Abstract
The role of intraluminal thrombus (ILT) in the rupture of abdominal aortic aneurysms (AAA) is controversial, and it is unclear whether it increases or decreases the risk of rupture. This research aims to find a clear answer to this question. Previous computer modelling suggests that an ILT lowers oxygen dissemination to the AAA wall, contributing to wall thinning. The methodology used in this study determines the amount of oxygen reaching the aneurysm wall after passing through the ILT by using the porous nature of the ILT to recreate the condition as closely as feasible. Using computed tomographic images, patient-specific three-dimensional (3D) AAA geometries were recreated. Modelling blood and oxygen flow in AAA was obtained using a computational fluid dynamics (CFD) approach. Our findings indicated that the oxygen volume percentage had completely reached the aneurysm wall. Only at the inlet and outflow did the greatest wall shear stress (WSS) occur, with a significant drop in the central region of the aneurysm wall. CFD was used to calculate the velocity, pressure, and WSS of aortic blood flow. ILT had no effect on oxygen flow to the aneurysm wall, disproving the theory that it produces local hypoxia.
1. Introduction
The abdominal aorta is one of the largest arteries in the human body and the major blood vessel emerging from the left ventricle of the heart [1]. In addition, it is the channel for distributing oxygenated blood from the heart to arterial branches that transmit the blood to all organs of the body [2]. Flow development in the aorta is complex due to the pulsatile nature of blood flow and statistical variations such as robust, torsion, tapering, twisting, curving, and branching. Theoretical and experimental analysis was detailed studying steady and unsteady flow patterns in a curved tube, which provides an understanding of flow patterns, expansion of secondary flow, dean vortices, and the WSS at the inner and outer walls of the aorta [3,4,5,6,7,8,9]. Computational fluid dynamics (CFD) was used to investigate blood flows in the aorta due to its high resolution and straightforward representation of flow parameters. CFD has vast applications for the treatment and diagnosis of CDs such as aneurysms, atherosclerosis, arteriosclerosis, and stenosis [10].
Abdominal aortic aneurysm (AAA) is a degenerative illness that causes the aorta to dilate and weaken [11]. AAA is also a life-threatening condition characterized by ballooning in the distal section of the abdominal aorta. This expansion continues until the aneurysm ruptures, which is a catastrophic event. Surgical repair is the sole option for this problem, and AAA diameter is the most important consideration in deciding whether or not to repair it. The aorta’s typical diameter is nearly 2 cm, and when it reaches or exceeds 5 cm, repair is recommended. Extensive investigations have recently been undertaken to evaluate if observing AAAs with a maximal diameter of 5.5 cm is safe [12,13]. The fundamental problem with this condition is that it is unpredictable or asymptomatic, and the only method to diagnose a patient or track the disease’s course is to perform CT scans on them annually or upon request. Untreated abdominal aortic aneurysms are dangerously close to rupturing. A rupture is a biomechanical event that occurs when stress in the dilating and degenerating aneurysm wall surpasses its strength at any point along its length [14]. The AAA frequently employs stress analysis to investigate stress distributions. The AAA is sensitive or prone to rupture if local tension is excessive, or there is stress concentration [15]. Furthermore, an intraluminal thrombus (ILT) is present in 75% of AAAs [16]. A blood clot is a three-dimensional (3D) fibrin structure incorporating blood cells, platelets, blood proteins, and cellular debris that forms between circulating blood and the aorta wall in varying degrees [17]. The role of the ILT in AAA is debatable, and it is unclear whether an ILT enhances or diminishes the chance of AAA rupture. The ILT is a porous substance, namely, a low-density material with pores.
However, the majority of earlier studies, particularly those based on three-dimensional geometries, modelled the ILT as a solid nonpermeable substance, which does not reflect reality. ILT was linked to an increased risk of rupture by some researchers [16,18]. Others feel that the ILT can reduce AAA wall tension and thus protect the AAA from rupture [14,19,20,21]. Despite this, numerous studies imply that ILT has no effect on the risk of rupture [22,23].
According to Wolf et al., an increased AAA thrombus burden was linked to a higher risk of fast growth [18]. Another study by Satta et al. linked the thickness of the endoluminal thrombus to the risk of rupture [24]. Ruptured and nonruptured aneurysms had similar dimensions, but the ruptured aneurysms had a thicker thrombus than the nonruptured ones. One study investigated if different flow patterns in aneurysms could be recognized by magnetic resonance imaging [25]. However, the recirculation zone produced inside the aneurysm cavity caused conditions that encourage thrombus formation and increase the risk of rupture. In contrast with these findings, a study by Faggioli et al. found no relation between thrombus thickness and rupture [26]. Intraluminal thrombus, a theoretic mathematic aneurysm model, can significantly reduce aneurysm wall stress and thereby protect against rupture [19,20]. Investigators concluded on the basis of AAA stress assessments using simplified hypothetic models that the presence of ILT decreases stress to the AAA wall [19,20,21,27]. However, in this research, the mechanical properties of ILT were predicted using a basic linear elastic model for this material, or simpler forms for the AAA and ILT were utilized.
This study focuses on whether the ILT provokes the rupture of the AAA wall by using CFD simulation with real ILT modelling. A 3D aorta geometric model with a single inlet and two outlets was reconstructed from each patient’s CT images. The 3D reconstructed model was imported to ANSYS v15 software for computation using FLUENT solvers. This locates the ILT by applying the porous property, studying pressure changes on the aneurysm wall, and oxygen and blood flow velocity.
2. Materials and Methods
2.1. Geometry
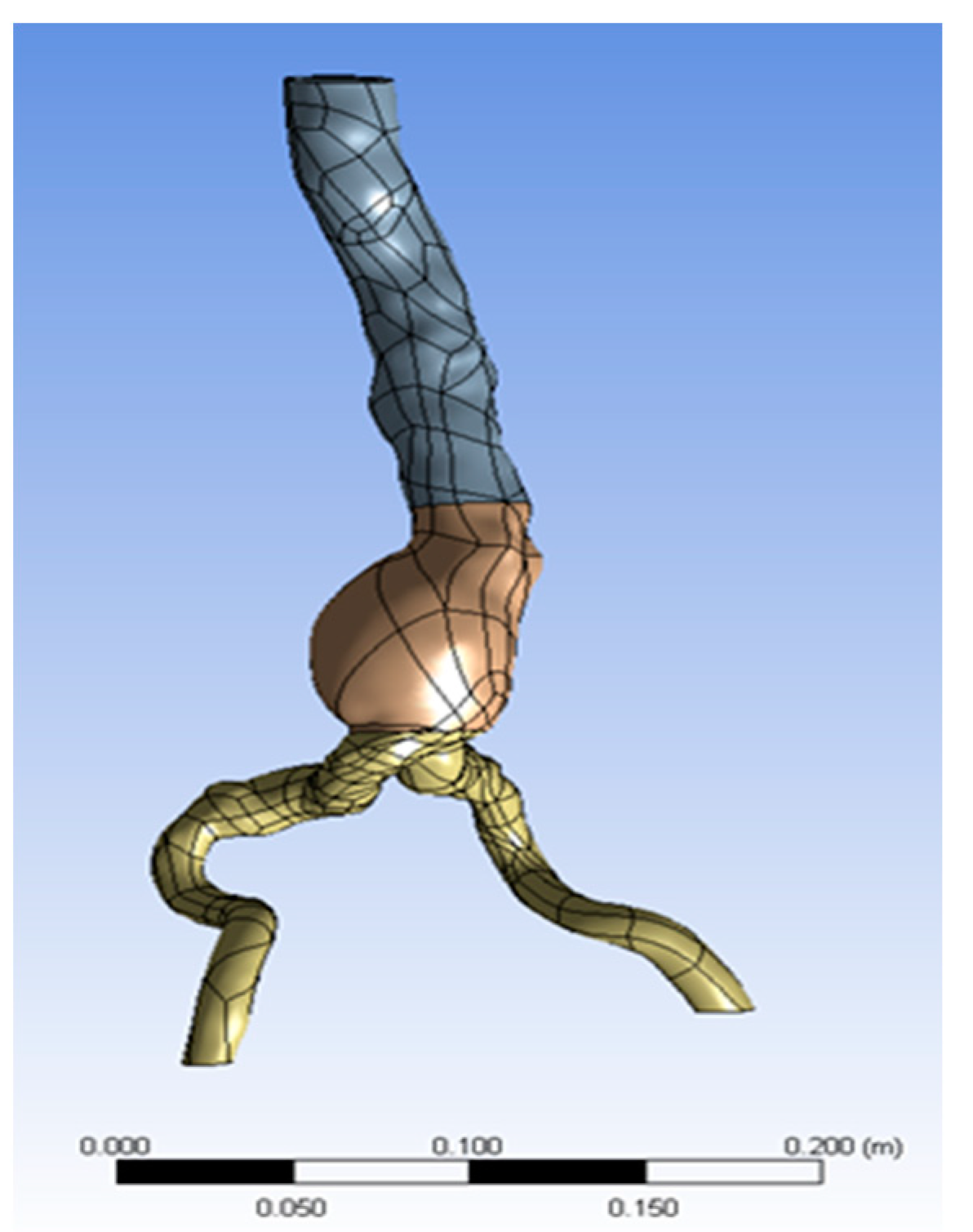
A three-dimensional patient-specific module of an abdominal aortic aneurysm (AAA) including a 1.5 mm thick intraluminal thrombus (ILT) was used here. The ILT was located inside the aneurysm that was inside the inflated part of the abdominal aorta. Thus, a 3D aorta geometric model with a single inlet and two outlets was reconstructed from each patient’s CT images, as shown in Figure 1 (available online: https://radiopaedia.org/articles/draped-aorta-sign?lang=us (accessed on 20 April 2021). However, the aneurysm geometry was mainly simulated for the following reasons:
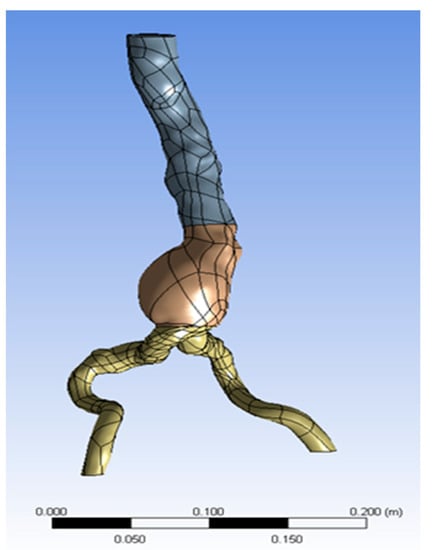
Figure 1.
