Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
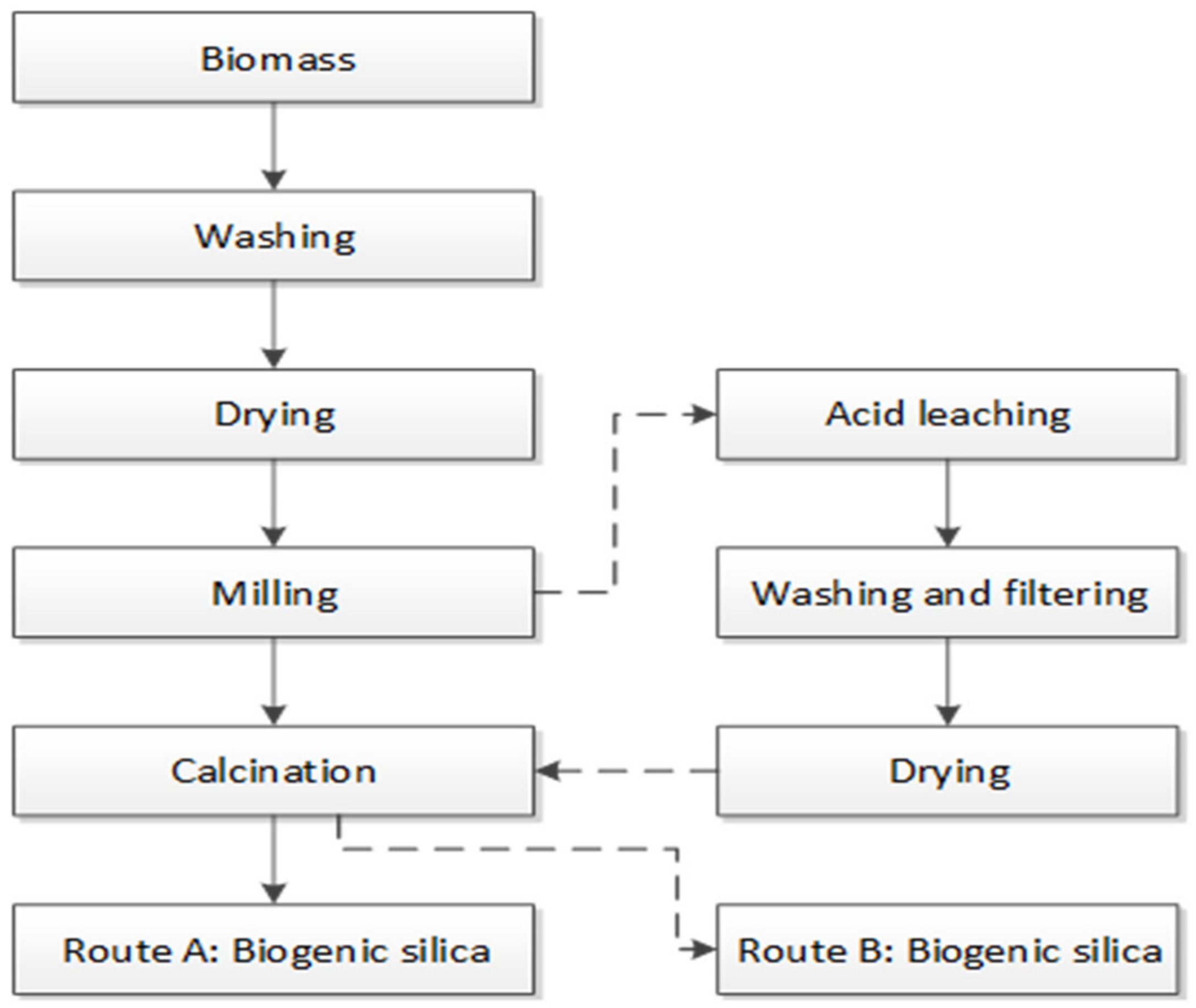
2.1. Material Used and Sample Preparation
2.2. Analysis of Physical and Chemical Properties
2.3. Thermal Analysis of Biomass Fuels
3. Results and Discussion
3.1. Solid Fuel Analysis
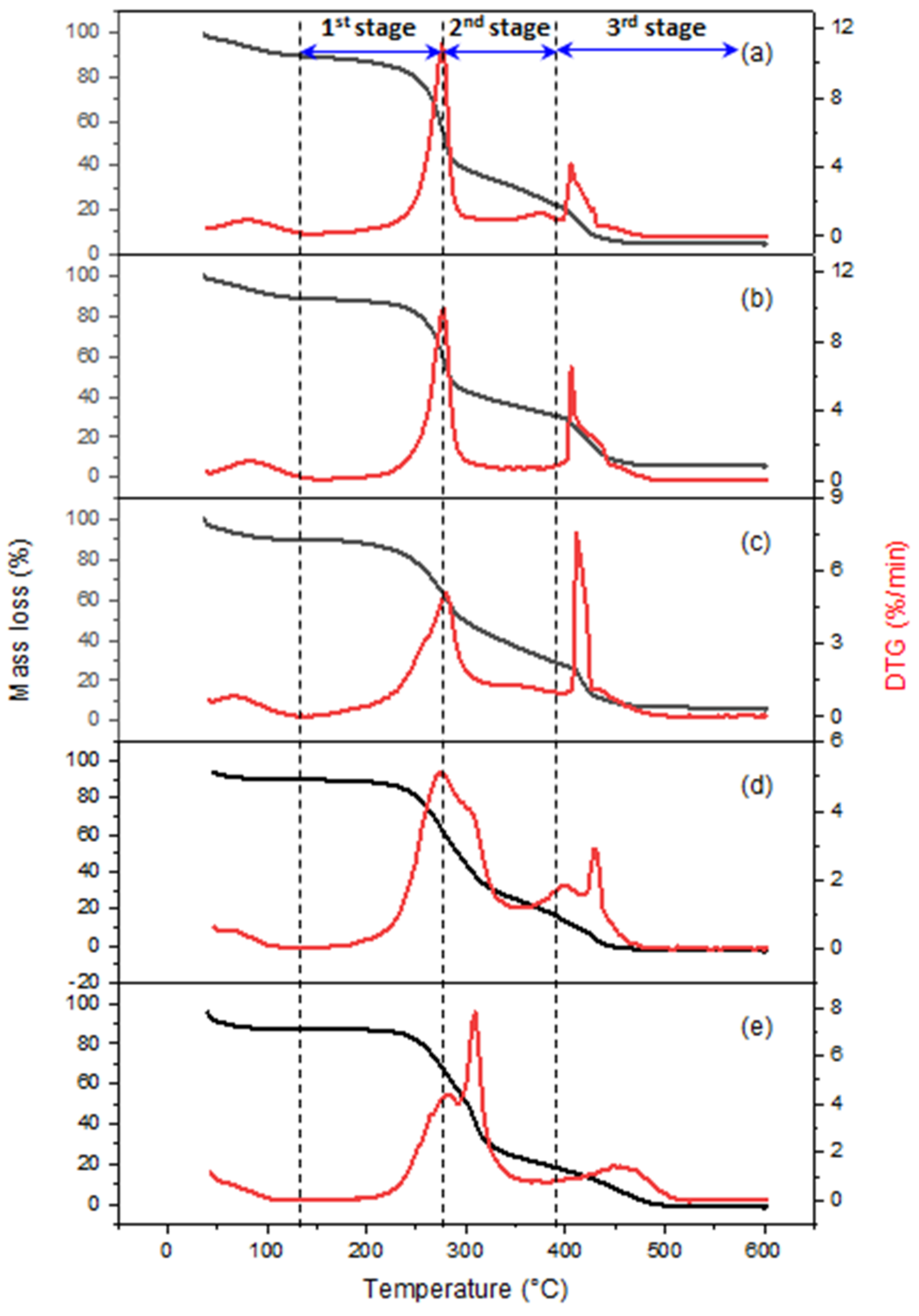
3.2. TGA Analysis of Biomass Fuels
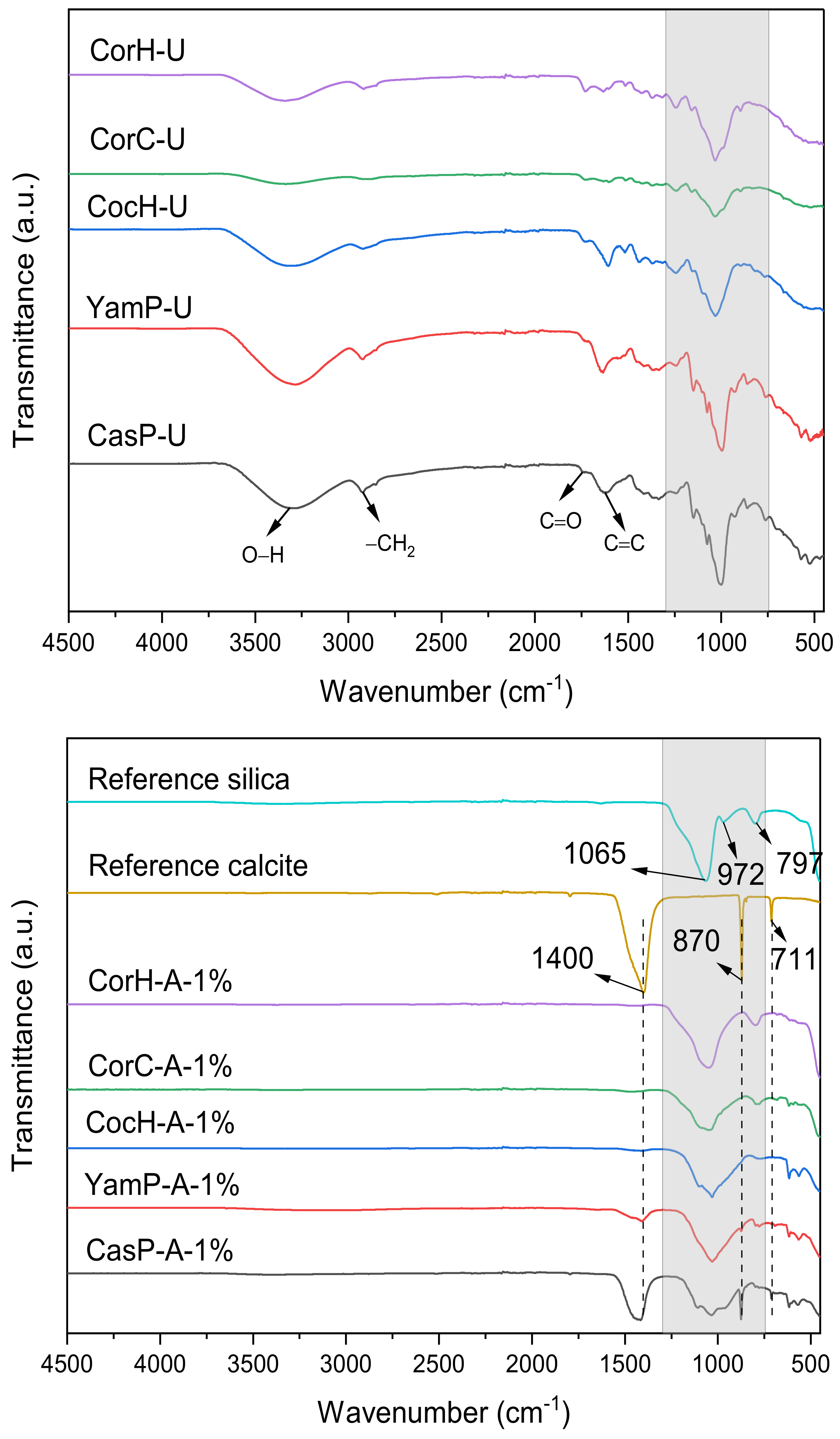
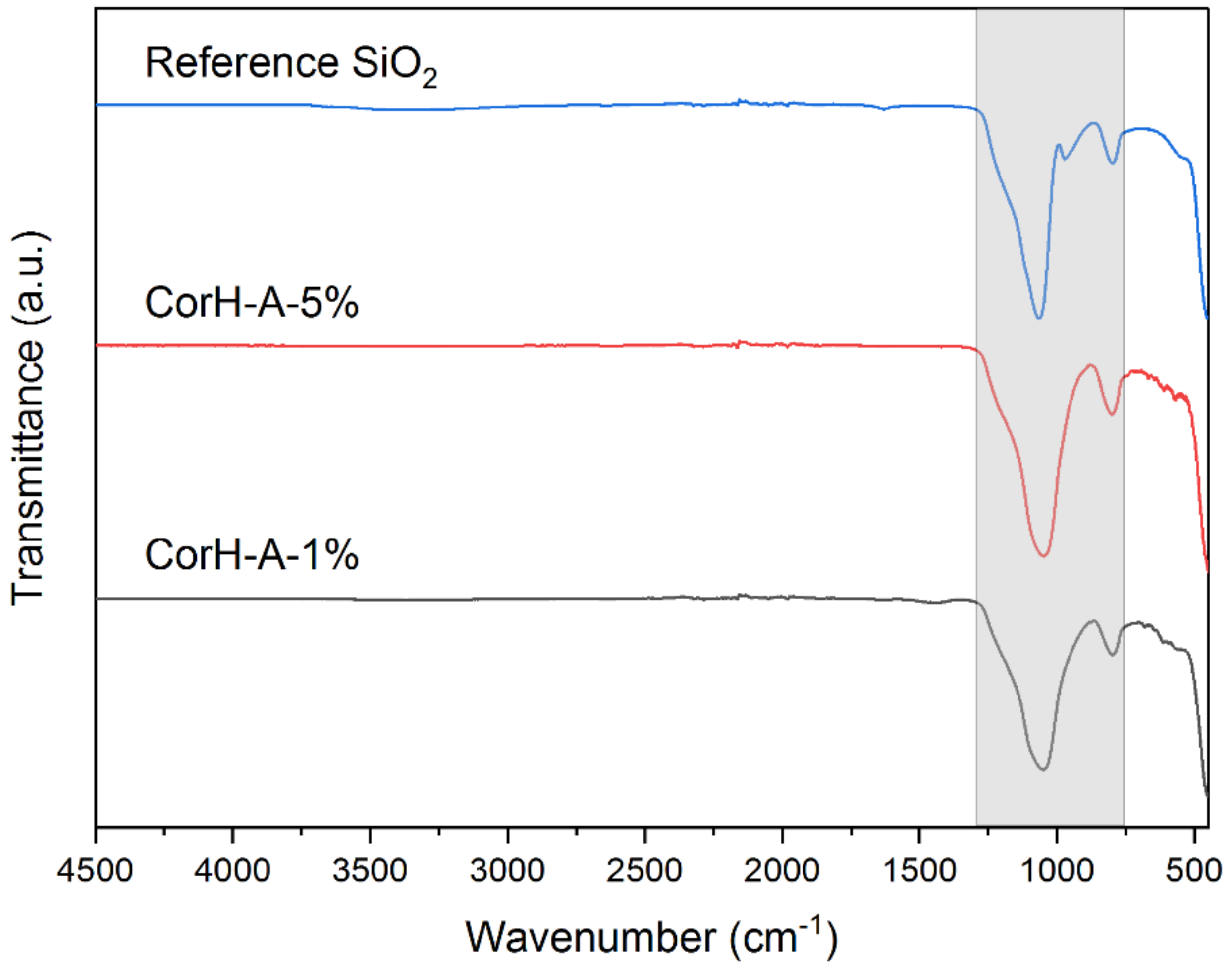
3.3. FTIR Analysis
3.4. Ash Analysis
3.5. Comparative Study of Physical Morphology, Textural Properties, and Phase Analysis of Ashes
3.5.1. Physical Morphology of Ashes from Untreated and Acid-Treated Biomass Fuels
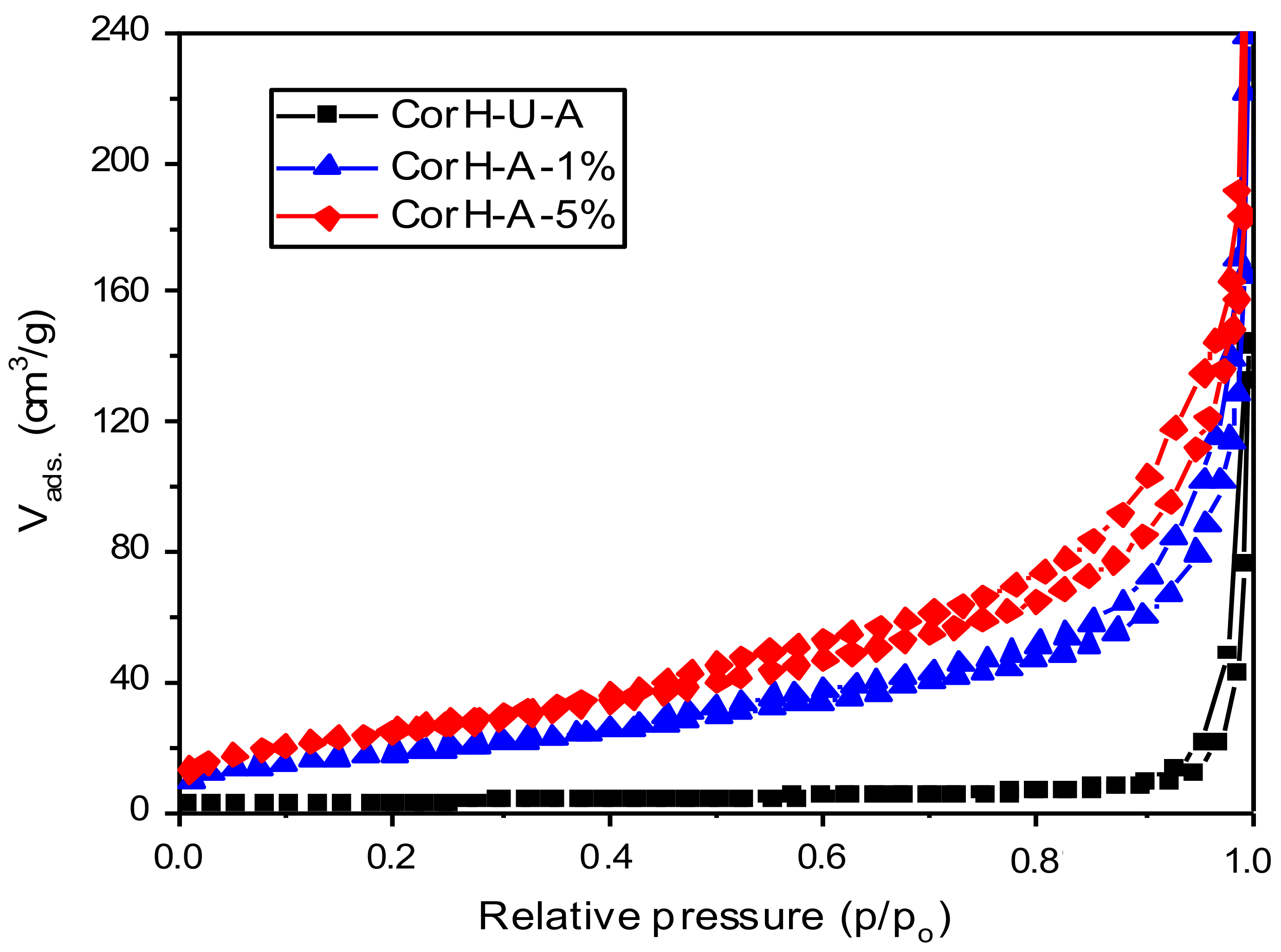
3.5.2. Nitrogen Gas Adsorption-Desorption Measurements
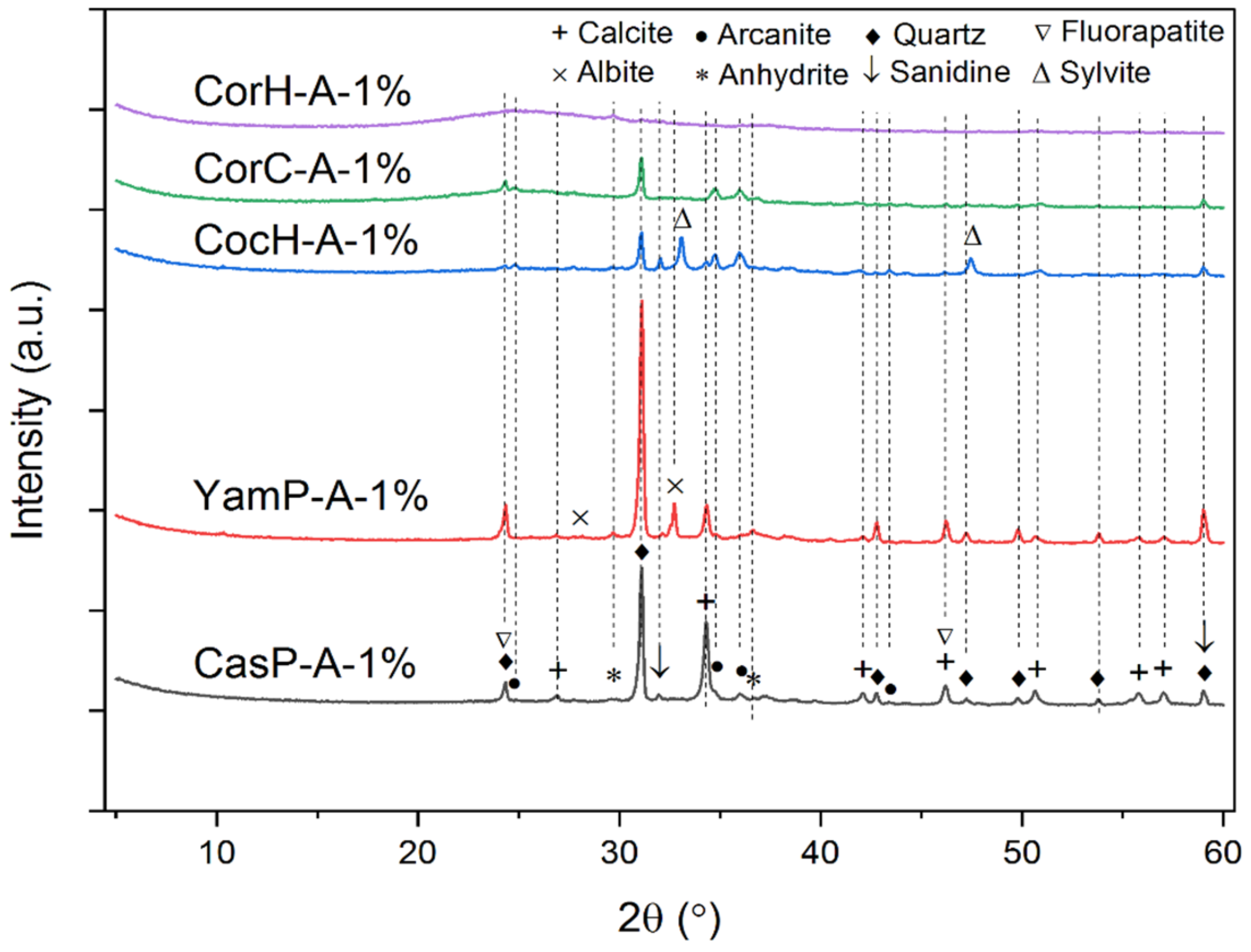
3.5.3. Phase Analysis of the Biomass Ash Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CasP | Cassava peelings |
| YamP | Yam peelings |
| CocH | Coconut husk |
| CorC | Corncob |
| CorH | Cornhusk |
| CasP-U | Untreated cassava peelings |
| YamP-U | Untreated yam peelings |
| CocH-U | Untreated coconut husk |
| CorC-U | Untreated cornhusk |
| CorH-U | Untreated cornhusk |
| YamP-1% | Yam peelings leached in 1 w/v% citric acid |
| CocH-1% | Coconut husk leached in 1 w/v% citric acid |
| CorC-1% | Corncob leached in 1 w/v% citric acid |
| CorH-1% | Cornhusk leached in 1 w/v% citric acid |
| CasP-U-A | Ash of untreated cassava peelings |
| YamP-U-A | Ash of untreated yam peelings |
| CocH-U-A | Ash of untreated coconut husk |
| CorC-U-A | Ash of untreated corncob |
| CorH-U-A | Ash of untreated cornhusk |
| CasP-A-1% | Ash of 1 w/v% acid-leached cassava peelings |
| YamP-A-1% | Ash of 1 w/v% acid-leached yam peelings |
| CocH-A-1% | Ash of 1 w/v% acid-leached coconut husk |
| CorC-A-1% | Ash of 1 w/v% acid-leached corncob |
