrocenemethanol on carbon nano-onion modified electrodes. J. Electroanal. Chem. 2020, 871, 114314. [Google Scholar] [CrossRef]
- Ritcharoon, B.; Sallabhan, R.; Toewiwat, N.; Mongkolsuk, S.; Loprasert, S. Detection of 2,4-dichlorophenoxyacetic acid herbicide using a FGE-sulfatase based whole-cell Agrobacterium biosensor. J. Microbiol. Meth. 2020, 175, 105997. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Sotomayor, M. Biomimetic sensor based on 5,10,15,20-tetrakis(pentafluorophenyl)-21H,23H-porphyrin iron (III) chloride and MWCNT for selective detection of 2,4-D. Sens. Actuat. B 2013, 181, 332–339. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Wu, X.; Fu, J.; Kang, Q.; Shen, D.; Li, J.; Chen, L. A molecular imprinting-based turn-on Ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens. Bioelectron. 2016, 81, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Dzantiev, B.; Zherdev, A.; Yulaev, M.; Sitdikov, R.; Dmitrieva, N.; Moreva, I. Electrochemical immunosensors for determination of the pesticides 2,4-dichlorophenoxyacetic and 2,4,5-tricholorophenoxyacetic acids. Biosens. Bioelectron. 1996, 11, 179–185. [Google Scholar] [CrossRef]
- Tomassetti, M.; Martini, E.; Campanella, L. New immunosensors for 2,4-D and 2,4,5-T pesticides determination. Int. J. Environ. Anal. Chem. 2012, 92, 417–431. [Google Scholar] [CrossRef]
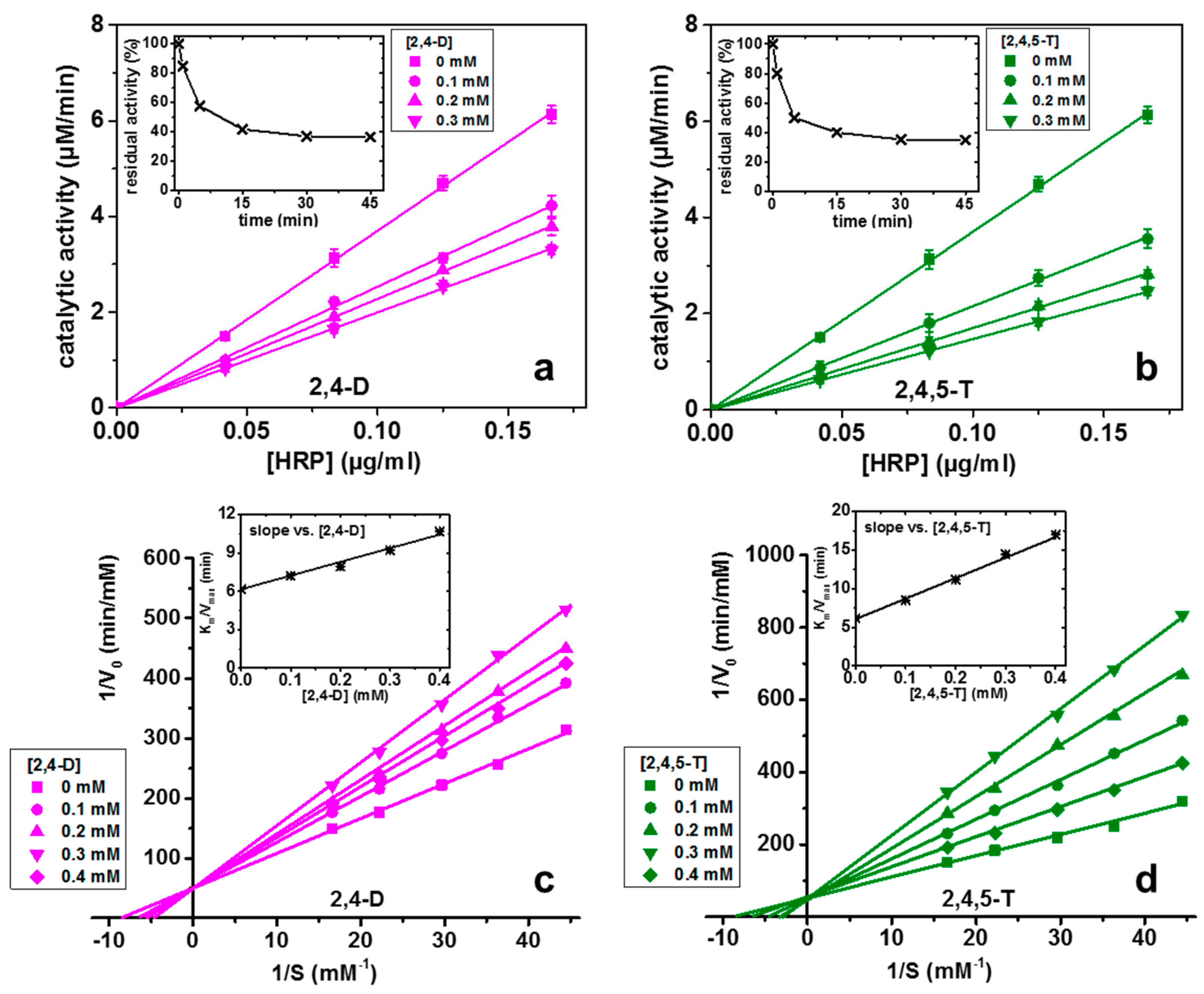
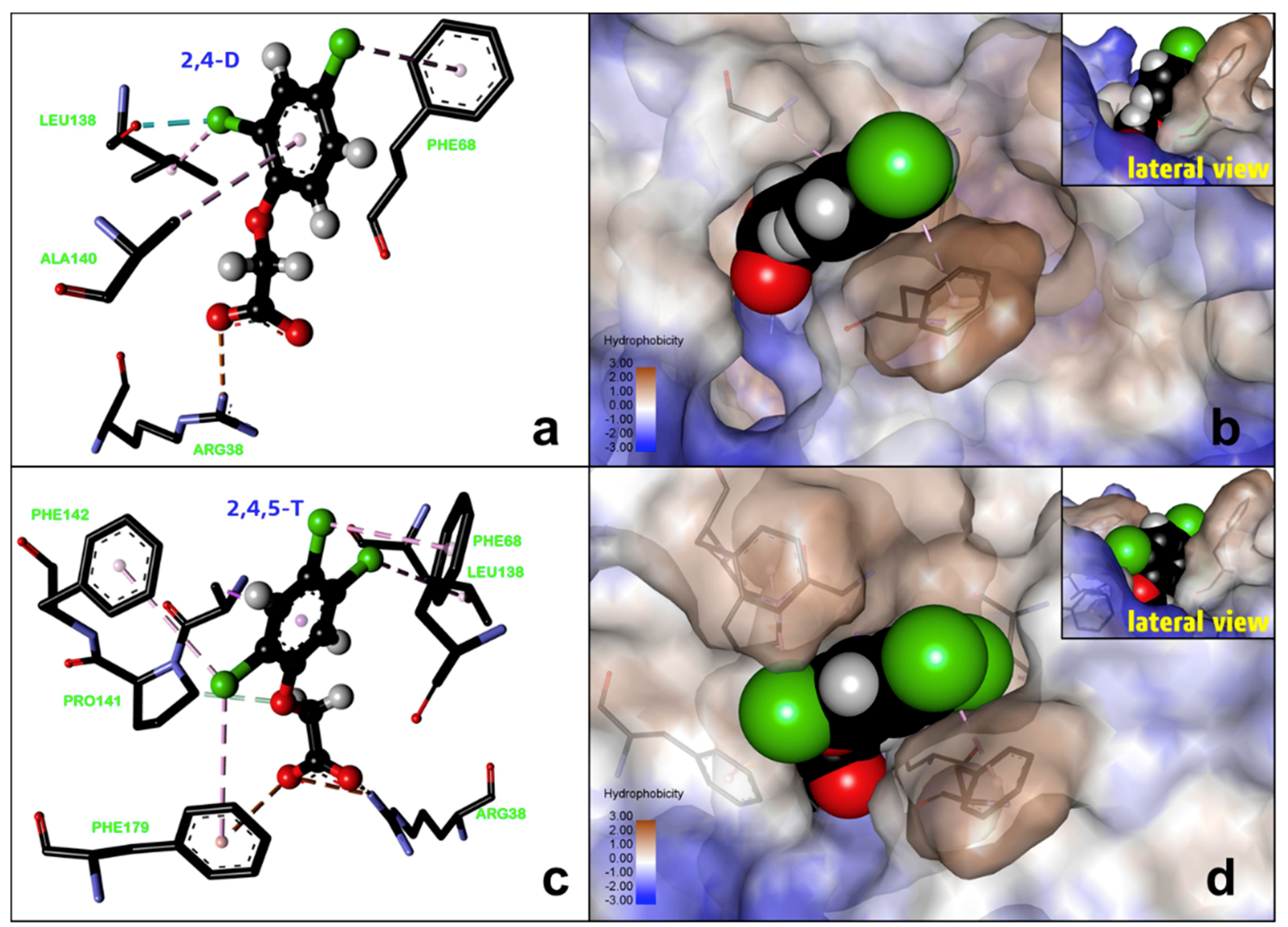
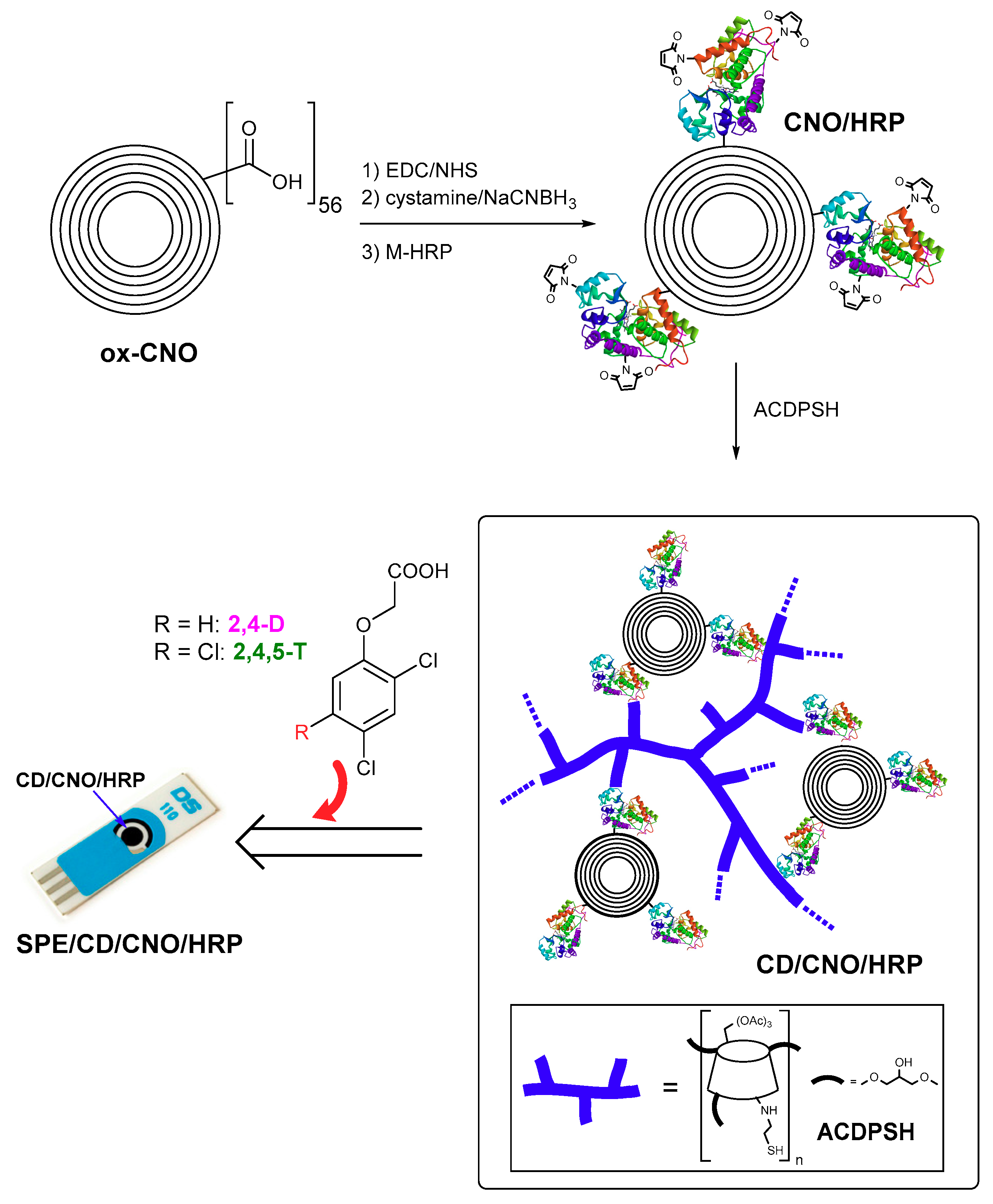
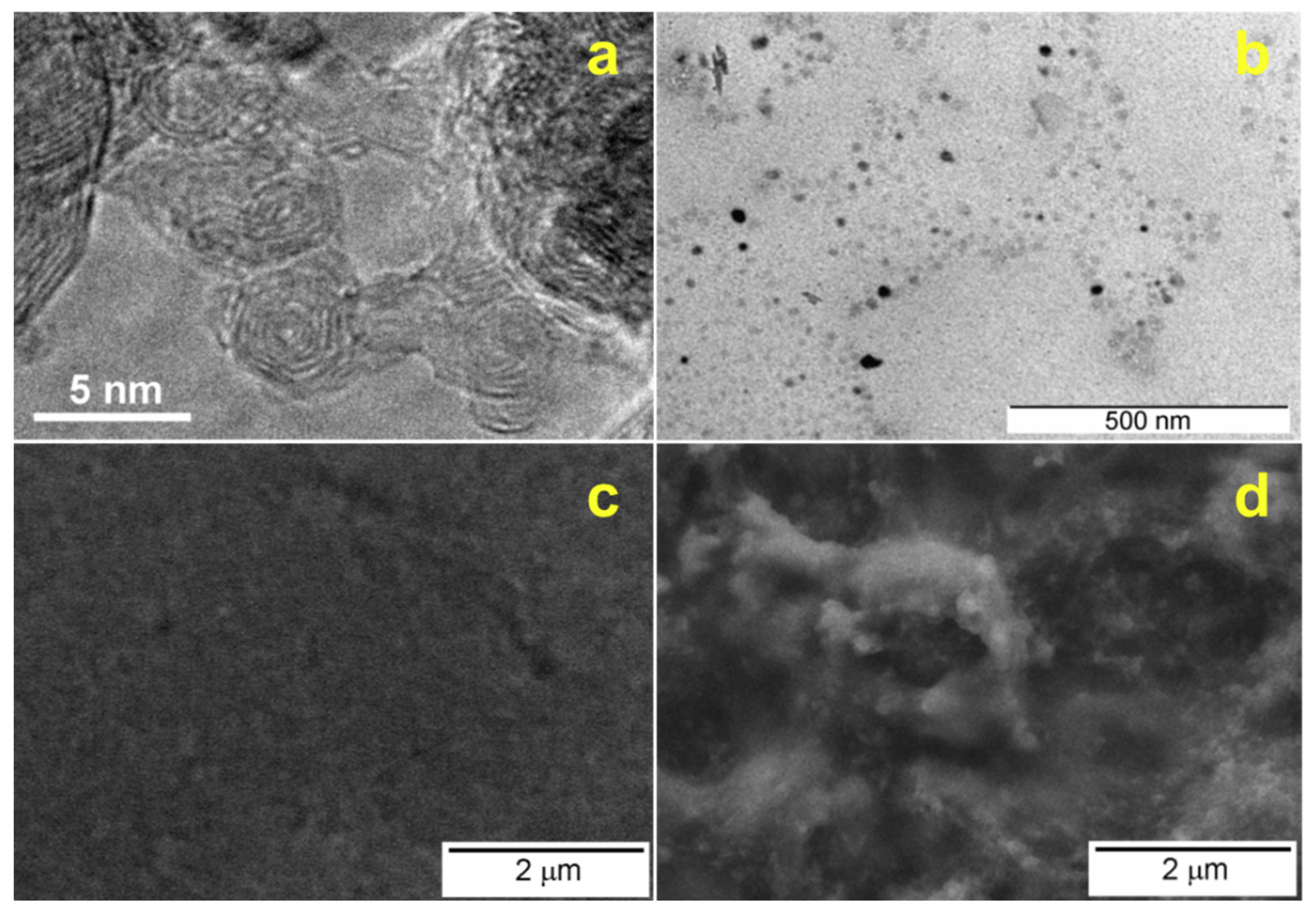


| Sensor | Herbicide | Sensitivity (μM−1) | LOD (nM) | LOD (μg/L) |
|---|---|---|---|---|
| SPE/CD/CNO/HRP | 2,4-D | 17 ± 2 | 23 ± 3 | 5.1 |
| SPE/CD/HRP | 2,4-D | 5.4 ± 0.6 | 33 ± 4 | 7.3 |