AAA geometry.
The ILT was located by applying the porous property to study changes in the aneurysm wall, reduce computational errors and time, and increase computational accuracy.
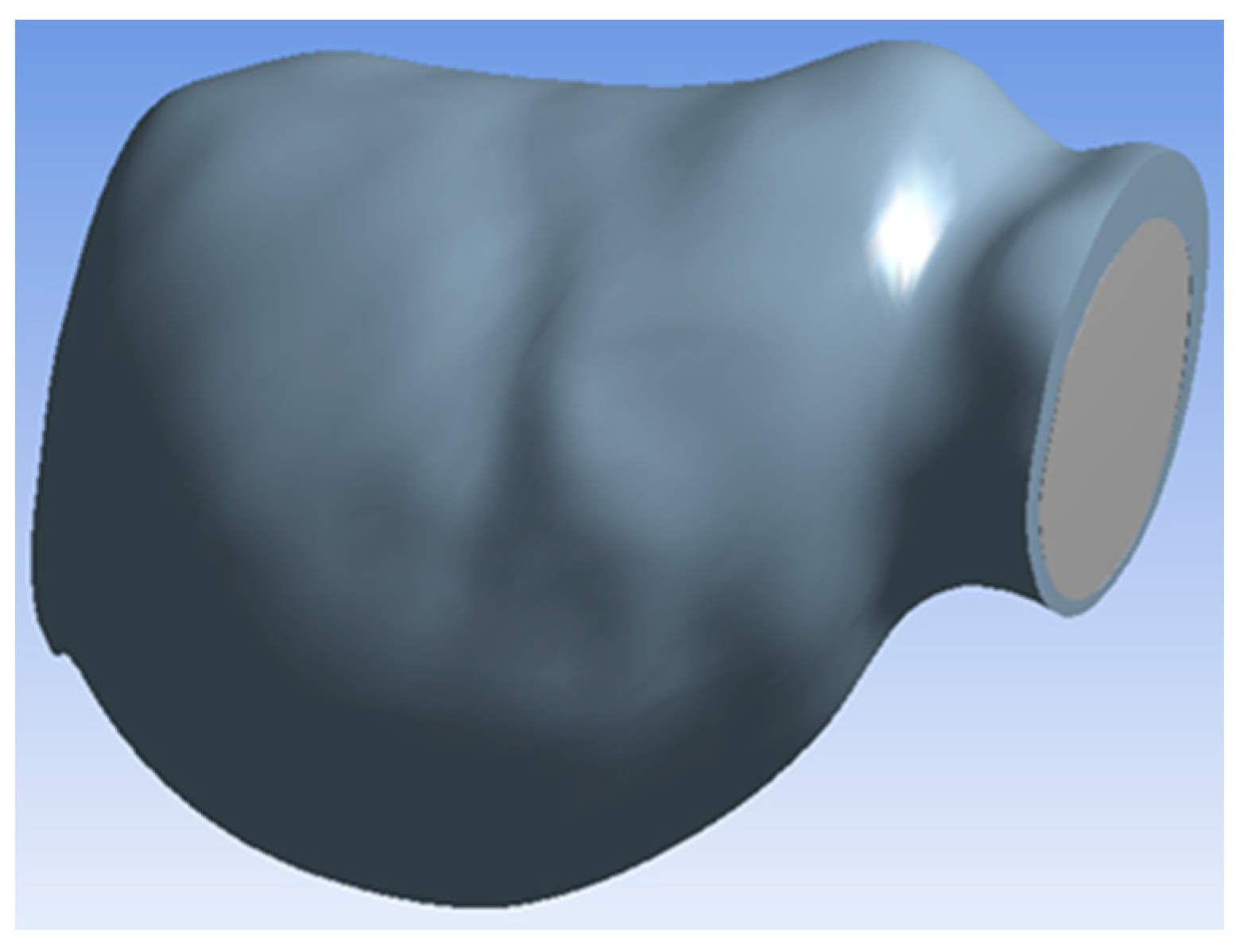
Figure 2 depicts the aneurysm geometry used in the simulation. Inlet and outlet diameters were approximately 3.5 cm, and the maximal aneurysm diameter was approximately 6.3 cm. The geometry had a single inlet and a single outlet for blood and oxygen flow. The 3D reconstructed model was imported to ANSYS v15 software (ANSYS, Canonsburg, PA, USA) for computation using FLUENT solvers.
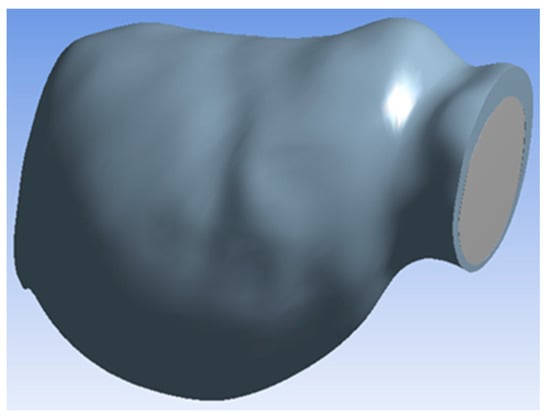
Figure 2.
Aneurysm geometry.
2.2. Mesh Generation
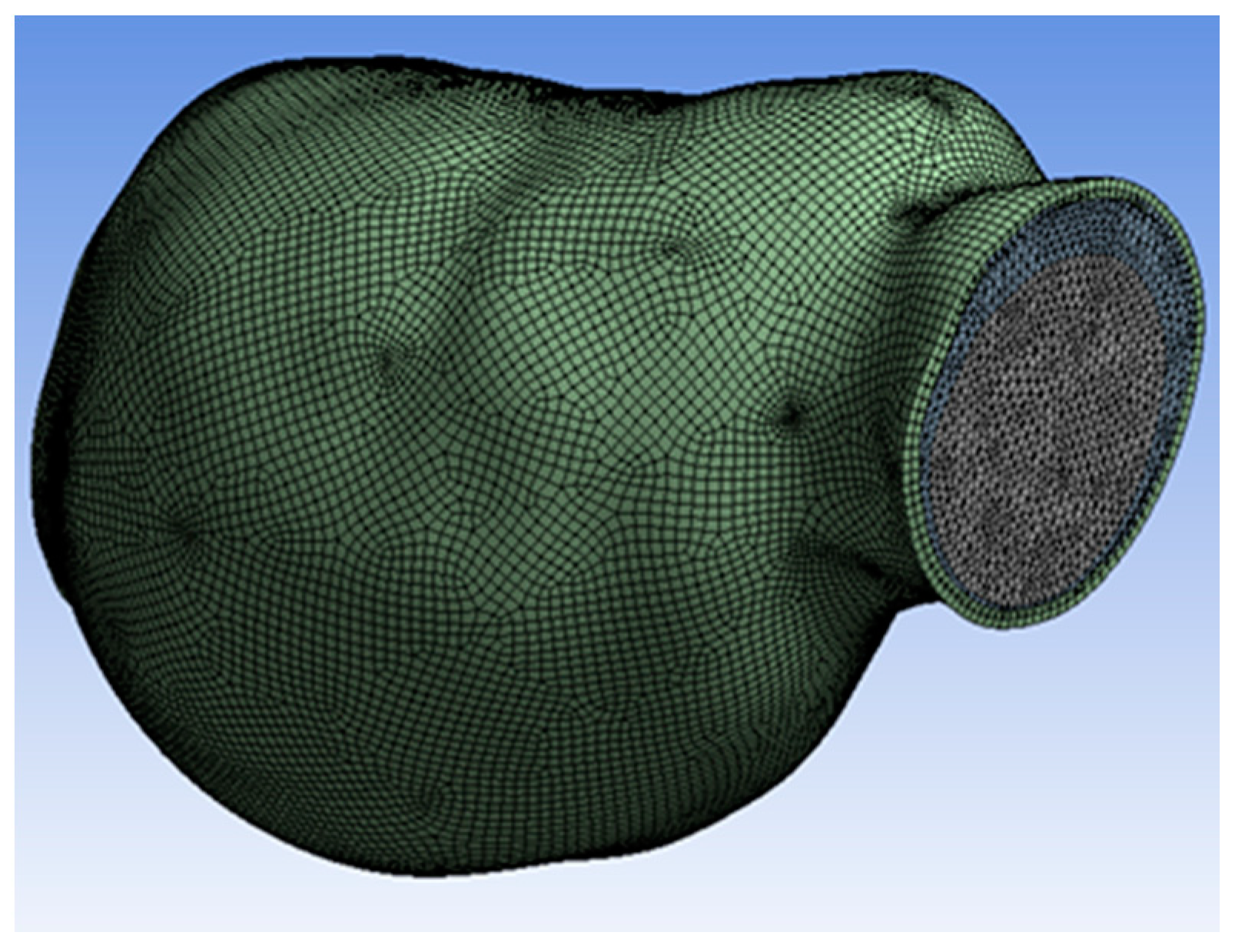
The 3D model of AAA was reconstructed and meshed, as shown in Figure 3. Mesh independence was studied to determine the optimal number of mesh elements, as meshing divides the geometry into many discretized volume elements or cells. This geometry meshed into 1,593,429 elements and 304,321 nodes. Thus, the change in the number of elements and nodes was due to changing the mesh size settings for higher computation accuracy.
