Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mueller Matrix Polar Decomposition (MMPD)
2.2. Mueller Matrix Transformation (MMT) Parameters
2.3. Materials and Measurement Setup
2.4. Quantitative Indicators for MMT and MMPD Parameters Comparison
3. Results and Discussion
3.1. Quantitative Comparison of Diattenuation Related MMPD and MMT Parameters for Transmission Imaging of Thin Tissue Samples
3.2. Quantitative Comparison of Diattenuation Related MMPD and MMT Parameters for Backscattering Imaging of Bulk Tissue Samples
3.3. Quantitative Comparison of Linear Retardance Related MMPD and MMT Parameters for Transmission Imaging of Thin Tissue Samples
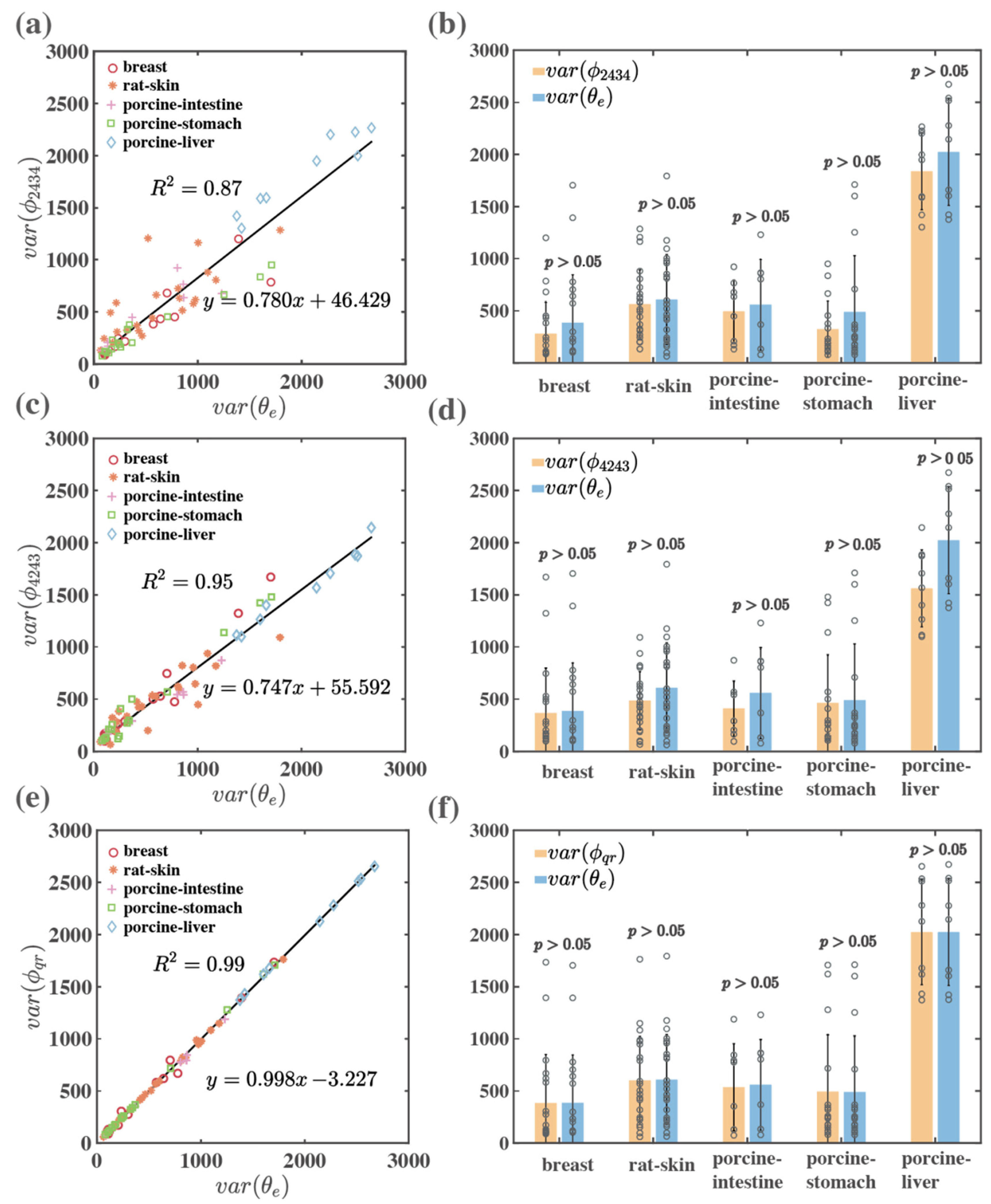
3.4. Quantitative Comparison of Linear Retardance Related MMPD and MMT Parameters for Backscattering Imaging of Bulk Tissue Samples
3.5. Quantitative Comparison of Linear Birefringence Orientation Related MMPD and MMT Parameters for Transmission Imaging of Thin Tissue Samples
3.6. Quantitative Comparison of Linear Birefringence Orientation Related MMPD and MMT Parameters for Backscattering Imaging of Bulk Tissue Samples
3.7. Quantitative Comparison of Depolarization MMPD and MMT Parameters for Transmission Imaging of Thin Tissue Samples
3.8. Quantitative Comparison of Linear Birefringence Orientation Related MMPD and MMT Parameters for Backscattering Imaging of Bulk Tissue Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, N.; Vitkin, A.I. Tissue polarimetry: Concepts, challenges, applications, and outlook. J. Biomed. Opt. 2011, 16, 110801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; He, H.; Chang, J.; Chen, B.; Ma, H.; Booth, M.J. Polarisation optics for biomedical and clinical applications: A review. Light Sci. Appl. 2021, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Ramella-Roman, J.; Saytashev, I.; Piccini, M. A review of polarization-based imaging technologies for clinical and pre-clinical applications. J. Opt. 2020, 22, 123001. [Google Scholar] [CrossRef]