| CorH-A-1% | Ash of 1 w/v% acid-leached cornhusk |
| CasP-A-5% | Ash of 5 w/v% acid-leached cassava peelings |
| YamP-A-5% | Ash of 5 w/v% acid-leached yam peelings |
| CocH-A-5% | Ash of 5 w/v% acid-leached coconut husk |
| CorC-A-5% | Ash of 5 w/v% acid-leached corncob |
| CorH-A-5% | Ash of 5 w/v% acid-leached cornhusk |
| VM | Volatile matter |
| AC | Ash content |
| MC | Moisture content |
| LHV | Lower heating value. |
| wb | Wet basis |
| wt.% | Weight percentage |
| db | Dry basis |
| n.d. | Not detected |
| CA | Citric acid |
References
- Shah, M.A.; Khan, M.N.S.; Kumar, V. Biomass residue characterization for their potential application as biofuels. J. Therm. Anal. Calorim. 2018, 134, 2137–2145. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.-S.; Su, S.; Xu, K.; He, L.-M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Marland, G. Accounting for Carbon Dioxide Emissions from Bioenergy Systems. J. Ind. Ecol. 2010, 14, 866–869. [Google Scholar] [CrossRef]
- Duku, M.H.; Gu, S.; Ben Hagan, E. A comprehensive review of biomass resources and biofuels potential in Ghana. Renew. Sustain. Energy Rev. 2011, 15, 404–415. [Google Scholar] [CrossRef]
- Lim, J.S.; Manan, Z.A.; Alwi, S.R.W.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Kemausuor, F.; Kamp, A.; Thomsen, S.T.; Bensah, E.; Østergård, H. Assessment of biomass residue availability and bioenergy yields in Ghana. Resour. Conserv. Recycl. 2014, 86, 28–37. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change. Adoption of the Paris Agreement: Proposal by the President. In Proceedings of the Paris Climate Change Conference, Paris, France, 30 November–12 December 2015. [Google Scholar]
- African Climate Policy Centre (ACPC). 2010. Overview of the ClimDev Africa Programme. Available online: http://www.climdev-africa.org/afrian-climate-policy-center (accessed on 28 September 2021).