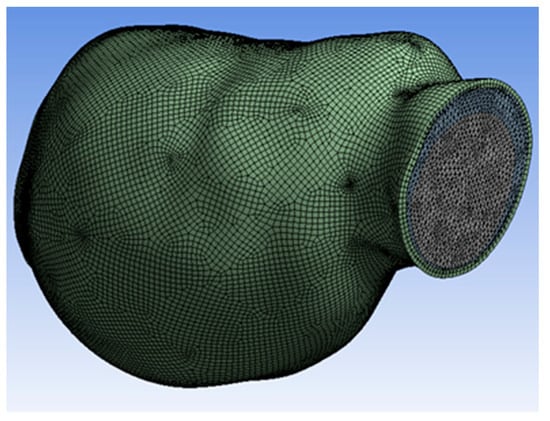
Figure 3.
Meshed model.
2.3. Material Properties
The geometry consisted of three bodies: the aneurysm walls as a solid material, the ILT as a porous material, and the lumen as a fluid material for the flow of oxygen and blood.
Wall properties:
ILT properties:
2.4. Intraluminal Thrombus Porosity
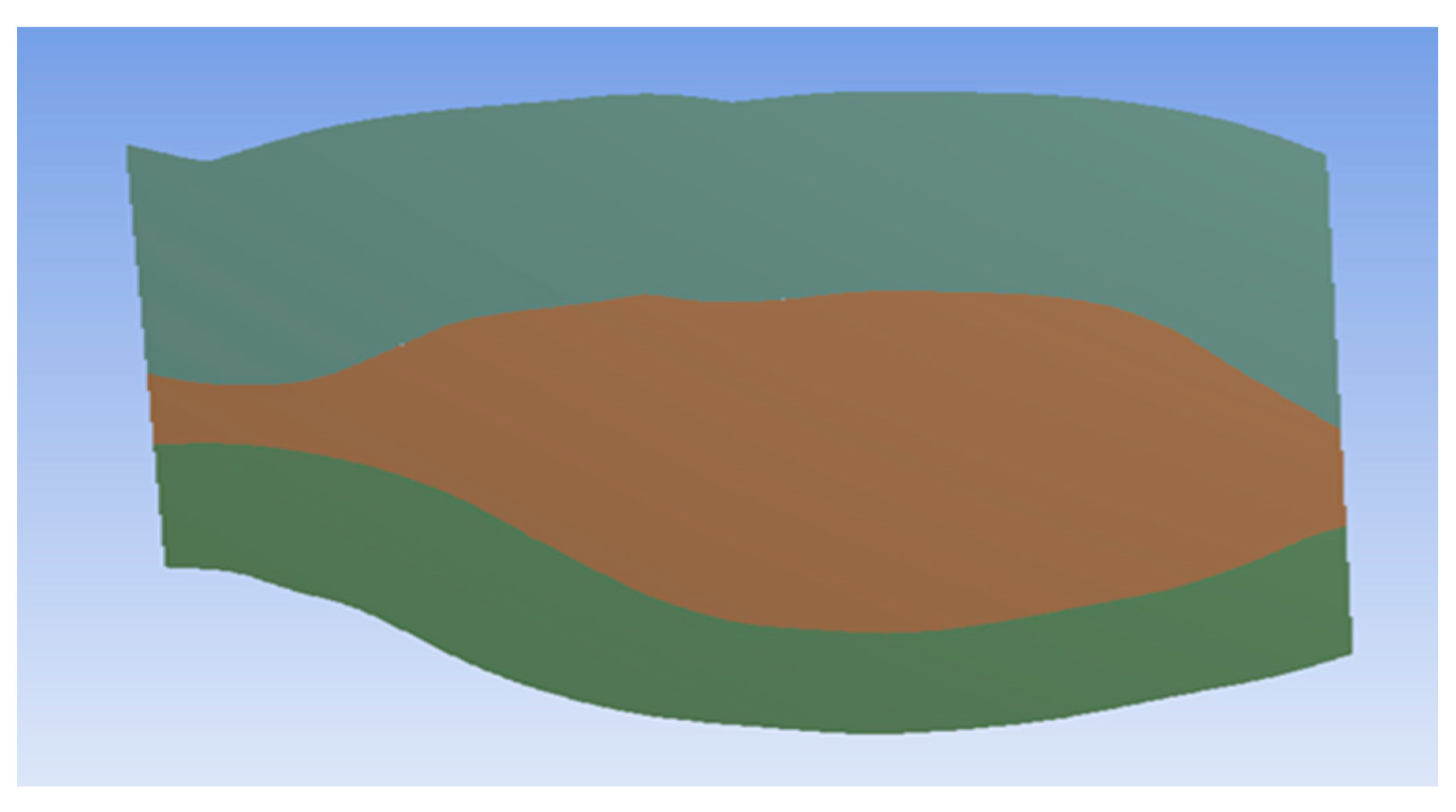
The ILT is a porous material, so this study applied this property to the ILT geometry to mimic reality. The 3D geometry was thus converted into a two-dimensional (2D) geometry to understand how to properly apply the porous property in ANSYS software. In Figure 4, brown represents the lumen where blood and oxygen flow, and green represents the ILT. The top and bottom surfaces represent the aneurysm wall. The perpendicular left surface represents the inlet, and the perpendicular right surface represent the outlet.
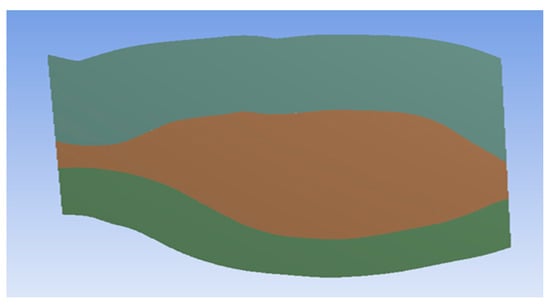
Figure 4.
AAA geometry after conversion from 3D to 2D.
Porosity was established by representing ILT permeability as the inverse of the viscous resistance in ANSYS software in the X, Y, and Z directions. The value of permeability (3.7 × 10−7 m2) was used in a study by Adolph et al. [28], so viscous resistance is (2,700,000 m−2), and we used the same value. Similarly, after applying porosity to the 2D geometry, the porous property of the ILT was established on the 3D geometry using the same approach as that used on the 2D geometry.
2.5. Blood and Oxygen Flow
Flow was simulated as turbulent Eulerian multiphase flow to display the volume of each phase separately or in combination. According to a study by Collins et al., oxygen was the second phase with a volume fraction of 20%, and the primary phase was blood [29].
Moreover, oxygen properties (density, 1.2999 kg/m3; viscosity, 1.919 × 10−5 kg/m−3) were found in ANSYS fluent database, and blood properties were manually inserted (density, 1080 kg/m3; viscosity, 0.0035 kg/m−3) [30].
In addition, multiphase flow over the single-phase flow was selected for the following reasons:
- to give a specific volume fraction for each material, as oxygen volume is lower than blood volume;
- to separately or in combination analyze the effect of both materials.
2.6. Boundary Conditions
The inlet was configured as a velocity inlet with a constant value of 0.3 m/s, and the outlet as a pressure outlet with a value of 13,335 Pa. No-slip boundary criteria were utilized to keep the walls immobile, as described in [31].
2.7. Initialization and Calculation
Without initialization, results in ANSYS software cannot be effectively obtained, so flow variables need initial values. Because we simulated porous media on the ILT, hybrid initialization was not utilized because it cannot manage the porous formulation that produces inaccurate results, so normal initialization was selected instead. Results were calculated in 50 max iterations, 50 time steps, time step sizes = 0.001, and the total calculation time for a single analysis was 5 h.