- Tuchin, V.V. Polarized light interaction with tissues. J. Biomed. Opt. 2016, 21, 071114. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Liao, R.; Zeng, N.; Li, P.; Chen, Z.; Liu, X.; Ma, H. Mueller matrix polarimetry—An emerging new tool for characterizing the microstructural feature of complex biological specimen. J. Lightwave Technol. 2019, 37, 2534–2548. [Google Scholar] [CrossRef]
- Qi, J.; Elson, D.S. Mueller polarimetric imaging for surgical and diagnostic applications: A review. J. Biophotonics 2017, 10, 950–982. [Google Scholar] [CrossRef] [Green Version]
- Alali, S.; Vitkin, I.A. Polarized light imaging in biomedicine: Emerging Mueller matrix methodologies for bulk tissue assessment. J. Biomed. Opt. 2015, 20, 061104. [Google Scholar] [CrossRef]
- Ossikovski, R. Analysis of depolarizing Mueller matrices through a symmetric decomposition. J. Opt. Soc. Am. A 2009, 26, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Vizet, J.; Ossikovski, R. Symmetric decomposition of experimental depolarizing Mueller matrices in the degenerate case. Appl. Opt. 2018, 57, 1159–1167. [Google Scholar] [CrossRef]
- Ortega-Quijano, N.; Arce-Diego, J.L. Depolarizing differential Mueller matrices. Opt. Lett. 2011, 36, 2429–2431. [Google Scholar] [CrossRef] [Green Version]
- Ossikovski, R. Differential matrix formalism for depolarizing anisotropic media. Opt. Lett. 2011, 36, 2330–2332. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Chipman, R.A. Interpretation of Mueller matrices based on polar decomposition. J. Opt. Soc. Am. A. 1996, 13, 1106–1113. [Google Scholar] [CrossRef]
- Ghosh, N.; Wood, M.F.G.; Vitkin, I.A. Mueller matrix decomposition for extraction of individual polarization parameters from complex turbid media exhibiting multiple scattering, optical activity, and linear birefringence. J. Biomed. Opt. 2008, 13, 044036. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zeng, N.; Du, E.; Guo, Y.; Li, D.; Liao, R.; Ma, H. A possible quantitative Mueller matrix transformation technique for anisotropic scattering media. Photonics Lasers Med. 2013, 2, 129–137. [Google Scholar] [CrossRef]