- Catuti, M.; Elkerbout, M.; Alessi, M.; Egenhofer, C. Biomass and Climate Neutrality: CEPS Policy Insights. No 2020-19. August 2020. Available online: https://www.ceps.eu/wp-content/uploads/2020/08/PI2020-19_Biomass-and-climate-neutrality.pdf (accessed on 28 September 2021).
- Guimarães, J.; Frollini, E.; da Silva, C.G.; Wypych, F.; Satyanarayana, K. Characterization of banana, sugarcane bagasse and sponge gourd fibers of Brazil. Ind. Crop. Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Milbrandt, A. Assessment of Biomass Resources in Liberia; No. NREL/TP-6A2-44808; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2009. [Google Scholar]
- Smith, O.B. Utilization of Crop Residues in the Nutrition of Sheep and Goats in the Humid Tropics of West Africa. In Sheep and Goat Meat Production in Humid Tropics of West Africa; FAO Animal Production and Health Paper; Atta Krah, A.N., Reyholds, L., Eds.; FAO: Rome, Italy, 1987; Available online: http://www.fao.org/library/library-home/en/ (accessed on 29 September 2021).
- Abass, A.; Mlingi, N.; Ranaivoson, R.; Zulu, M.; Mukuka, I.; Abele, S.; Bachwenkizi, B.; Cromme, N. Potential for Commercial Production and Marketing of Cassava: Experiences from the Small-Scale Cassava Processing Project in East and Southern Africa; IITA: Ibadan, Nigeria, 2013. [Google Scholar]
- Titiloye, J.; Abu Bakar, M.S.; Odetoye, T. Thermochemical characterisation of agricultural wastes from West Africa. Ind. Crop. Prod. 2013, 47, 199–203. [Google Scholar] [CrossRef]
- Adepoju, A.D.; Adebisi, J.A.; Odusote, J.K.; Ahmed, I.I.; Hassan, S.B. Preparation of Silica from Cassava Periderm. J. Solid Waste Technol. Manag. 2016, 42, 216–221. [Google Scholar] [CrossRef]
- Jekayinfa, S.O.; Scholz, V. Potential Availability of Energetically Usable Crop Residues in Nigeria. Energy Sources Part A Recover. Util. Environ. Eff. 2009, 31, 687–697. [Google Scholar] [CrossRef]