3. Results
Results were measured using a four central processing unit computer, and the time for an analysis was up to 5 h.
3.1. Porosity
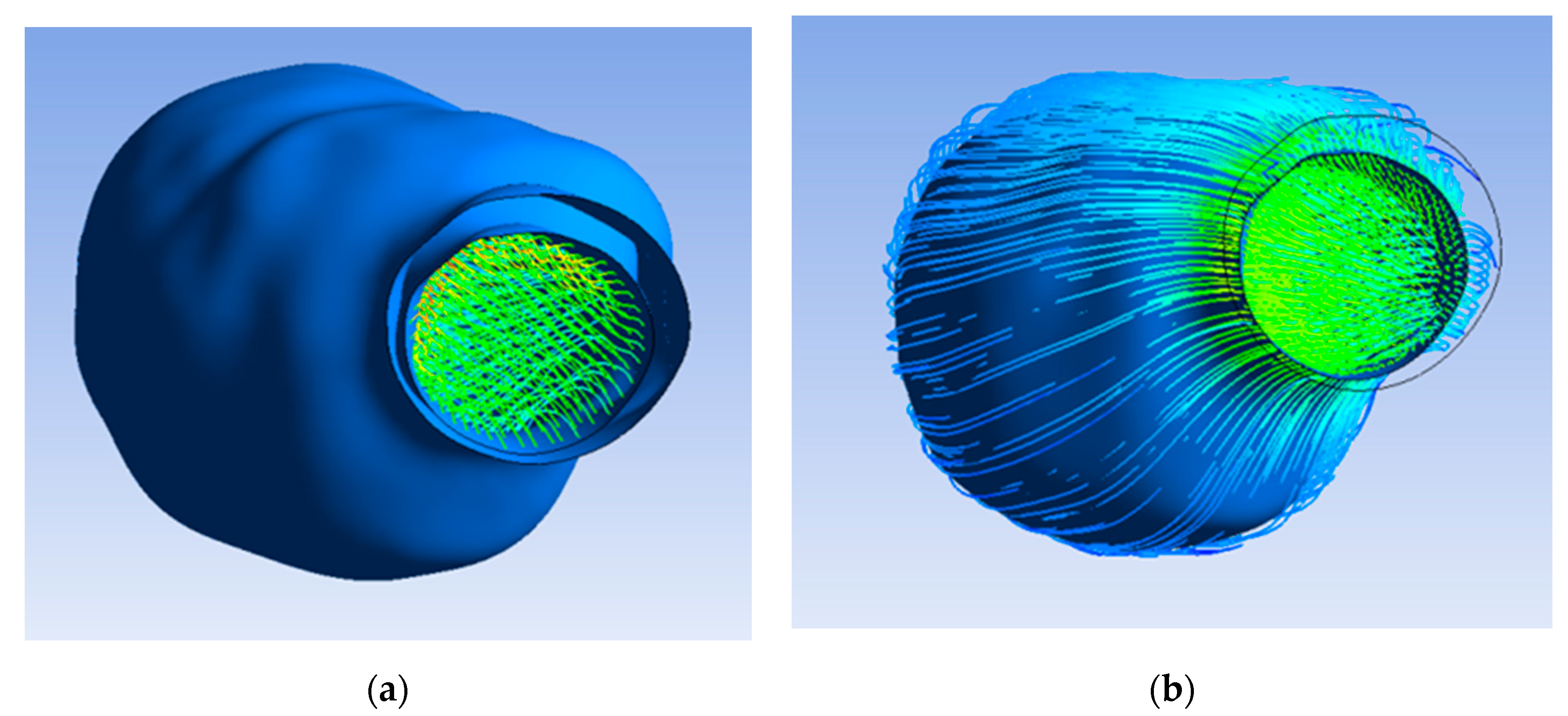
First, the flow pattern inside the lumen and ILT before applying porosity was found. Therefore, the flow of blood and oxygen did not penetrate the ILT surface and the aneurysm wall, as the porous property was not yet applied, and the wall was impermeable, as shown in Figure 5a.
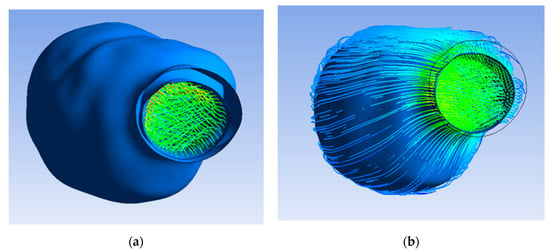
Figure 5.
(a) Inner ILT surface and aneurysm wall; (b) flow pattern inside lumen.
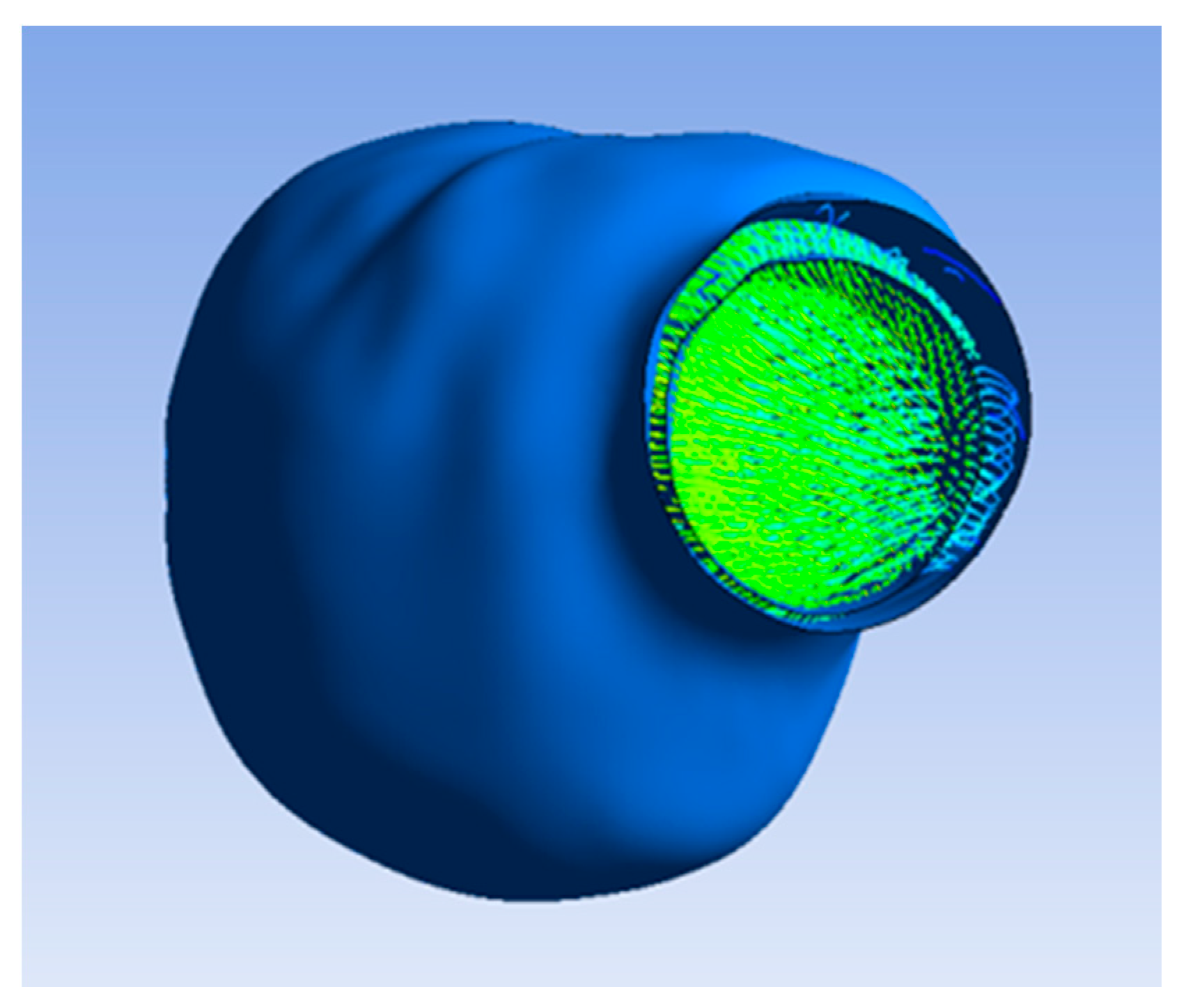
The flow pattern inside the lumen and ILT after applying porosity with a permeability value of 3.7 × 10−7 m2 is illustrated in Figure 5b, which indicates the flow of blood and oxygen penetrating the ILT surface due to the porous property applied on the ILT. Figure 6 shows that the flow did not penetrate the aneurysm wall, as it was impermeable.
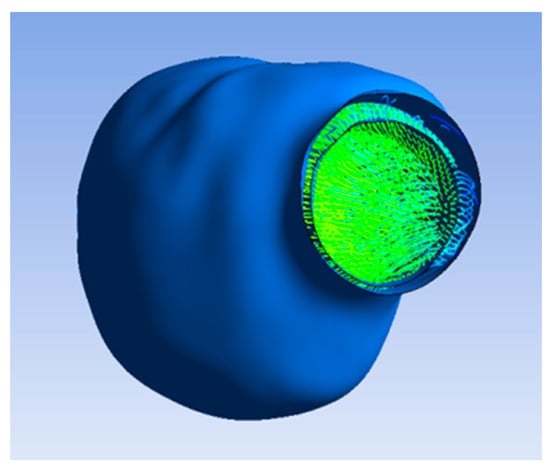
Figure 6.