- Dong, Y.; Qi, J.; He, H.; He, C.; Liu, S.; Wu, J.; Elson, D.S.; Ma, H. Quantitatively characterizing the microstructural features of breast ductal carcinoma tissues in different progression stages by Mueller matrix microscope. Biomed. Opt. Express 2017, 8, 3643–3655. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Liu, S.; Shen, Y.; He, H.; Ma, H. Probing variations of fibrous structures during the development of breast ductal carcinoma tissues via Mueller matrix imaging. Biomed. Opt. Express 2020, 11, 4960–4975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, H.; Chang, J.; He, C.; Liu, S.; Li, M.; Zeng, N.; Wu, J.; Ma, H. Mueller matrix microscope: A quantitative tool to facilitate detections and fibrosis scorings of liver cirrhosis and cancer tissues. J. Biomed. Opt. 2016, 21, 071112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Lu, M.; Chen, B.; Zhong, Q.; Li, J.; He, H.; Ma, H. Distinguishing structural features between Crohn’s disease and gastrointestinal luminal tuberculosis using Mueller matrix derived parameters. J. Biophotonics 2019, 12, e201900151. [Google Scholar] [CrossRef]
- Pierangelo, A.; Nazac, A.; Benali, A.; Validire, P.; Cohen, H.; Novikova, T.; Ibrahim, B.H.; Manhas, S.; Fallet, C.; Antonelli, M.; et al. Polarimetric imaging of uterine cervix: A case study. Opt. Express 2013, 21, 14120–14130. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Chang, J.; Zeng, N.; Liu, S.; Li, M.; Ma, H. Differentiating characteristic microstructural features of cancerous tissues using Mueller matrix microscope. Micron 2015, 79, 8–15. [Google Scholar] [CrossRef]
- Iqbal, M.; Ahmad, I.; Khaliq, A.; Khan, S. Comparative study of Mueller matrix transformation and polar decomposition for optical characterization of turbid media. Optik 2020, 224, 165508. [Google Scholar] [CrossRef]
- Khaliq, A.; Ashraf, S.; Gul, B.; Ahmad, I. Comparative study of 3 × 3 Mueller matrix transformation and polar decomposition. Opt. Commun. 2021, 485, 126756. [Google Scholar] [CrossRef]