| SPE/CD/CNO/HRP | 2,4,5-T | 22 ± 2 | 10 ± 3 | 2.6 |
| SPE/CD/HRP | 2,4,5-T | 8.5 ± 0.7 | 17 ± 2 | 4.4 |
| Herbicide | Surface Chemistry | Detection Technique | Assay Time (min) | LOD (μM) | Reference |
|---|---|---|---|---|---|
| 2,4-D | Catalase immobilized on porous graphene | Amperometry, enzyme inhibition | 10 | 0.15 | [16] |
| 2,4-D | Sulfatase based whole-cell Agrobacterium biosensor | Fluorescence, enzyme activity | overnight | 1.56 | [32] |
| 2,4-D | Iron (III) porphyrin on immobilized on MWCNT | Amperometry, electrocatalysis | 3 | 2.1 | [33] |
| 2,4-D | Quantum dot modified molecule imprinting polymer | Fluorescence of nitrobenzoxadiazole | 5 | 0.14 | [34] |
| 2,4-D | Carbon nano-onion/HRP conjugate on cyclodextrin polymer | Amperometry, enzyme inhibition | 5 | 0.023 | This work |
| 2,4,5-T | Alkaline phosphatase/sol-gel chitosan film on carbon paste electrode | Amperometry, enzyme inhibition | 15 | 1.9 | [15] |
| 2,4,5-T | Polyclonal antibody-based immunoassay | Colorimetric, ELISA | 60 | 0.005 | [14] |
| 2,4,5-T | Polyclonal antibody on graphite electrode | Potentiometry, competitive assay | 10 | 200 | [35] |
| 2,4,5-T | Albumin-2,4,5-T immobilized in membrane | Colorimetric, competitive ELISA | 30 | 0.0028 | [36] |
| 2,4,5-T | Carbon nano-onion/HRP conjugate on cyclodextrin polymer | Amperometry, enzyme inhibition | 5 | 0.010 | This work |
| Herbicide | Sample | Method | Concentration |
|---|---|---|---|
| 2,4-D | Soil treated with 2,4-D | SPE/CD/CNO/HRP biosensor | (11 ± 3) mg/kg |
| ELISA | (9.6 ± 0.5) mg/kg | ||
| 2,4,5-T | River water spiked with 2,4,5-T | SPE/CD/CNO/HRP biosensor | 3.0 μM (added) |
| (2.85 ± 0.05) μM (found) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sok, V.; Fragoso, A. Carbon Nano-Onion Peroxidase Composite Biosensor for Electrochemical Detection of 2,4-D and 2,4,5-T. Appl. Sci. 2021, 11, 6889. https://doi.org/10.3390/app11156889
Sok V, Fragoso A. Carbon Nano-Onion Peroxidase Composite Biosensor for Electrochemical Detection of 2,4-D and 2,4,5-T. Applied Sciences. 2021; 11(15):6889. https://doi.org/10.3390/app11156889
Chicago/Turabian StyleSok, Vibol, and Alex Fragoso. 2021. "Carbon Nano-Onion Peroxidase Composite Biosensor for Electrochemical Detection of 2,4-D and 2,4,5-T" Applied Sciences 11, no. 15: 6889. https://doi.org/10.3390/app11156889
APA StyleSok, V., & Fragoso, A. (2021). Carbon Nano-Onion Peroxidase Composite Biosensor for Electrochemical Detection of 2,4-D and 2,4,5-T. Applied Sciences, 11(15), 6889. https://doi.org/10.3390/app11156889