Flow not penetrating aneurysm wall.
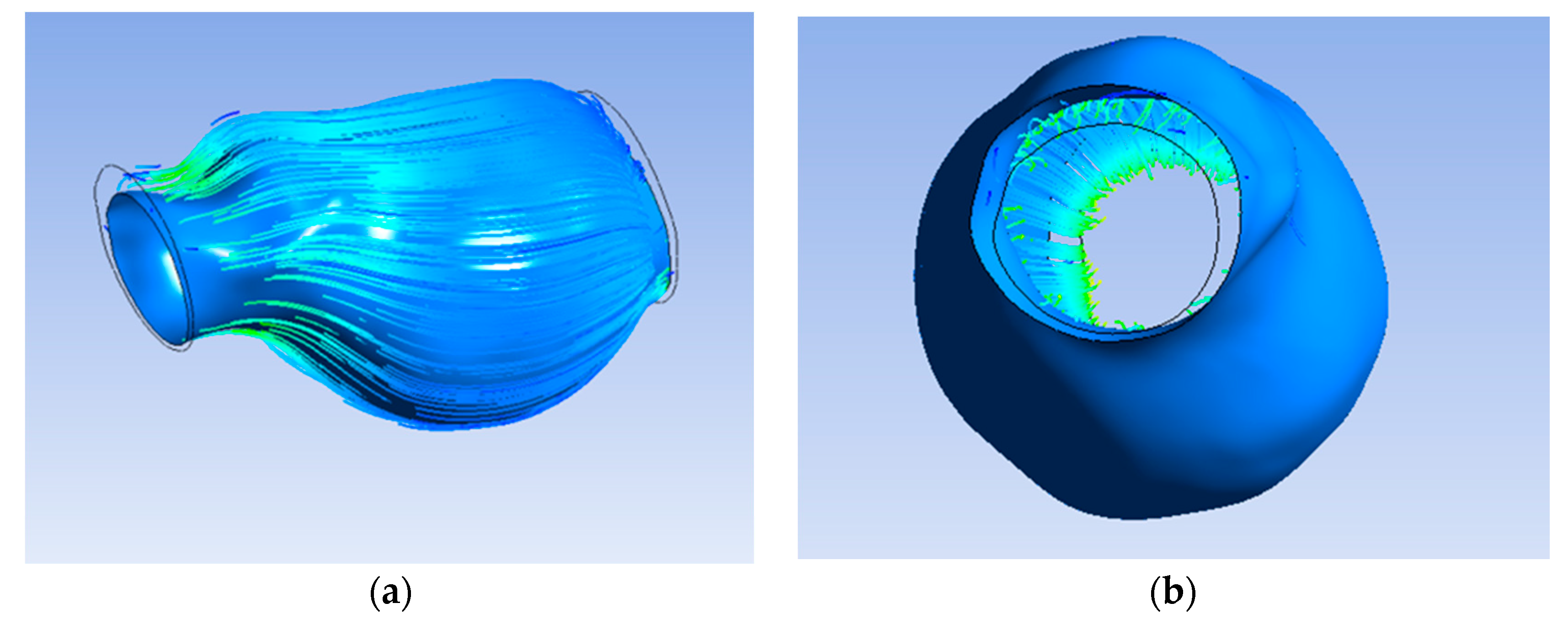
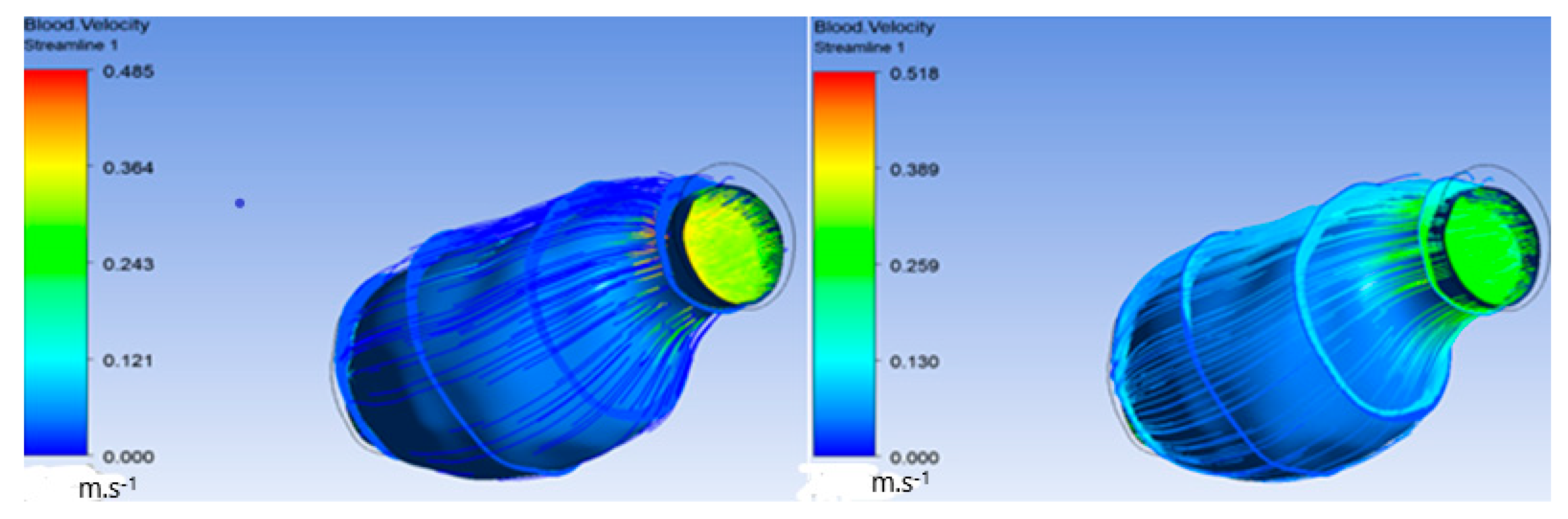
Furthermore, Figure 7a exhibit the flow of blood and oxygen inside the ILT only without flow inside the lumen. Figure 7b shows the inlet on the left and the outlet on the right.
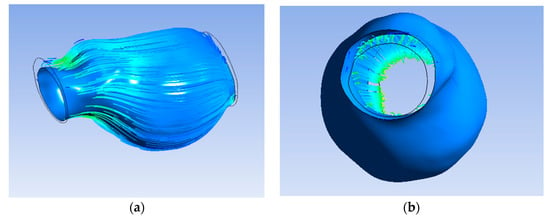
Figure 7.
(a) Streamline penetrating ILT surface; (b) streamline only inside ILT.