- Iqbal, M.; Gul, B.; Khan, S.; Ashraf, S.; Ahmad, I. Isolating individual polarization effects from the Mueller matrix: Comparison of two non-decomposition techniques. Biomed. Opt. Express 2021, 12, 3743–3759. [Google Scholar] [CrossRef]
- Sheng, W.; Li, W.; Qi, J.; Liu, T.; Dong, Y.; Liu, S.; Wu, J.; Ma, H.; He, H.; Elson, D.S. Quantitative analysis of 4 × 4 Mueller matrix transformation parameters for biomedical imaging. Photonics 2019, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Liu, T.; He, H.; Wu, J.; Ma, H. Distinguishing anisotropy orientations originated from scattering and birefringence of turbid media using Mueller matrix derived parameters. Opt. Lett. 2018, 43, 4092–4095. [Google Scholar] [CrossRef]
- Chen, B.; Li, W.; He, H.; He, C.; Guo, J.; Shen, Y.; Liu, S.; Sun, T.; Wu, J.; Ma, H. Analysis and calibration of linear birefringence orientation parameters derived from Mueller matrix for multi-layered tissues. Opt. Lasers Eng. 2021, 146, 106690. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, R.; He, H.; Liu, S.; Dong, Y.; Wu, J.; Ma, H. Comparative study of the influence of imaging resolution on linear retardance parameters derived from the Mueller matrix. Biomed. Opt. Express 2021, 12, 211–225. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chang, J.; Hu, Q.; Wang, J.; Antonello, J.; He, H.; Liu, S.; Lin, J.; Dai, B.; Elson, D.S.; et al. Complex vectorial optics through gradient index lens cascades. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Chang, J.; He, C.; Ma, H. Transformation of full 4 × 4 Mueller matrices: A quantitative technique for biomedical diagnosis. Proc. SPIE 2016, 9707, 97070K. [Google Scholar]
- Li, P.; Lv, D.; He, H.; Ma, H. Separating azimuthal orientation dependence in polarization measurements of anisotropic media. Opt. Express 2018, 26, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Meng, R.; Qi, J.; Liu, Y.; Wang, X.; Chen, Y.; Liao, R.; Ma, H. Fast Mueller matrix microscope based on dual DoFP polarimeters. Opt. Lett. 2021, 46, 1676–1679. [Google Scholar] [CrossRef]