- Cooper, C.; Laing, C. A macro analysis of crop residue and animal wastes as a potential energy source in Africa. J. Energy S. Afr. 2007, 18, 10–19. [Google Scholar] [CrossRef]
- Dasappa, S. Potential of biomass energy for electricity generation in sub-Saharan Africa. Energy Sustain. Dev. 2011, 15, 203–213. [Google Scholar] [CrossRef]
- Stecher, K.; Brosowski, A.; Thrän, D. Biomass Potential in Africa. Irena 2013. Available online: https://www.dbfz.de/fileadmin/user_upload/Referenzen/Broschueren/IRENA-DBFZ_Biomass_Potential_in_Africa.pdf (accessed on 4 October 2021).
- Alyosef, H.A.; Schneider, D.; Wassersleben, S.; Roggendorf, H.; Weiß, M.; Eilert, A.; Denecke, R.; Hartmann, I.; Enke, D. Meso/Macroporous Silica from Miscanthus, Cereal Remnant Pellets, and Wheat Straw. ACS Sustain. Chem. Eng. 2015, 3, 2012–2021. [Google Scholar] [CrossRef]
- Nitsch, J.; Krewitt, W.; Langniss, O. Renewable Energy in Europe. Encycl. Energy 2004, 5, 313–331. [Google Scholar] [CrossRef]
- Adebisi, J.A.; Agunsoye, J.O.; Bello, S.A.; Kolawole, F.O.; Ramakokovhu, M.M.; Daramola, M.O.; Hassan, S.B. Extraction of Silica from Sugarcane Bagasse, Cassava Periderm and Maize Stalk: Proximate Analysis and Physico-Chemical Properties of Wastes. Waste Biomass Valorization 2017, 10, 617–629. [Google Scholar] [CrossRef]
- Omohimi, C.I.; Piccirillo, C.; Roriz, M.; Ferraro, V.; Vasconcelos, M.W.; Sanni, L.; Tomlins, K.; Pintado, M.M.; Abayomi, L.A. Study of the proximate and mineral composition of different Nigerian yam chips, flakes and flours. J. Food Sci. Technol. 2017, 55, 42–51. [Google Scholar] [CrossRef]
- FAOSTAT. The State of Food and Agriculture; Food and Agriculture Organisation of the UN: Rome, Italy, 2007; Available online: http://www.fao.org/3/a1200e/a1200e.pdf (accessed on 21 August 2021).