3.2. Velocity
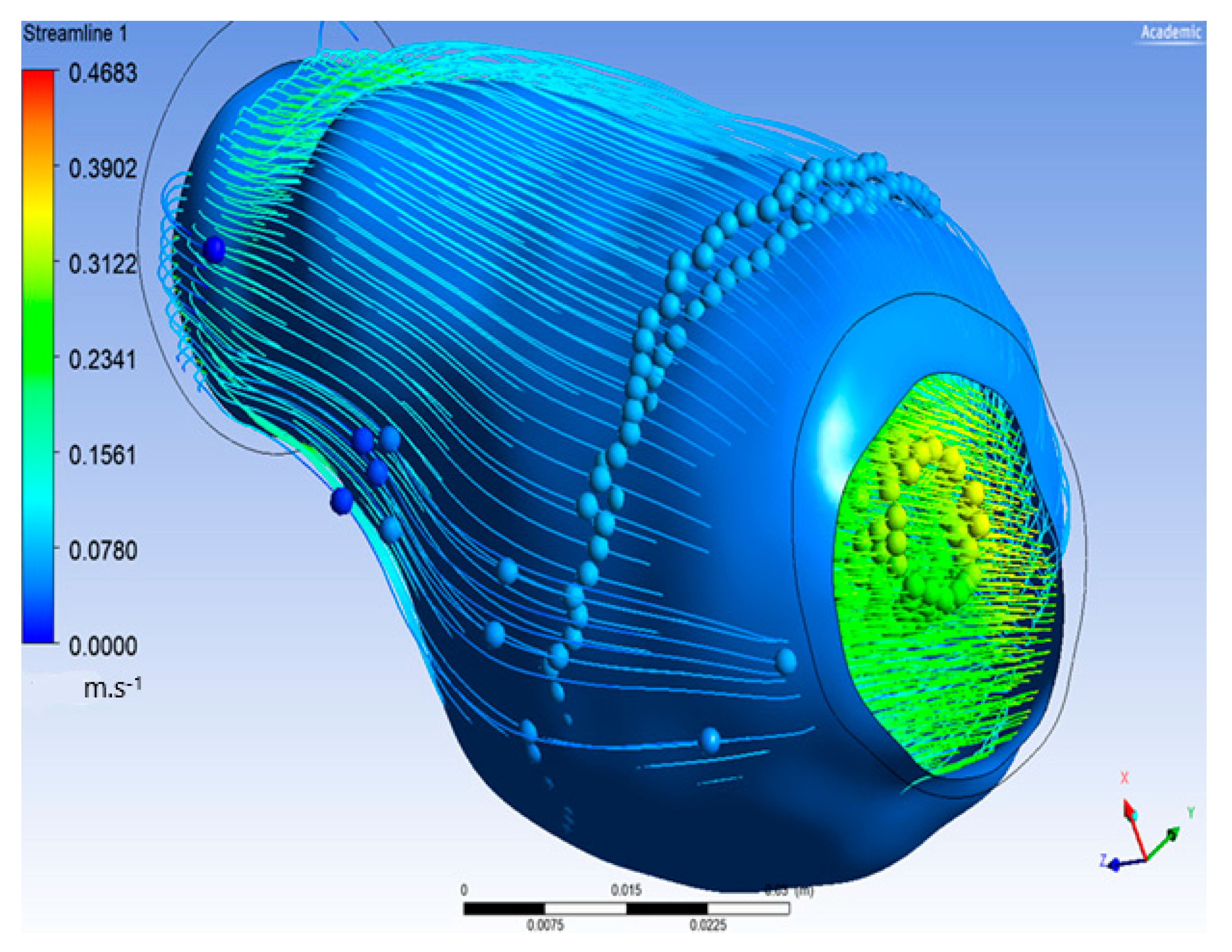
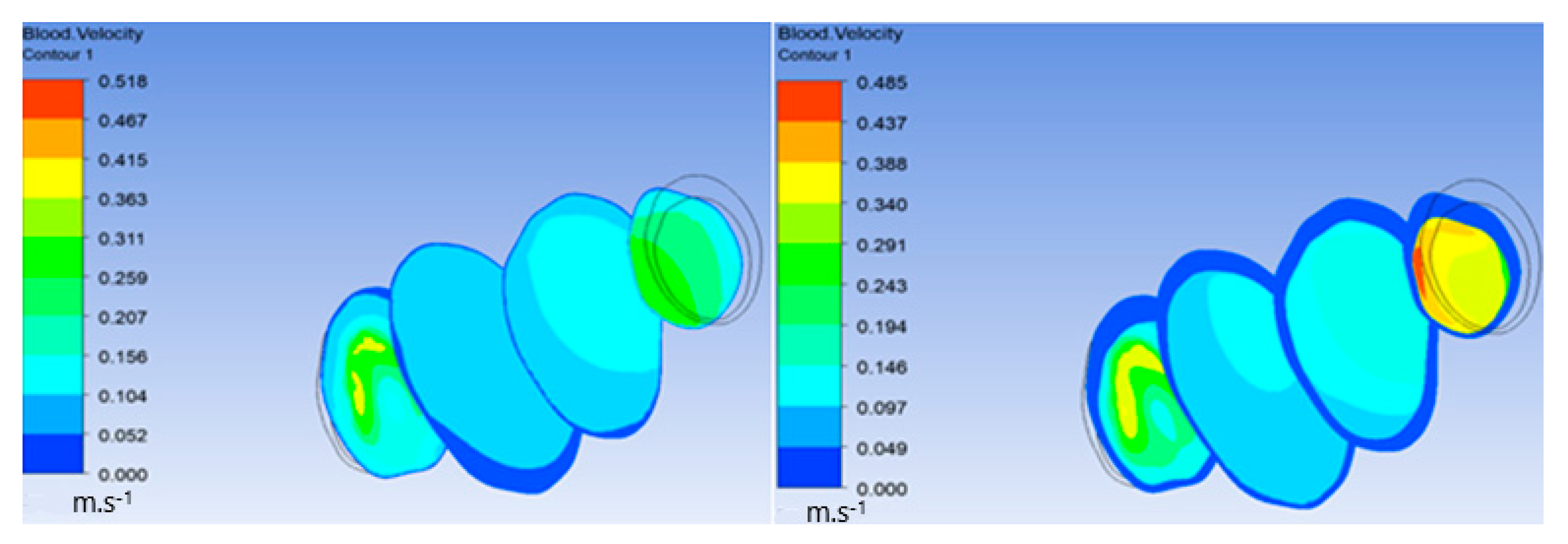
The flow of oxygen and blood particles inside the lumen and ILT is shown in Figure 8. Particles flowed from the inlet on the left to the outlet on the right with maximal velocity reaching 0.483 m/s and minimal value of 0, and it appeared inside the ILT.
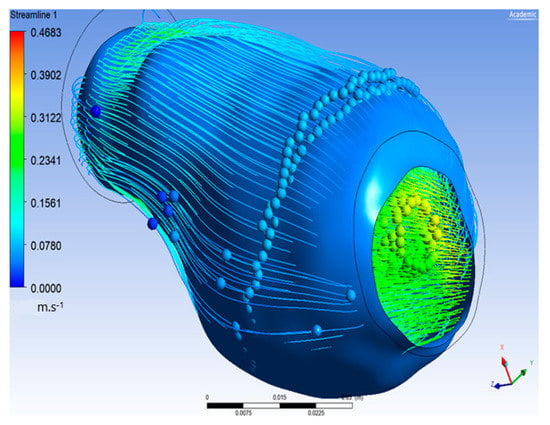
Figure 8.
Particle flow inside aneurysm.
Additionally, in the following figures, velocity inside the ILT before and after increasing the viscous resistance was compared. Figure 9 shows a significant decrease in velocity in the ILT region due to high viscous resistance. Figure 10 demonstrates the streamlines penetrating the ILT surface, which indicates that the ILT was permeable. There was also a significant decrease in velocity streamlines flowing inside the ILT after increasing the viscous resistance of the ILT. The previous comparison proves that the porosity of the ILT functioned and highly affected flow velocity inside the ILT when using high viscous resistance.
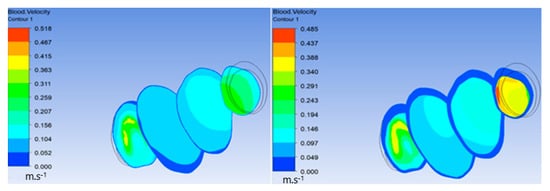
Figure 9.
Decrease in velocity in the ILT region.
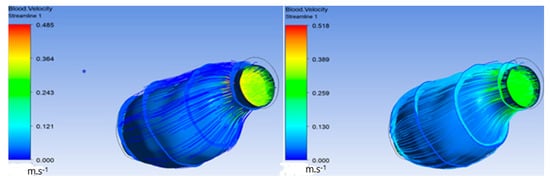
Figure 10.
Streamlines penetrating ILT surface.
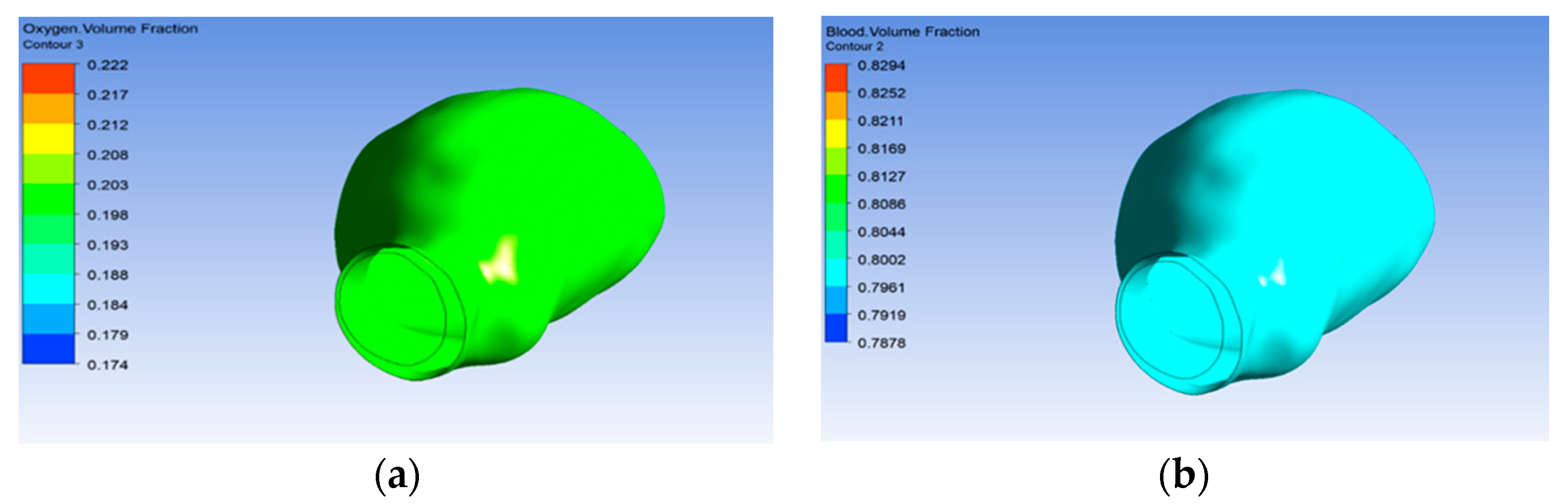
3.3. Oxygen Volume Reaching the Aneurysm Wall
The initial oxygen volume fraction used in this research was 20% of the total flow, and the majority of the flow was blood, which was about 80% of the flow. Figure 11a shows the volume of oxygen reaching the aneurysm wall. Therefore, the oxygen volume reached the aneurysm wall due to the porous property, as shown in Figure 11b.