- Zhou, J.; He, H.; Chen, Z.; Wang, Y.; Ma, H. Modulus design multiwavelength polarization microscope for transmission Mueller matrix imaging. J. Biomed. Opt. 2018, 23, 016007. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; He, H.; Wang, Y.; Huang, Y.; Li, X.; He, C.; Liao, R.; Zeng, N.; Liu, S.; Ma, H. Division of focal plane polarimeter-based 3 × 4 Mueller matrix microscope: A potential tool for quick diagnosis of human carcinoma tissues. J. Biomed. Opt. 2016, 21, 056002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Zeng, N.; Du, E.; Guo, Y.; Li, D.; Liao, R.; He, Y.; Ma, H. Two-dimensional and surface backscattering Mueller matrices of anisotropic sphere-cylinder scattering media: A quantitative study of influence from fibrous scatterers. J. Biomed. Opt. 2013, 18, 046002. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, R.; Zhu, Y.; Ma, H. A collinear reflection Mueller matrix microscope for backscattering Mueller matrix imaging. Opt. Lasers Eng. 2020, 129, 106055. [Google Scholar] [CrossRef]
- Azzam, R.M.A. Photopolarimetric measurement of the Mueller matrix by Fourier analysis of a single detected signal. Opt. Lett. 1978, 2, 148–150. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; Garcia-Caurel, E.; Laude, B.; Drévillon, B. General methods for optimized design and calibration of Mueller polarimeters. Thin Solid Films 2004, 455, 112–119. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, X.; Li, M. Error analysis and calibration improvement of the imaging section in a Mueller matrix microscope. Appl. Sci. 2020, 10, 4422. [Google Scholar] [CrossRef]
- Sedgwick, P. Pearson’s correlation coefficient. BMJ 2012, 345, e4483. [Google Scholar] [CrossRef] [Green Version]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Krzywinski, M.; Altman, N. Significance, P values and t-tests. Nat. Methods 2013, 10, 1041–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.H.; Collett, E. Polarized Light; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]