- Bonfim, W.B.; de Paula, H.M. Characterization of different biomass ashes as supplementary cementitious material to produce coating mortar. J. Clean. Prod. 2021, 291, 125869. [Google Scholar] [CrossRef]
- Andreola, F.; Martín, M.; Ferrari, A.; Lancellotti, I.; Bondioli, F.; Rincón, J.; Romero, M.; Barbieri, L. Technological properties of glass-ceramic tiles obtained using rice husk ash as silica precursor. Ceram. Int. 2013, 39, 5427–5435. [Google Scholar] [CrossRef] [Green Version]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of rice husk ash as filler in natural rubber vulcanizates: In comparison with other commercial fillers. J. Appl. Polym. Sci. 2002, 83, 2485–2493. [Google Scholar] [CrossRef]
- Ajmal, M.; Rao, R.A.K.; Anwar, S.; Ahmad, J.; Ahmad, R. Adsorption studies on rice husk: Removal and recovery of Cd(II) from wastewater. Bioresour. Technol. 2003, 86, 147–149. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Miricioiu, M.; Geana, E.-I.; Ionete, R.-E.; Paun, N.; Parvulescu, V. Silica Mesoporous Materials -an Efficient Sorbent for Wine Polyphenols Separation. Rev. Chim. 2019, 70, 1513–1517. [Google Scholar] [CrossRef]
- Liu, D.; Seeburg, D.; Kreft, S.; Bindig, R.; Hartmann, I.; Schneider, D.; Enke, D.; Wohlrab, S. Rice Husk Derived Porous Silica as Support for Pd and CeO2 for Low Temperature Catalytic Methane Combustion. Catalyst 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Azadi, M.; Bahrololoom, M.E.; Heidari, F. Enhancing the mechanical properties of an epoxy coating with rice husk ash, a green product. J. Coat. Technol. Res. 2010, 8, 117–123. [Google Scholar] [CrossRef]
- Dai, H.; Yang, J.; Ma, J.; Chen, F.; Fei, Z.; Zhong, M. A green process for the synthesis of controllable mesoporous silica materials. Microporous Mesoporous Mater. 2012, 147, 281–285. [Google Scholar] [CrossRef]
- Adebisi, J.; Agunsoye, J.; Bello, S.; Ahmed, I.; Ojo, O.; Hassan, S. Potential of producing solar grade silicon nanoparticles from selected agro-wastes: A review. Sol. Energy 2017, 142, 68–86. [Google Scholar] [CrossRef]
- Florek, J.; Guillet-Nicolas, R.; Kleitz, F. Ordered mesoporous silica: Synthesis and applications. In Functional Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2014; pp. 61–100. [Google Scholar]
- Schneider, D. Biogenic Silica from Regional Feedstocks—Sustainable Synthesis and Characterization. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2019. [Google Scholar]
- Beidaghy Dizaji, H.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of High Quality Biogenic Silica by Combustion of Rice Husk and Rice Straw Combined with Pre- and Post-Treatment Strategies—A Review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef] [Green Version]
- Quispe, I.; Navia, R.; Kahhat, R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Manag. 2017, 59, 200–210. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zemnukhova, L.A.; Egorov, A.G.; Fedorishcheva, G.A.; Barinov, N.N.; Sokol’Nitskaya, T.A.; Botsul, A.I. Properties of amorphous silica produced from rice and oat processing waste. Inorg. Mater. 2006, 42, 24–29. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Nikolic, M.; Ye, M.-J.; Xiao, Z.-X.; Liang, Y.-C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Vaibhav, V.; Vijayalakshmi, U.; Roopan, S.M. Agricultural waste as a source for the production of silica nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, R.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Miricioiu, M.G.; Niculescu, V.-C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, M.; Datye, A.K. Catalysis for the Conversion of Biomass and Its Derivatives; Max Planck Research Library for History and Development of Knowledge: Berlin, Germany, 2013. [Google Scholar]
- Long, J.; Song, H.; Jun, X.; Sheng, S.; Lun-Shi, S.; Kai, X.; Yao, Y. Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour. Technol. 2012, 116, 278–284. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized Procedure for the Production of High-Grade, Porous Biogenic Silica. Waste Biomass Valorization 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Umeda, J.; Imai, H.; Kondoh, K. Polysaccharide hydrolysis and metallic impurities removal behaviour of rice husks in citric aicd leaching treatment. Trans. JWRI 2009, 38, 13–18. [Google Scholar]
- Umeda, J.; Kondoh, K. High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J. Mater. Sci. 2008, 43, 7084–7090. [Google Scholar] [CrossRef]
- Beidaghy Dizaji, H.; Zeng, T.; Hölzig, H.; Bauer, J.; Klöß, G.; Enke, D. Ash transformation mechanism during combustion of rice husk and rice straw. Fuel 2022, 307, 121768. [Google Scholar] [CrossRef]
- Utsev, J.T.; Taku, J.K. Coconut Shell Ash as Partial Replacement of Ordinary Portland Cement In Concrete Production. Int. J. Sci. Technol. Res. 2012, 1, 86–89. [Google Scholar]
- Samanta, A.K.; Basu, G.; Mishra, L. Role of major constituents of coconut fibres on absorption of ionic dyes. Ind. Crop. Prod. 2018, 117, 20–27. [Google Scholar] [CrossRef]
- Asuquo, E.D.; Martin, A.D.; Nzerem, P. Evaluation of Cd(II) Ion Removal from Aqueous Solution by a Low-Cost Adsorbent Prepared from White Yam (Dioscorea rotundata) Waste Using Batch Sorption. ChemEngineering 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Schliermann, T.; Hartmann, I.; Beidaghy Dizaji, H.; Zeng, T.; Schneider, D.; Wassersleben, S.; Enke, D.; Jobst, T.; Lange, A.; Roelofs, F.; et al. High Quality Biogenic Silica from Combined Energetic and Material Utilization of Agricultural Residues. In Proceedings of the 7th International Symposium on Energy from Biomass and Waste, Venice, Italy, 15–18 October 2018; ISBN 978-8-86-265013-7. [Google Scholar]