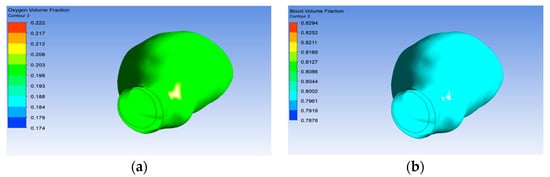
Figure 11.
Volume of (a) oxygen and (b) blood.
3.4. Wall Shear Stress (WSS) and Pressure
Finding the WSS and pressure was part of studying the effect of a porous ILT on the aneurysm wall. The maximal WSS was 7.54 Pascal, and the minimal WSS was 0.0176 Pascal. The maximal pressure value was approximately 13,548.593 Pascal, and the minimal value was 13,335 Pascal.
4. Discussion
The presence of ILT complicates the already difficult disease of abdominal aortic aneurysm. Many researchers used numerical analysis of AAAs to investigate the wall shear stress (WSS) distributions and flow patterns within the aneurysm sac [32,33] to investigate blood flow in AAAs [34,35,36]. Wall stress is linked to AAA shape and its components. The shape and maximal diameter of the aneurysm, the thickness of the aneurysm walls, and the thickness and distribution of the ILT inside the aneurysm are all parameters that influence simulation results. The role of abnormal low-density lipoprotein (LDL) build-up inside the arterial wall and hypoxia was well-documented in the development and progression of atherosclerosis [37]. By releasing many types of harmful enzymes, inflammation triggers structural disintegration [38,39]. Pathogenesis is a major topic of debate. AAAs start with inflammation and weakening of the wall (e.g., the first loss of elastin), leading to atherosclerotic involvement and intraluminal thrombus, according to some studies.
ILT’s effect on the risk of AAA rupture is controversial. An ILT limited oxygen transport to the AAA wall, perhaps resulting in local hypoxia and wall weakening [16]. The aorta wall’s mechanical failure to resist the sign, which occurs when the tangential stress within the wall exceeds the vessel wall’s sturdiness at any point, causes aneurysm rupture. Wall stress is affected by pressure level, vessel diameter, and wall thickness [40]. The two most important predictors of AAA rupture are wall stress and strength. The AAA ruptures when local stress surpasses local wall strength, and bursts only when local stress exceeds local wall strength. Figure 5 and Figure 6 show the flow pattern before and after applying porosity to the ILT to provide a better understanding of the overall geometry, ILT surface, and aneurysm wall. In realistic AAA geometries, the CFD study also reveals unknown information on the link between blood flow hemodynamics and rupture site.
There is a strong suggestion in the literature that an ILT decreases the tension acting on the aneurysm wall before to rupture, stress known as Von Mises stress. This study could only compute the WSS because it used computational fluid dynamics rather than the finite-element method, and it presumed that the ILT also reduced the WSS. WSS results were compared with those in a previous study by Boyd et al. [41,42,43,44]. The geometry’s maximal diameter was roughly 6.3 cm, indicating that it was highly prone to rupture, and they used a ruptured abdominal aortic aneurysm model without ILT in their investigation. The aneurysm wall boundary conditions in their study were the same as those in this study. Lower WSS values than their values were expected, since their flow was directly touching the aneurysm wall. Their maximal WSS value was 5 Pascal, and it occurred at the aneurysm wall’s inlet, outlets, and central region. The aneurysm wall’s center in this study had substantially less shear stress than the aneurysm walls did. Similarly, WSS results were compared to those in [31], where a geometry with an intact abdominal aorta was employed. The boundary conditions of their aneurysm wall and exit were identical to those in this study. Their maximal WSS value (8.91 Pascal) was higher than the maximal WSS value obtained in this study (7.54 Pascal). Moreover, to further study the effect of the porous ILT, results were compared with those of previous studies. In terms of pressure, these results and those in [23] showed similar findings, with their maximal pressure measured being approximately 110 mmHg, which was slightly different to our maximal pressure value, which was 13,548.593 Pascal (13,548.593 Pascal = 101.62279 mmHg).
In addition, a realistic material model is crucial in the modeling of AAA wall stress, and it is the cornerstone of the computer simulation of AAA stability. Low WSS is unlikely to directly cause aortic rupture, but ILT deposition may continue in locations where velocity and WSS are low. Alterations in the local environment, such as inflammatory [28,45] or ischemic changes [16,46], may result in adventitial integrity loss as a result of ILT deposition. The aortic rupture happened in low-flow areas and, in the vast majority of cases, in recirculation zones with low WSS and ILT deposition. In this investigation, a significant decline in velocity was found when comparing particles penetrating and flowing inside the ILT to particles flowing inside the lumen. The change in velocity was caused by the porous characteristic of the ILT and the change in aneurysm diameter. The ILT’s viscous resistance was increased to examine if the porous property was effective and affecting the flow.
Despite the encouraging results obtained from the present computational model created in this study, the proposed models are unable to adequately mimic in vivo conditions, and hence do not account for several very complicated local issues that occur over the course of thrombus development. This also assumes tissue homogeneity, which is not usually the case. Furthermore, intraluminal thrombi locally form along the wall, whereas the model assumed flow around the thrombus. Furthermore, the aneurysm wall is separated from a luminal oxygen supply; the actuality of this trait is thrombus metaplasia that may occur over time.
These may be caused by persistently hypoxic tissue. Another factor that may contribute to artery weakening is variations in blood flow within artery walls.
Another factor to consider in future research is the type of dynamic flow around the thrombus, as laminar flow is not always a perfect simulation, and wall damage can locally occur as a result of disturbed flow, increasing local oxygen demand while decreasing oxygen supply, partly due to tissue thickening. A thrombus having high porosity may be validated early in thrombus formation; however, as thrombi become denser, the percentage of porosity decreases, and this theory may have a margin of error.
5. Conclusions
ILT causes a slight reduction in pressure and a considerable reduction in shear stress acting on the aneurysm wall. The maximal stress and WSS acting on the aneurysm wall were similarly lowered. Furthermore, because the oxygen volume almost completely reached the aneurysm wall, the findings of this study refute the notion that an ILT is the source of local hypoxia. The results of CFD simulations showed that developing a geometric model utilizing meshing and mesh independency check is a realistic technique for studying human physiology and illness in vitro at a fixed period.
While computational models are unable to adequately simulate in vivo conditions, as mentioned in the discussion, findings in this paper may be obtained with a margin of error.
Future research should focus on improving computer modeling by incorporating local and time-varying factors, such as the kind of dynamic flow surrounding the thrombus, tissue homogeneity assumption, and decreasing porosity over time.
Author Contributions
M.A. confirms sole responsibility for the study conception and design, data collection, analysis, results interpretation of, and manuscript preparation. The author has read and agreed to the published version of the manuscript.
Funding
College of Applied Medical Sciences Research Centre and the Deanship of Scientific Research at King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The author would like to thank Omar Altwijri, Mahdi Alqahtani and, Ali Saad for their valuable comments and suggestions, and Eng. Abdullah Alaskar, Eng. Abdullah Al-abdulwahed, and Eng. Ravish Khan for the data collection. The author would like to extend his appreciation to the College of Applied Medical Sciences Research Centre and the Deanship of Scientific Research at King Saud University for funding this research.
Conflicts of Interest
The author declares no competing interests.