| Type | Sample Description | Experimental Setup | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Thickness (μm) | Number | Light Source | Power (W) | Geometry | |||
| Thin | colorectal | 12 | 14 | LED | 633 | 1 | Forward | 86 |
| ITB | 12 | 14 | LED | 633 | 1 | |||
| liver | 12 | 6 | LED | 633 | 1 | |||
| CD | 12 | 10 | LED | 633 | 1 | |||
| bladder | 12 | 14 | LED | 633 | 1 | |||
| breast | 12 | 14 | LED | 633 | 1 | |||
| adenoma | 12 | 14 | LED | 633 | 1 | |||
| Bulk | breast | - | 7 | LED | 633 | 1 | Backward | 63 |
| rat-skin | - | 23 | LED | 633 | 1 | |||
| porcine-intestine | - | 8 | LED | 633 | 1 | |||
| porcine-stomach | - | 16 | LED | 633 | 1 | |||
| porcine-liver | - | 9 | LED | 633 | 1 | |||
| Type | Tissues | Diattenuation | |||
|---|---|---|---|---|---|
| R2 | p | ||||
| D—t1213 | D—t121314 | D—t1213 | D—t121314 | ||
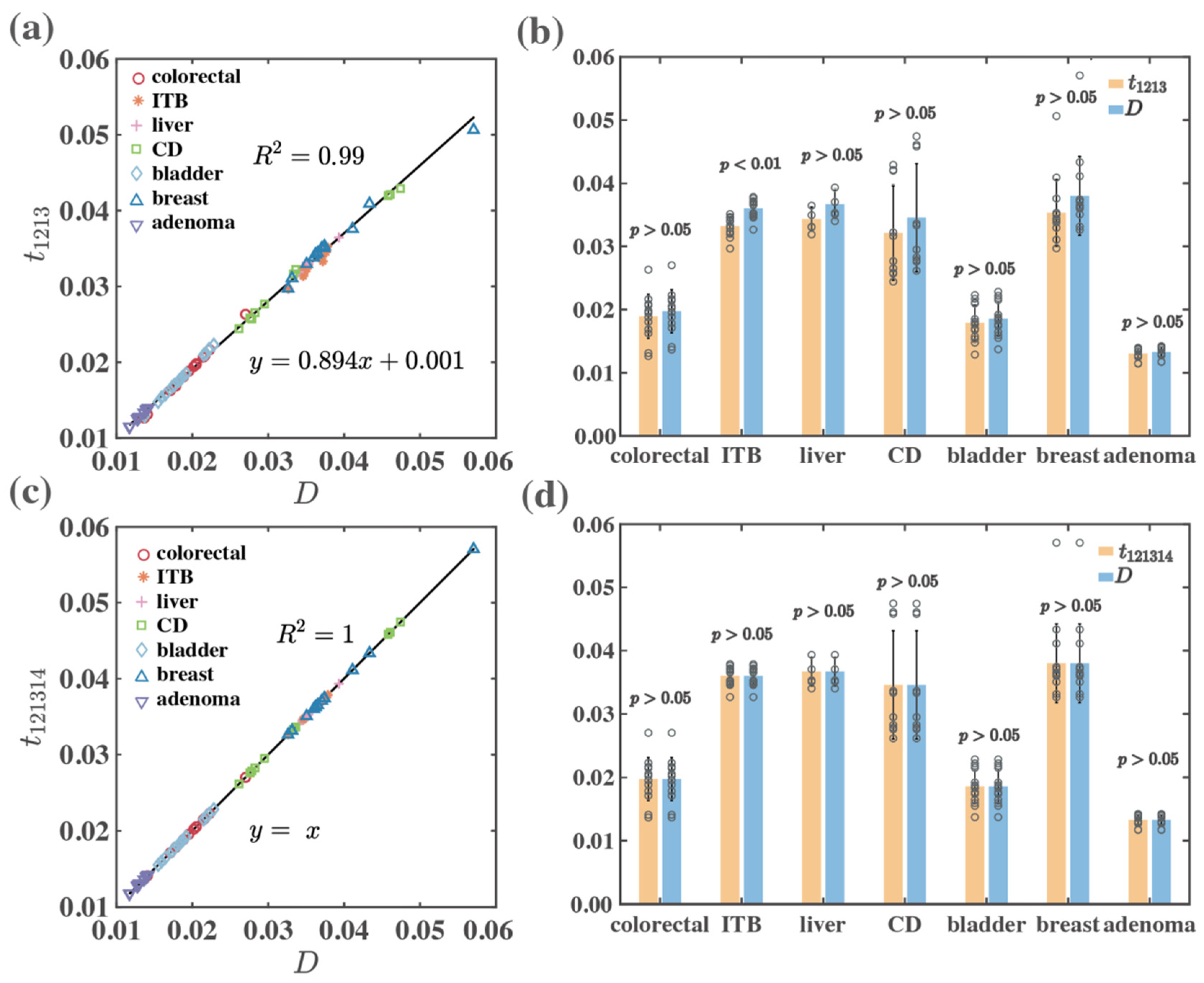
| Forward | colorectal | 0.99 | 1.00 | >0.05 | >0.05 |
| ITB | 0.90 | 1.00 | <0.01 | >0.05 | |
| liver | 0.99 | 1.00 | >0.05 | >0.05 | |
| CD | 0.99 | 1.00 | >0.05 | >0.05 | |
| bladder | 0.99 | 1.00 | >0.05 | >0.05 | |
| breast | 0.98 | 1.00 | >0.05 | >0.05 | |
| adenoma | 0.99 | 1.00 | >0.05 | >0.05 | |
| Total | 0.99 | 1.00 | >0.05 | >0.05 | |
| Type | Tissues | Diattenuation | |||
|---|---|---|---|---|---|
| R2 | p | ||||
| D—t1213 | D—t121314 | D—t1213 | D—t121314 | ||
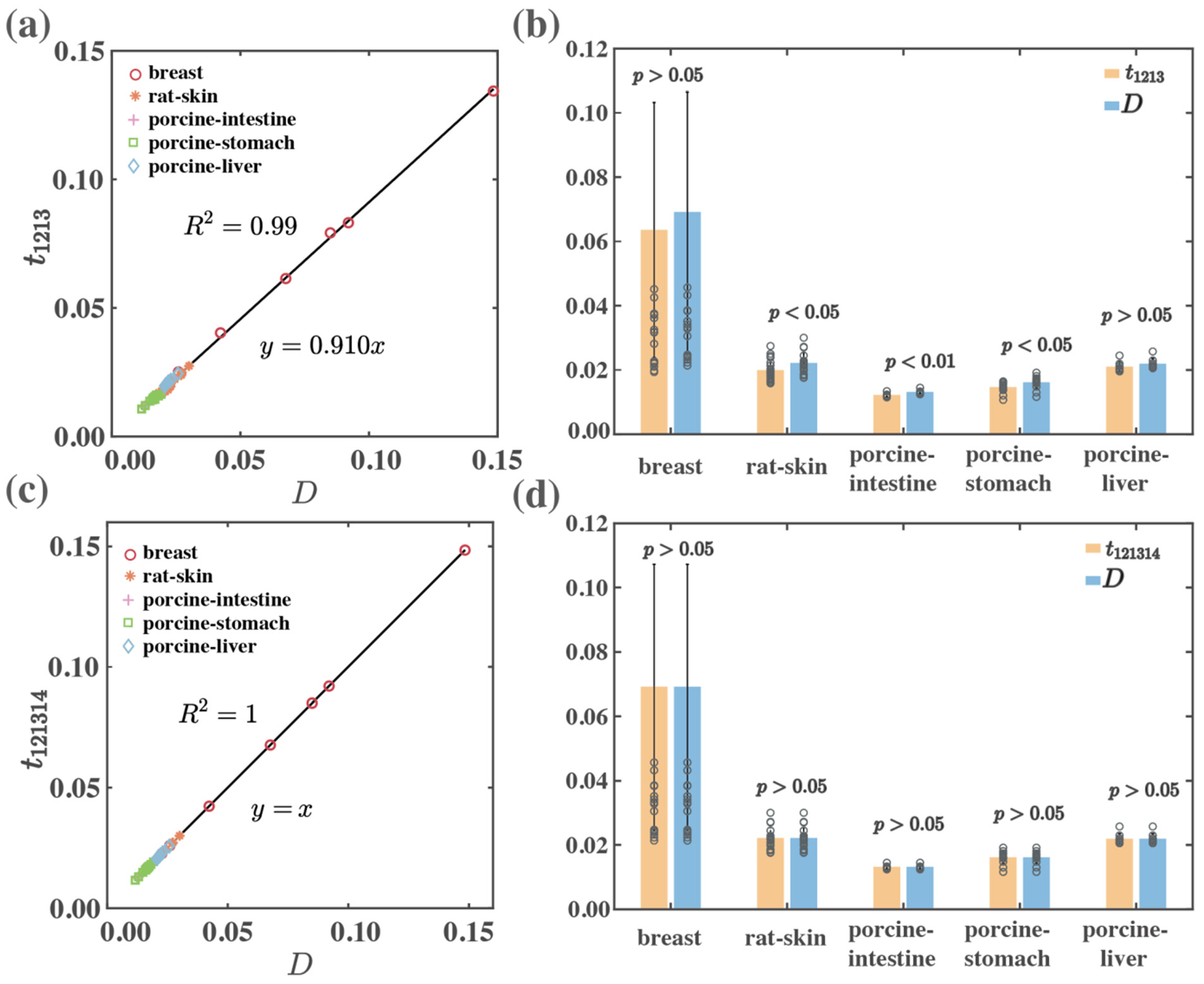
| Backward | breast | 0.99 | 1.00 | >0.05 | >0.05 |
| rat-skin | 0.94 | 1.00 | <0.05 | >0.05 | |
| porcine-intestine | 0.99 | 1.00 | <0.01 | >0.05 | |
| porcine-stomach | 0.92 | 1.00 | <0.05 | >0.05 | |
| porcine-liver | 0.99 | 1.00 | >0.05 | >0.05 | |
| Total | 0.99 | 1.00 | <0.05 | >0.05 | |
| Type | Tissues | Linear Retardance | |||||
|---|---|---|---|---|---|---|---|
| R2 | p | ||||||
| δ—t2434 | δ—t4243 | δ—tqr | δ—t2434 | δ—t4243 | δ—tqr | ||
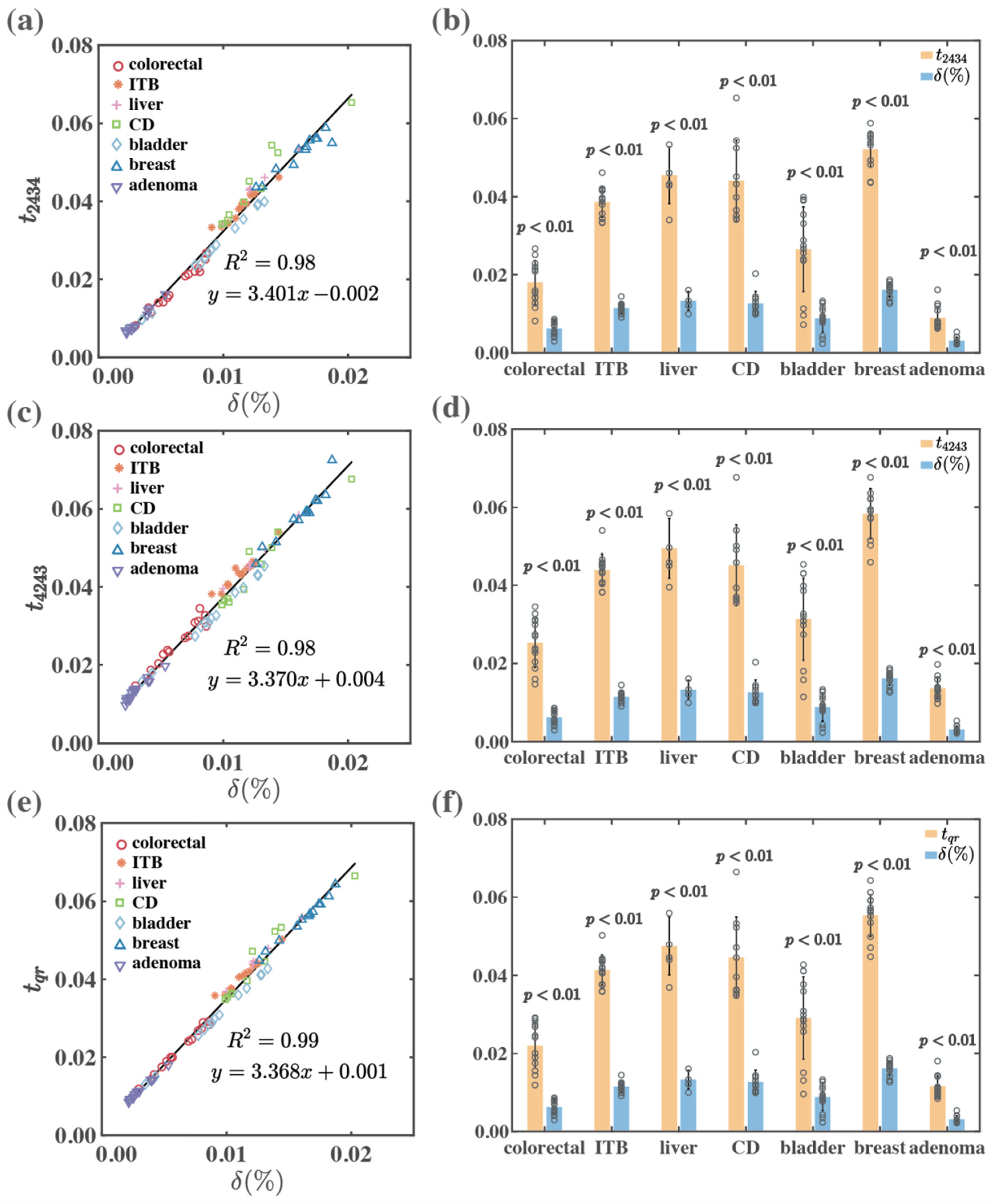
| Forward | colorectal | 0.97 | 0.94 | 0.99 | <0.01 | <0.01 | <0.01 |
| ITB | 0.93 | 0.93 | 0.97 | <0.01 | <0.01 | <0.01 | |