- Hilbers, T.J.; Wang, Z.; Pecha, B.; Westerhof, R.J.; Kersten, S.R.; Pelaez-Samaniego, M.R.; Garcia-Perez, M. Cellulose-Lignin interactions during slow and fast pyrolysis. J. Anal. Appl. Pyrolysis 2015, 114, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, D.; Gu, J.; Bao, B.; Zhang, Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energy Convers. Manag. 2015, 89, 251–259. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, A.H.; Zheng, C. Thermogravimetric Analysis−Fourier Transform Infrared Analysis of Palm Oil Waste Pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Mendes, C.A.; Adnet, F.A.; Leite, M.C.A.M.; Furtado, C.; Furtado, M. Chemical, physical, mechanical, thermal and morphological characterization of corn husk residue. Cellul. Chem. Technol. 2015, 49, 727–735. [Google Scholar]
- Ceylan, S.; Topçu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ma, X.; Li, L.; Liu, C.; Cheng, K.; Li, Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py–GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Z.; Fei, B.; Cai, Z.; Yu, Y.; Liu, X. The pyrolysis characteristics of moso bamboo. J. Anal. Appl. Pyrolysis 2012, 94, 48–52. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, L.; Dzenis, Y.A.; Jones, D.D.; Hanna, M.A. Thermogravimetric characterization of corn stover as gasification and pyrolysis feedstock. Biomass Bioenergy 2008, 32, 460–467. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. In-depth investigation on the pyrolysis kinetics of raw biomass. Part I: Kinetic analysis for the drying and devolatilization stages. Bioresour. Technol. 2013, 131, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Mansaray, A.E.G.K.G. Kinetics of the Thermal Degradation of Rice Husks in Nitrogen Atmosphere. Energy Sources 1999, 21, 773–784. [Google Scholar] [CrossRef]
- Jose, A.; Nivitha, M.; Krishnan, M.; Robinson, R. Characterization of cement stabilized pond ash using FTIR spectroscopy. Constr. Build. Mater. 2020, 263, 120136. [Google Scholar] [CrossRef]
- Jung, H.; Kwak, H.; Chun, J.; Oh, K. Alkaline Fractionation and Subsequent Production of Nano-Structured Silica and Cellulose Nano-Fibrils for the Comprehensive Utilization of Rice Husk. Sustainability 2021, 13, 1951. [Google Scholar] [CrossRef]
- Nakason, K.; Khemthong, P.; Kraithong, W.; Chukaew, P.; Panyapinyopol, B.; Kitkaew, D.; Pavasant, P. Upgrading properties of biochar fuel derived from cassava rhizome via torrefaction: Effect of sweeping gas atmospheres and its economic feasibility. Case Stud. Therm. Eng. 2021, 23, 100823. [Google Scholar] [CrossRef]
- Pollard, Z.A.; Goldfarb, J.L. Valorization of cherry pits: Great Lakes agro-industrial waste to mediate Great Lakes water quality. Environ. Pollut. 2021, 270, 116073. [Google Scholar] [CrossRef]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Dalai, A.K.; Koziński, J.A. Chemical composition, character and reactivity of renewable fuel ashes. Fuel 2016, 176, 135–145. [Google Scholar] [CrossRef]
- Nieves, L.J.J.; Elyseu, F.; Goulart, S.; Pereira, M.D.S.; Valvassori, E.Z.; Bernardin, A.M. Use of fly and bottom ashes from a thermoelectrical plant in the synthesis of geopolymers: Evaluation of reaction efficiency. Energy Geosci. 2021, 2, 167–173. [Google Scholar] [CrossRef]
- Nana, A.; Epey, N.; Rodrique, K.C.; Deutou, J.G.N.; Djobo, J.N.Y.; Tomé, S.; Alomayri, T.S.; Ngouné, J.; Kamseu, E.; Leonelli, C. Mechanical strength and microstructure of metakaolin/volcanic ash-based geopolymer composites reinforced with reactive silica from rice husk ash (RHA). Materialia 2021, 16, 101083. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, B.; Gao, W.; Qu, Y.; Wang, L.; Wang, Z.; Zhu, Y. A recyclable method for production of pure silica from rice hull ash. Powder Technol. 2012, 217, 497–501. [Google Scholar] [CrossRef]
- Bathla, A.; Narula, C.; Chauhan, R.P. Hydrothermal synthesis and characterization of silica nanowires using rice husk ash: An agricultural waste. J. Mater. Sci. Mater. Electron. 2018, 29, 6225–6231. [Google Scholar] [CrossRef]
- Morrow, B.A.; McFarlan, A.J. Surface vibrational modes of silanol groups on silica. J. Phys. Chem. 1992, 96, 1395–1400. [Google Scholar] [CrossRef]
- Mohanraj, K.; Kannan, S.; Barathan, S.; Sivakumar, G. Preparation and Characterization of Nano SiO2 from Corn Cob Ash by Precipitation Method. Optoelectron. Adv. Mater. Rapid Commun. 2012, 6, 394–397. [Google Scholar]
- Ennaciri, Y.; El Alaoui-Belghiti, H.; Bettach, M. Comparative study of K2SO4 production by wet conversion from phosphogypsum and synthetic gypsum. J. Mater. Res. Technol. 2019, 8, 2586–2596. [Google Scholar] [CrossRef]
- Medina, J.M.; Del Bosque, I.F.S.; Frías, M.; De Rojas, M.I.S.; Medina, C. Characterisation and valorisation of biomass waste as a possible addition in eco-cement design. Mater. Struct. 2017, 50, 207. [Google Scholar] [CrossRef]
- Liou, T.-H. Evolution of chemistry and morphology during the carbonization and combustion of rice husk. Carbon 2004, 42, 785–794. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Savastano, H. Brazilian sugar cane bagasse ashes from the cogeneration industry as active pozzolans for cement manufacture. Cem. Concr. Compos. 2011, 33, 490–496. [Google Scholar] [CrossRef]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Alyosef, H.A.; Ibrahim, S.; Welscher, J.; Inayat, A.; Eilert, A.; Denecke, R.; Schwieger, W.; Münster, T.; Kloess, G.; Einicke, W.-D.; et al. Effect of acid treatment on the chemical composition and the structure of Egyptian diatomite. Int. J. Miner. Process. 2014, 132, 17–25. [Google Scholar] [CrossRef]
- Keown, D.; Favas, G.; Hayashi, J.; Li, C.-Z. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass: Differences between sugar cane bagasse and cane trash. Bioresour. Technol. 2005, 96, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2012, 1, 254–259. [Google Scholar] [CrossRef]