References
- Kumar, D.; Vinoth, R.; Adhikari, R.; Shankar, V. Non-Newtonian and Newtonian blood flow in human aorta: A transient Analysis. Biomed. Res. 2017, 28, 3194–3203. [Google Scholar]
- White, H.J.; Bordes, S.; Borger, J. Anatomy, Abdomen and Pelvis, Aorta; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Yearwood, T.; Chandran, K. Physiological pulsatile flow experiments in a model of the human aortic arch. J. Biomech. 1982, 15, 683–704. [Google Scholar] [CrossRef]
- Kousera, C.A.; Wood, N.B.; Seed, W.A.; Torii, R.; O’Regan, D.; Xu, X.Y. A Numerical Study of Aortic Flow Stability and Comparison With In Vivo Flow Measurements. J. Biomech. Eng. 2013, 135, 011003 (9 pages). [Google Scholar] [CrossRef]
- Yao, L.-S.; Berger, S.A. Entry flow in a curved pipe. J. Fluid Mech. 1975, 67, 177–196. [Google Scholar] [CrossRef]
- Dean, W. Fluid motion in a curved pipe. Proc. R. Soc. Lond. Ser. A 1928, 121, 402. [Google Scholar]
- Dwyer, H.A.; Cheer, A.Y.; Rutaganira, T.; Shacheraghi, N. Calculation of Unsteady Flows in Curved Pipes. J. Fluids Eng. 2001, 123, 869–877. [Google Scholar] [CrossRef]
- Chandran, K.; Yearwood, T.; Wieting, D. An experimental study of pulsatile flow in a curved tube. J. Biomech. 1979, 12, 793–805. [Google Scholar] [CrossRef]
- Chandran, K.; Yearwood, T. Experimental study of physiological pulsatile flow in a curved tube. J. Fluid Mech. 1981, 111, 59–85. [Google Scholar] [CrossRef]
- Morris, P.D.; Narracott, A.; von Tengg-Kobligk, H.; Soto, D.A.S.; Hsiao, S.; Lungu, A.; Evans, P.; Bressloff, N.W.; Lawford, P.V.; Hose, D.R.; et al. Computational fluid dynamics modelling in cardiovascular medicine. Heart 2016, 102, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; U-King-Im, J.; Tang, T.Y.; Soh, E.; See, T.C.; Gillard, J.H. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J. Vasc. Surg. 2008, 47, 928–935. [Google Scholar] [CrossRef]
- Lederle, F.A.; Wilson, S.E.; Johnson, G.R.; Littooy, F.N.; Acher, C.; Messina, L.M.; Reinke, D.B.; Ballard, D.J. Design of the abdominal aortic Aneurysm Detection and Management Study. J. Vasc. Surg. 1994, 20, 296–303. [Google Scholar] [CrossRef]
- Brown, P.M.; Pattenden, R.; Vernooy, C.; Zelt, D.T.; Gutelius, J.R. Selective management of abdominal aortic aneurysms in a prospective measurement program. J. Vasc. Surg. 1996, 23, 213–222. [Google Scholar] [CrossRef]
- Wang, D.H.; Makaroun, M.S.; Webster, M.W.; Vorp, D. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J. Vasc. Surg. 2002, 36, 598–604. [Google Scholar] [CrossRef]
- Vorp, D.A.; Geest, J.P.V. Biomechanical Determinants of Abdominal Aortic Aneurysm Rupture. Arter. Thromb. Vasc. Biol. 2005, 25, 1558–1566. [Google Scholar] [CrossRef]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef]
- Harter, L.P.; Gross, B.H.; Callen, P.W.; Barth, R.A. Ultrasonic evaluation of abdominal aortic thrombus. J. Ultrasound Med. 1982, 1, 315–318. [Google Scholar] [CrossRef]
- Wolf, Y.G.; Thomas, W.S.; Brennan, F.J.; Goff, W.G.; Sise, M.J.; Bernstein, E.F. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J. Vasc. Surg. 1994, 20, 529–538. [Google Scholar] [CrossRef][Green Version]
- Inzoli, F.; Boschetti, F.; Zappa, M.; Longo, T.; Fumero, R. Biomechanical factors in abdominal aortic aneurysm rupture. Eur. J. Vasc. Surg. 1993, 7, 667–674. [Google Scholar] [CrossRef]
- Mower, W.R.; Quiñones, W.J.; Gambhir, S.S. Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J. Vasc. Surg. 1997, 26, 602–608. [Google Scholar] [CrossRef]
- Di Martino, E.; Mantero, S.; Inzoli, F.; Melissano, G.; Astore, D.; Chiesa, R.; Fumero, R. Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: Experimental characterisation and structural static computational analysis. Eur. J. Vasc. Endovasc. Surg. 1998, 15, 290–299. [Google Scholar] [CrossRef]
- Dobrin, P.B. Pathophysiology and Pathogenesis of Aortic Aneurysms: Current Concepts. Surg. Clin. N. Am. 1989, 69, 687–703. [Google Scholar] [CrossRef]
- Schurink, G.W.H.; Van Baalen, J.M.; Visser, M.J.T.; Van Bockel, J.H. Thrombus within an aortic aneurysm does not reduce pressure on the aneurysmal wall. J. Vasc. Surg. 2000, 31, 501–506. [Google Scholar] [CrossRef]
- Satta, J.; Läärä, E.; Juvonen, T. Intraluminal thrombus predicts rupture of an abdominal aortic aneurysm. J. Vasc. Surg. 1996, 23, 737–739. [Google Scholar] [CrossRef]
- Laustsen, J.; Paaske, W.P.; Oyre, S.; Pedersen, E.M. Dynamic quantification, visualisation and animation of blood velocities and flows in infrarenal aortic aneurysms in vivo by three-dimensional MR phase velocity encoding. Eur. J. Vasc. Endovasc. Surg. 1995, 9, 383–388. [Google Scholar] [CrossRef][Green Version]
- Faggioli, G.L.; Stella, A.; Gargiulo, M.; Tarantini, S.; D’Addato, M.; Ricotta, J.J. Morphology of small aneurysms: Definition and impact on risk of rupture. Am. J. Surg. 1994, 168, 131–135. [Google Scholar] [CrossRef]
- Vorp, D.A.; Wang, D.H.J.; Raghavan, M.L.; Webster, M.W. Effect of shape of intraluminal thrombus on wall stress in abdominal aortic aneurysm. Biomedical Engineering Society. Ann. Biomed. Eng. 1998, 26, 68. [Google Scholar]
- Adolph, R.; Vorp, D.; Steed, D.L.; Webster, M.W.; Kameneva, M.V.; Watkins, S. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J. Vasc. Surg. 1997, 25, 916–926. [Google Scholar] [CrossRef]
- Polzer, S.; Gasser, T.C.; Markert, B.; Bursa, J.; Skacel, P. Impact of poroelasticity of intraluminal thrombus on wall stress of abdominal aortic aneurysms. Biomed. Eng. Online 2012, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.-A.; Rudenski, A.; Gibson, J.; Howard, L.; O’Driscoll, R. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Dwidmuthe, P.; Mathpati, C.; Joshi, J. CFD Simulation of Blood Flow Inside the Human Artery: Aorta. In Proceedings of the 7th International and 45th National Conference on Fluid Mechanics and Fluid Power (FMFP), Mumbai, India, 10–12 December 2018. [Google Scholar]
- Perktold, K. On the paths of fluid particles in an axisymmetrical aneurysm. J. Biomech. 1987, 20, 311–317. [Google Scholar] [CrossRef]
- Finol, E.A.; Keyhani, K.; Amon, C.H. The Effect of Asymmetry in Abdominal Aortic Aneurysms Under Physiologically Realistic Pulsatile Flow Conditions. J. Biomech. Eng. 2003, 125, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.C.; Auer, M.; Labruto, F.; Swedenborg, J.; Roy, J. Biomechanical rupture risk assessment of abdominal aortic aneurysms: Model complexity versus predictability of finite element simulations. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 176–185. [Google Scholar] [CrossRef]
- Bonert, M.; Leask, R.L.; Butany, J.; Ethier, C.R.; Myers, J.G.; Johnston, K.W.; Ojha, M. The relationship between wall shear stress distributions and intimal thickening in the human abdominal aorta. Biomed. Eng. Online 2003, 2, 1–14. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Li, Z. Analysis and computer program for rupture-risk prediction of abdominal aortic aneurysms. Biomed. Eng. Online 2006, 5, 1–13. [Google Scholar] [CrossRef]
- Okamoto, R.; Hatani, M.; Tsukitani, M.; Suehiro, A.; Fujino, M.; Imai, N.; Takano, S.; Watanabe, Y.; Fukuzaki, H. The effect of oxygen on the development of atherosclerosis in WHHL rabbits. Atherosclerosis 1983, 47, 47–53. [Google Scholar] [CrossRef]
- Crawford, T. Some Observations on the Pathogenesis and Natural History of Intracranial Aneurysms. J. Neurol. Neurosurg. Psychiatry 1959, 22, 259–266. [Google Scholar] [CrossRef]
- Crompton, M.R. Mechanism of Growth and Rupture in Cerebral Berry Aneurysms. BMJ 1966, 1, 1138–1142. [Google Scholar] [CrossRef]
- Couch, N.P. Hemodynamics for Surgeons. Arch. Surg. 1976, 111, 929–930. [Google Scholar] [CrossRef]
- Boyd, A.; Kuhn, D.C.; Lozowy, R.J.; Kulbisky, G.P. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J. Vasc. Surg. 2016, 63, 1613–1619. [Google Scholar] [CrossRef]
- Zhu, C.; Leach, J.R.; Wang, Y.; Gasper, W.; Saloner, D.; Hope, M.D. Intraluminal Thrombus Predicts Rapid Growth of Abdominal Aortic Aneurysms. Vasc. Interv. Radiol. 2020, 294, 707–713. [Google Scholar] [CrossRef]
- Boyd, A.J. Intraluminal thrombus: Innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc. Sci. 2021, 2, 159. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.; Zhou, M.; Cai, L.; Tang, H.; Xie, T.; Shi, Z.; Fu, W. Factor Xa inhibitor rivaroxaban suppresses experimental abdominal aortic aneurysm progression via attenuating aortic inflammation. Vasc. Pharmacol. 2021, 136, 106818. [Google Scholar] [CrossRef]
- Kazi, M.; Thyberg, J.; Religa, P.; Roy, J.; Eriksson, P.; Hedin, U.; Swedenborg, J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J. Vasc. Surg. 2003, 38, 1283–1292. [Google Scholar] [CrossRef]
- Vorp, D.A.; Wang, D.H.J.; Webster, M.W.; Federspiel, W.J. Effect of Intraluminal Thrombus Thickness and Bulge Diameter on the Oxygen Diffusion in Abdominal Aortic Aneurysm. J. Biomech. Eng. 1998, 120, 579–583. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).