| liver | 0.98 | 0.99 | 0.99 | <0.01 | <0.01 | <0.01 | |
| CD | 0.91 | 0.93 | 0.93 | <0.01 | <0.01 | <0.01 | |
| bladder | 0.99 | 0.99 | 0.99 | <0.01 | <0.01 | <0.01 | |
| breast | 0.90 | 0.91 | 0.99 | <0.01 | <0.01 | <0.01 | |
| adenoma | 0.97 | 0.94 | 0.99 | <0.01 | <0.01 | <0.01 | |
| Total | 0.98 | 0.98 | 0.99 | <0.01 | <0.01 | <0.01 | |
| Type | Tissues | Linear Retardance | |||||
|---|---|---|---|---|---|---|---|
| R2 | p | ||||||
| δ—t2434 | δ—t4243 | δ—tqr | δ—t2434 | δ—t4243 | δ—tqr | ||
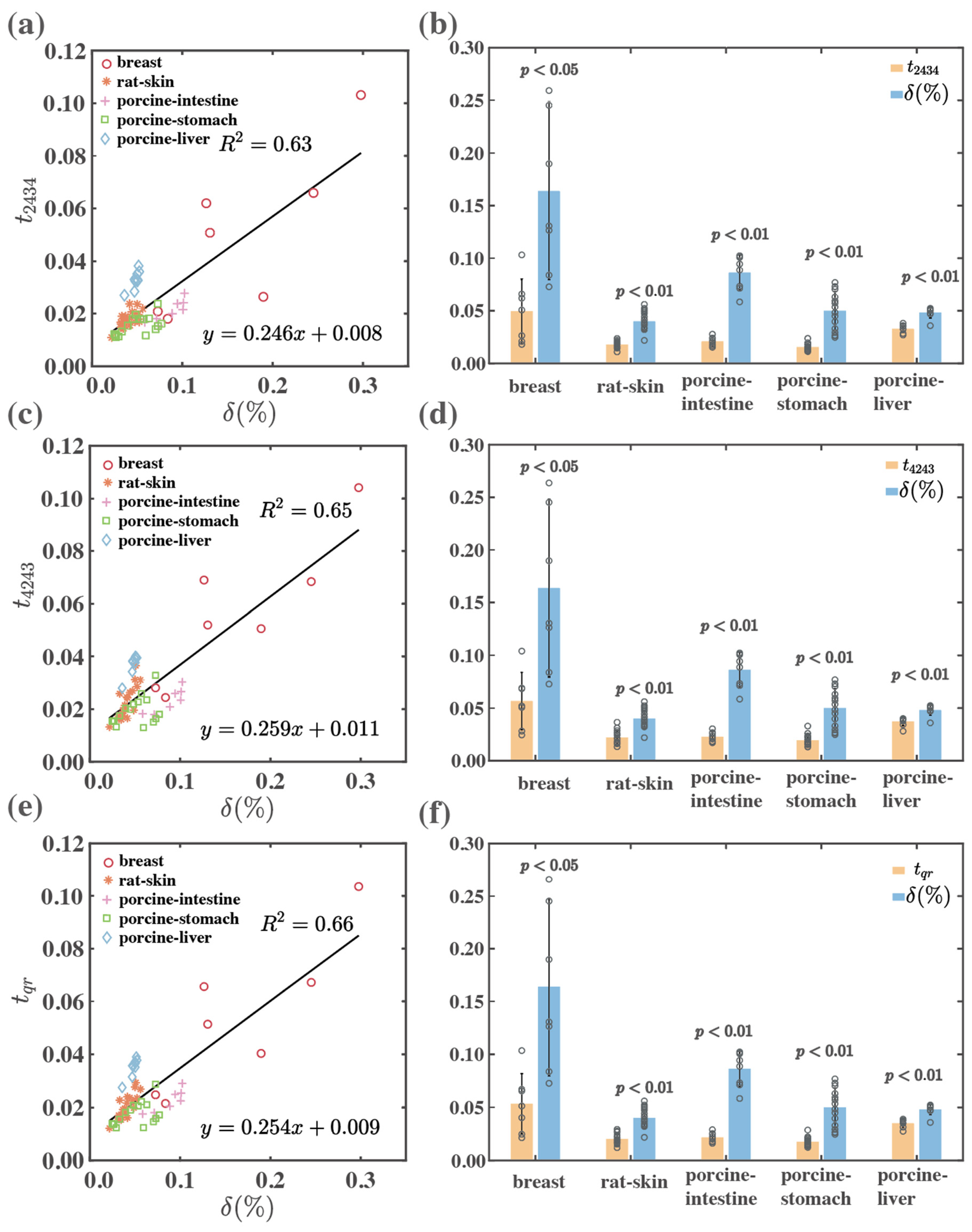
| Backward | breast | 0.65 | 0.76 | 0.73 | <0.05 | <0.05 | <0.05 |
| rat-skin | 0.46 | 0.65 | 0.68 | <0.01 | <0.01 | <0.01 | |
| porcine-intestine | 0.73 | 0.74 | 0.74 | <0.01 | <0.01 | <0.01 | |
| porcine-stomach | 0.33 | 0.16 | 0.22 | <0.01 | <0.01 | <0.01 | |
| porcine-liver | 0.65 | 0.89 | 0.84 | <0.01 | <0.01 | <0.01 | |
| Total | 0.63 | 0.65 | 0.66 | <0.01 | <0.01 | <0.01 | |
| Type | Tissues | Linear Birefringence Fast Axis Orientation | |||||
|---|---|---|---|---|---|---|---|
| R2 | p | ||||||
| θe—ϕ2434 | θe—ϕ4243 | θe—ϕqr | θe—ϕ2434 | θe—ϕ4243 | θe—ϕqr | ||
| Forward | colorectal | 0.97 | 0.97 | 1.00 | >0.05 | >0.05 | >0.05 |
| ITB | 0.94 | 0.82 | 0.99 | >0.05 | <0.01 | >0.05 | |
| liver | 0.95 | 0.44 | 0.99 | <0.01 | >0.05 | >0.05 | |
| CD | 0.97 | 0.95 | 0.99 | >0.05 | >0.05 | >0.05 | |
| bladder | 0.97 | 0.93 | 0.99 | >0.05 | >0.05 | >0.05 | |
| breast | 0.94 | 0.98 | 0.99 | >0.05 | >0.05 | >0.05 | |
| adenoma | 0.98 | 0.97 | 1.00 | >0.05 | >0.05 | >0.05 | |
| Total | 0.88 | 0.92 | 0.99 | >0.05 | >0.05 | >0.05 | |
| Type | Tissues | Linear Birefringence Fast Axis Orientation | |||||
|---|---|---|---|---|---|---|---|
| R2 | p | ||||||
| θe—ϕ2434 | θe—ϕ4243 | θe—ϕqr | θe—ϕ2434 | θe—ϕ4243 | θe—ϕqr | ||
| Backward | breast | 0.97 | 0.97 | 0.99 | >0.05 | >0.05 | >0.05 |
| rat-skin | 0.58 | 0.82 | 0.99 | >0.05 | >0.05 | >0.05 | |
| porcine-intestine | 0.75 | 0.97 | 0.99 | >0.05 | >0.05 | >0.05 | |
| porcine-stomach | 0.96 | 0.97 | 0.99 | >0.05 | >0.05 | >0.05 | |