- Dunlop, A.P. Furfural Formation and Behavior. Ind. Eng. Chem. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Olsson, J.G.; Jäglid, U.; Pettersson, J.B.C.; Hald, P. Alkali Metal Emission during Pyrolysis of Biomass. Energy Fuels 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Hunt, L.P.; Dismukes, J.P.; Amick, J.A.; Schei, A.; Larsen, K. Rice Hulls as a Raw Material for Producing Silicon. J. Electrochem. Soc. 1984, 131, 1683–1686. [Google Scholar] [CrossRef]
- Amick, J.A. Purification of Rice Hulls as a Source of Solar Grade Silicon for Solar Cells. J. Electrochem. Soc. 1982, 129, 864–866. [Google Scholar] [CrossRef]
- Umeda, J.; Katsuyoshi, K. Process Optimization to Prepare High-Purity Amorphous Silica from Rice Husks via Citric Acid Leaching Treatment. Trans. JWRI 2008, 37, 13–17. [Google Scholar]
- Currie, H.A.; Perry, C.C. Silica in Plants: Biological, Biochemical and Chemical Studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef]
- Singh, S.P.; Endley, N. Fabrication of nano-silica from agricultural residue and their application. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–134. [Google Scholar]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of Biogenic Silica Generated by Thermo Chemical Treatment of Rice Husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.G.; Pramada, P.N.; Raghavan, P.; Gupta, T.N. Review Processing, properties and applications of reactive silica from rice husk—An overview. J. Mater. Sci. 2003, 38, 3159–3168. [Google Scholar] [CrossRef]
- Ma, J.F.; Higashitani, A.; Sato, K.; Takeda, K. Genotypic variation in silicon concentration of barley grain. Plant Soil 2003, 249, 383–387. [Google Scholar] [CrossRef]
- Takahashi, E.; Ma, J.F.; Miyake, Y. The Possibility of Silicon as an Essential Element for Higher Plants. Comments Agric. Food Chem. 1990, 2, 99–122. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Ma, J.F.; Rengel, Z.; Zhao, F. Beneficial Elements; Elsevier: Amsterdam, The Netherlands, 2012; pp. 249–269. [Google Scholar]
- Deren, C. Chapter 8 Plant genotype, silicon concentration, and silicon-related responses. Inulin Inulin-Contain. Crop. 2001, 8, 149–158. [Google Scholar] [CrossRef]
- Krishnarao, R.; Subrahmanyam, J.; Kumar, T.J. Studies on the formation of black particles in rice husk silica ash. J. Eur. Ceram. Soc. 2001, 21, 99–104. [Google Scholar] [CrossRef]
- Zareihassangheshlaghi, A.; Beidaghy Dizaji, H.; Zeng, T.; Huth, P.; Ruf, T.; Denecke, R.; Enke, D. Behavior of Metal Impurities on Surface and Bulk of Biogenic Silica from Rice Husk Combustion and the Impact on Ash-Melting Tendency. ACS Sustain. Chem. Eng. 2020, 8, 10369–10379. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P.N.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pre-treatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pre-treatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, H.; Luque, R. Advances on biomass pre-treatment using ionic liquids: An overview. Energy Environ. Sci. 2011, 4, 3913–3929. [Google Scholar] [CrossRef]
- Hanna, S.; Farag, L.; Mansour, N. Pyrolysis and combustion of treated and untreated rice hulls. Thermochim. Acta 1984, 81, 77–86. [Google Scholar] [CrossRef]
- Chakraverty, A.; Mishra, P.; Banerjee, H.D. Investigation of combustion of raw and acid-leached rice husk for production of pure amorphous white silica. J. Mater. Sci. 1988, 23, 21–24. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Gautam, R.K.; Jaiswal, A.; Chattopadhyaya, M.C.; Sharma, Y.C. Rapid scavenging of methylene blue dye from a liquid phase by adsorption on alumina nanoparticles. RSC Adv. 2015, 5, 14425–14440. [Google Scholar] [CrossRef]
- Li, T.; Shen, J.; Huang, S.; Li, N.; Ye, M. Hydrothermal carbonization synthesis of a novel montmorillonite supported carbon nanosphere adsorbent for removal of Cr (VI) from waste water. Appl. Clay Sci. 2014, 93–94, 48–55. [Google Scholar] [CrossRef]
- Basu, P. Biomass Characteristics. In Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–91. [Google Scholar]
- Shen, J.; Liu, X.; Zhu, S.; Zhang, H.; Tan, J. Effects of calcination parameters on the silica phase of original and leached rice husk ash. Mater. Lett. 2011, 65, 1179–1183. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.; González, L. Review on the physicochemical treatments of rice husk for production of advanced materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Ozdemir, S.; Ozdemir, S.; Yetilmezsoy, K. Poultry abattoir sludge as bio-nutrient source for walnut plantation in low-fertility soil. Environ. Prog. Sustain. Energy 2019, 38, e13066. [Google Scholar] [CrossRef]
- Venezia, A.M.; La Parola, V.; Longo, A.; Martorana, A. Effect of Alkali Ions on the Amorphous to Crystalline Phase Transition of Silica. J. Solid State Chem. 2001, 161, 373–378. [Google Scholar] [CrossRef]
- Yang, H.; Liu, B.; Chen, Y.; Li, B.; Chen, H. Influence of Inherent Silicon and Metals in Rice Husk on the Char Properties and Associated Silica Structure. Energy Fuels 2015, 29, 7327–7334. [Google Scholar] [CrossRef]
- Gilbe, C.; Öhman, M.; Lindström, E.; Boström, D.; Backman, R.; Samuelsson, R.; Burvall, J. Slagging Characteristics during Residential Combustion of Biomass Pellets. Energy Fuels 2008, 22, 3536–3543. [Google Scholar] [CrossRef]
- Strandberg, A.; Skoglund, N.; Thyrel, M.; Lestander, T.A.; Broström, M.; Backman, R. Time-Resolved Study of Silicate Slag Formation During Combustion of Wheat Straw Pellets. Energy Fuels 2019, 33, 2308–2318. [Google Scholar] [CrossRef]
- Boström, D.; Skoglund, N.; Grimm, A.; Boman, C.; Öhman, M.; Broström, M.; Backman, R. Ash Transformation Chemistry during Combustion of Biomass. Energy Fuels 2012, 26, 85–93. [Google Scholar] [CrossRef]
- Deng, H.; Wang, X.-M.; Du, C.; Shen, X.-C.; Cui, F.-Z. Combined effect of ion concentration and functional groups on surface chemistry modulated CaCO3 crystallization. CrystEngComm 2012, 14, 6647–6653. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- World Health Organization. Agents Classified by the IARC Monographs, World Health Organization. International Agency for Research on Cancer. 2016. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 28 October 2021).


| Crop | Theoretical Potential of Residue (Mt/Year) | Technical Potential of Residue (Mt/Year) |
|---|---|---|
| Cassava peeling P | 3.6 | 0.72 |
| Yam straw F | 3.2 | 2.5 |
| Coconut husk P | 0.12 | 0.12 |
| Corncob P | 0.49 | 0.49 |
| Corn husk P | 0.34 | 0.34 |
| CasP | YamP | CocH | CorC | CorH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | U | L | U | L | U | L | U | L | U | L |
| VM | wt.% db | 77.1 | 79.7 | 79 | 82.4 | 68.6 | 79.2 | 79.9 | 86.6 | 82 | 87.4 |
| AC | wt.% db | 5.5 | 3.59 | 4.57 | 2.38 | 5.04 | 0.93 | 2.32 | 0.74 | 1.85 | 1.04 |
| MC | wt.% wb | 9.96 | 4.23 | 13.6 | 2.71 | 10.7 | 1.73 | 5.34 | 3.53 | 9.9 | 5.18 |
| LHV | MJ/kg db | 16.52 | 17.3 | 16.36 | 16.64 | 18.12 | 18.8 | 17.49 | 17.68 | 17.14 | 17.41 |
| C | wt.% db | 45 | 47.3 | 44.4 | 45.3 | 49.3 | 50.1 | 47.7 | 47.6 | 47.4 | 46.6 |
| H | wt.% db | 5.76 | 5.93 | 5.82 | 6.23 | 5.24 | 5.92 | 5.91 | 5.86 | 5.87 | 5.81 |
| N | wt.% db | 1.09 | 1.04 | 1.01 | 1.02 | 0.54 | 0.4 | 0.66 | 0.58 | 0.26 | 0.23 |
| O | wt.% db | 49.12 | 46.77 | 49.69 | 48.37 | 45.36 | 43.98 | 46.39 | 46.54 | 46.73 | 47.59 |
| S | wt.% db | 0.12 | n.d | 0.09 | 0.1 | 0.1 | n.d | n.d | n.d | n.d | n.d |
| Fuel ash analysis | |||||||||||
| Al2O3 | wt.% db | 5.67 | 8.46 | 1.76 | 2.87 | 0.84 | 1.57 | 0.51 | 0.79 | 1.00 | 0.68 |
| CaO | wt.% db | 14.80 | 30.31 | 3.91 | 8.07 | 4.96 | 16.76 | 2.23 | 9.82 | 6.65 | 10.69 |
| Fe2O3 | wt.% db | 2.23 | 3.03 | 0.65 | 2.11 | 0.92 | 2.07 | 0.87 | 2.98 | 0.02 | 0.84 |
| K2O | wt.% db | 40.84 | 1.83 | 46.66 | 17.67 | 61.98 | 4.24 | 62.44 | 8.21 | 33.60 | 1.61 |
| MgO | wt.% db | 3.96 | 2.62 | 2.22 | 1.97 | 4.67 | 3.07 | 2.97 | 2.49 | 0.00 | 2.67 |
| MnO | wt.% db | 0.14 | 0.08 | 0.08 | 0.12 | 0.06 | 0.04 | 0.10 | 0.05 | 8.50 | 0.05 |
| Na2O | wt.% db | 0.36 | 1.10 | 0.43 | 1.04 | 4.45 | 2.96 | 0.09 | 2.23 | 0.18 | 1.72 |
| P2O5 | wt.% db | 4.35 | 2.94 | 11.15 | 8.88 | 4.48 | 5.81 | 3.98 | 4.64 | 0.02 | 1.16 |
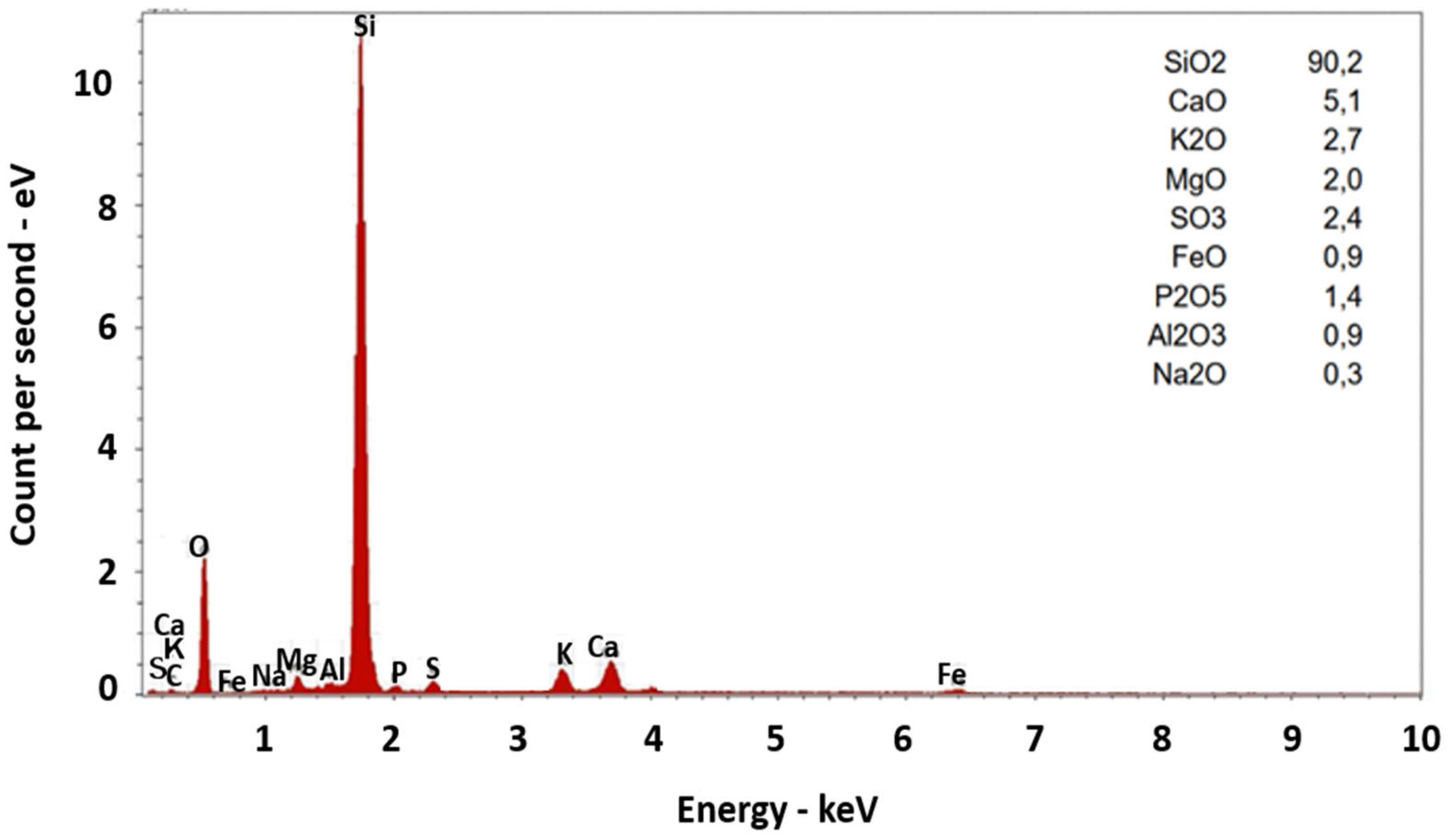
| SiO2 | wt.% db | 20.01 | 38.62 | 26.70 | 45.22 | 11.53 | 44.57 | 20.82 | 49.53 | 44.13 | 70.74 |
| SO3 | wt.% db | 6.82 | 9.68 | 5.98 | 11.23 | 5.86 | 18.17 | 5.67 | 18.52 | 5.27 | 9.37 |
| Others * | wt.% db | 0.82 | 1.33 | 0.45 | 0.82 | 0.25 | 0.74 | 0.31 | 0.75 | 0.61 | 0.47 |
| Wavenumber (cm−1) | Functional Groups | Compounds | Reported Values (cm−1) | References |
|---|---|---|---|---|
| 3600–3000 | O-H stretching | Acid, methanol | 3600–3000 | Yang et al. [60] |
| 2900–3000 | C-H stretching | Alkyl, aliphatic, aromatic | 2860–2970 | Yang et al. [60] |
| 1402–1415 | OH bending, CO32−, CH bending | Acid and CaCO3 | 1440–1400, 1400–1460, 1417–1425 | Magdziarz et al. [77] Nieves et al. [78] Ceylan et al. [65] Yang et al. [60] |
| 1159–1108; 1027–1051 | C–O–C stretching vibration | Pyranose ring skeletal | 1170, 1082 | Yang et al. [60] |
| 1035–1125 | C–O stretching, C–O deformation, OH association and Si–O–Si | C–OH (ethanol), SO42− and SiO2 | 1035–1065, 1108 | Nana et al. [79] Ma et al. [80] Nieves et al. [78] Yang et al. [60] |
| 956–972 | Si–O–Si | SiO2 | 956–972 | Bathla et al. [81] Morrow et al. [82] Mohanraj et al. [83] |
| C–H and stretching vibration of CO32− | Aromatic hydrogen and CaCO3 | 700–900 | Ennaciri et al. [84] Bonfim et al. [26] Medina et al. [85] Liou et al. [86] | |
| 778–799 | Si–O–Si | SiO2 | 796, 798 | Frías et al. [87] |
| 711 | Stretching vibration of C–O | CaCO3 | 713, 709 | Leng et al. [88] Ennaciri et al. [84] |
| 400–600 | C–C stretching | Aromatic hydrogen | 700–400 | Yang et al., 2007 [60] |
| Sample | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|
| Untreated | ||
| CasP-U-A | 3 | 0 |
| YamP-U-A | 2 | 0 |
| CocH-U-A | 0 | 0 |
| CorC-U-A | 2 | 0.01 |
| CorH-U-A | 9 | 0.04 |
| 1 w/v % CA | ||
| CasP-A-1% | 16 | 0.06 |
| YamP-A-1% | 7 | 0.16 |
| CocH-A-1% | 20 | 0.05 |
| CorC-A-1% | 47 | 0.09 |
| CorH-A-1% | 67 | 0.17 |
| 5 w/v % CA | ||
| CasP-A-5% | 26 | 0.10 |
| YamP-A-5% | 5 | 0.02 |
| CocH-A-5% | 56 | 0.14 |
| CorC-A-5% | 70 | 0.14 |
| CorH-A-5% | 91 | 0.21 |
| Ashes | |||||
|---|---|---|---|---|---|
| Phase | CasP-A-1% | YamP-A-1% | CocH-A-1% | CorC-A-1% | CorH-A-1% |
| Anhydrite [Ca(SO4)] | x | x | x | ||
| Quartz (SiO2) | x | x | x | x | x |
| Arcanite (K2SO4) | x | x | |||
| Sylvite (KCl) | x | ||||
| Calcite (CaCO3) | x | x | x | ||
| Albite [Na(AlSi3O8)] | x | ||||
| Sanidine (KAlSi3O8) | x | x | x | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prempeh, C.O.; Formann, S.; Schliermann, T.; Dizaji, H.B.; Nelles, M. Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa. Appl. Sci. 2021, 11, 10363. https://doi.org/10.3390/app112110363
Prempeh CO, Formann S, Schliermann T, Dizaji HB, Nelles M. Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa. Applied Sciences. 2021; 11(21):10363. https://doi.org/10.3390/app112110363
Chicago/Turabian StylePrempeh, Clement Owusu, Steffi Formann, Thomas Schliermann, Hossein Beidaghy Dizaji, and Michael Nelles. 2021. "Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa" Applied Sciences 11, no. 21: 10363. https://doi.org/10.3390/app112110363
APA StylePrempeh, C. O., Formann, S., Schliermann, T., Dizaji, H. B., & Nelles, M. (2021). Extraction and Characterization of Biogenic Silica Obtained from Selected Agro-Waste in Africa. Applied Sciences, 11(21), 10363. https://doi.org/10.3390/app112110363