| porcine-liver | 0.91 | 0.96 | 0.99 | >0.05 | >0.05 | >0.05 | |
| Total | 0.87 | 0.95 | 0.99 | >0.05 | >0.05 | >0.05 | |
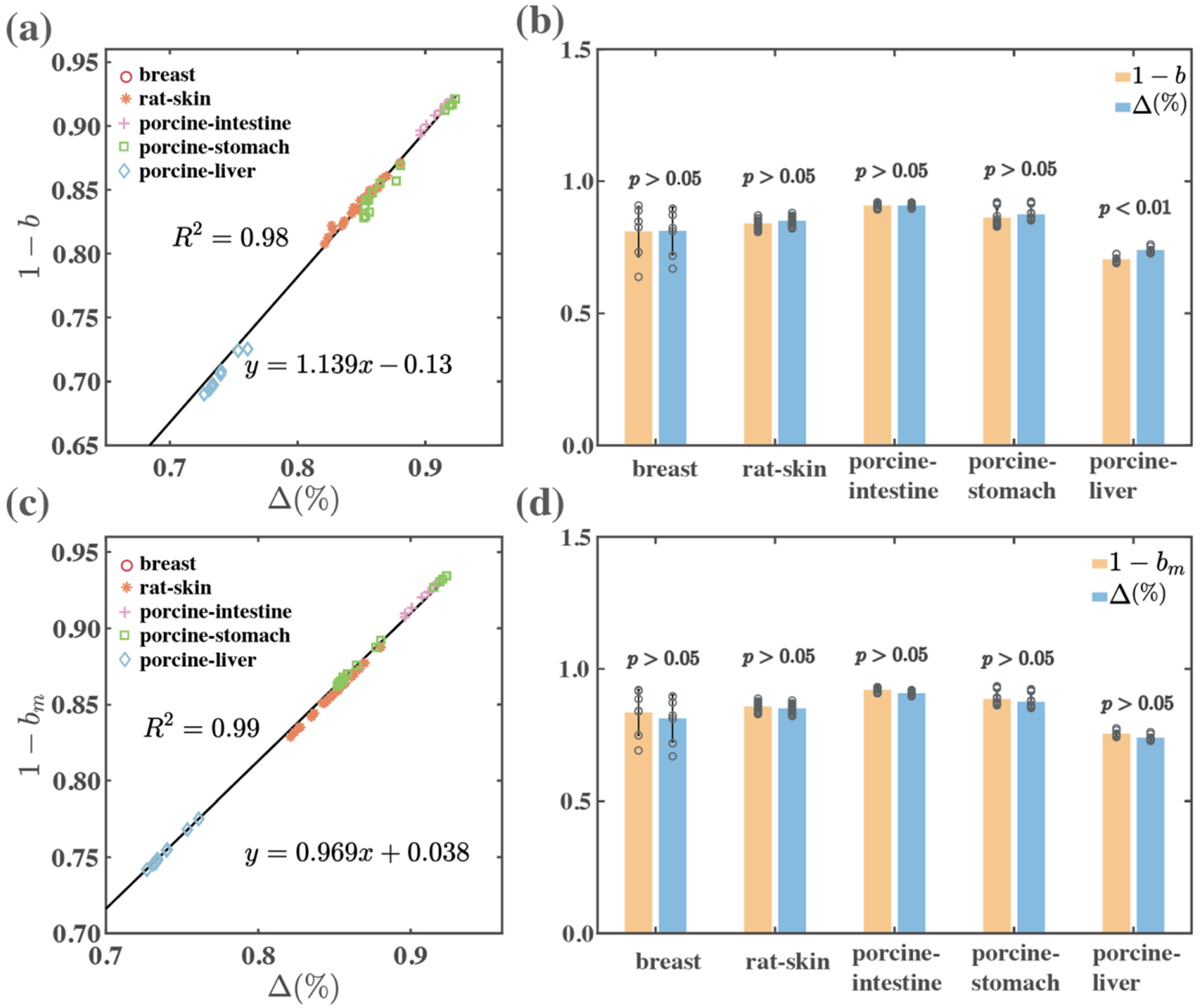
| Type | Tissues | Depolarization | |||
|---|---|---|---|---|---|
| R2 | p | ||||
| Δ—1 − b | Δ—1 − bm | Δ—1 − b | Δ—1 − bm | ||
| Forward | colorectal | 0.53 | 0.50 | <0.01 | <0.01 |
| ITB | 0.23 | 0.26 | <0.01 | <0.01 | |
| liver | 0.73 | 0.70 | <0.01 | <0.01 | |
| CD | 0.09 | 0.05 | >0.05 | <0.01 | |
| bladder | 0.43 | 0.44 | <0.01 | <0.05 | |
| breast | 0.49 | 0.70 | >0.05 | <0.01 | |
| adenoma | 0.17 | 0.11 | <0.01 | <0.05 | |
| Total | 0.49 | 0.49 | <0.01 | <0.01 | |
| Type | Tissues | Depolarization | |||
|---|---|---|---|---|---|
| R2 | p | ||||
| Δ—1 − b | Δ—1 − bm | Δ—1 − b | Δ—1 − bm | ||
| Backward | breast | 0.96 | 0.99 | >0.05 | >0.05 |
| rat-skin | 0.98 | 0.99 | >0.05 | >0.05 | |
| porcine-intestine | 0.98 | 0.99 | >0.05 | >0.05 | |
| porcine-stomach | 0.98 | 0.99 | >0.05 | >0.05 | |
| porcine-liver | 0.97 | 0.99 | <0.01 | >0.05 | |
| Total | 0.98 | 0.99 | >0.05 | >0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Lan, Y.; Zhai, H.; Deng, L.; He, H.; Mao, H.; Ma, H. Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries. Appl. Sci. 2021, 11, 10416. https://doi.org/10.3390/app112110416
Chen B, Lan Y, Zhai H, Deng L, He H, Mao H, Ma H. Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries. Applied Sciences. 2021; 11(21):10416. https://doi.org/10.3390/app112110416
Chicago/Turabian StyleChen, Binguo, Yuxiang Lan, Haoyu Zhai, Liangyu Deng, Honghui He, Hua Mao, and Hui Ma. 2021. "Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries" Applied Sciences 11, no. 21: 10416. https://doi.org/10.3390/app112110416
APA StyleChen, B., Lan, Y., Zhai, H., Deng, L., He, H., Mao, H., & Ma, H. (2021). Comparative Study of Modified Mueller Matrix Transformation and Polar Decomposition Parameters for Transmission and Backscattering Tissue Polarimetries. Applied Sciences, 11(21), 10416. https://doi.org/10.3390/app112110416







