A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
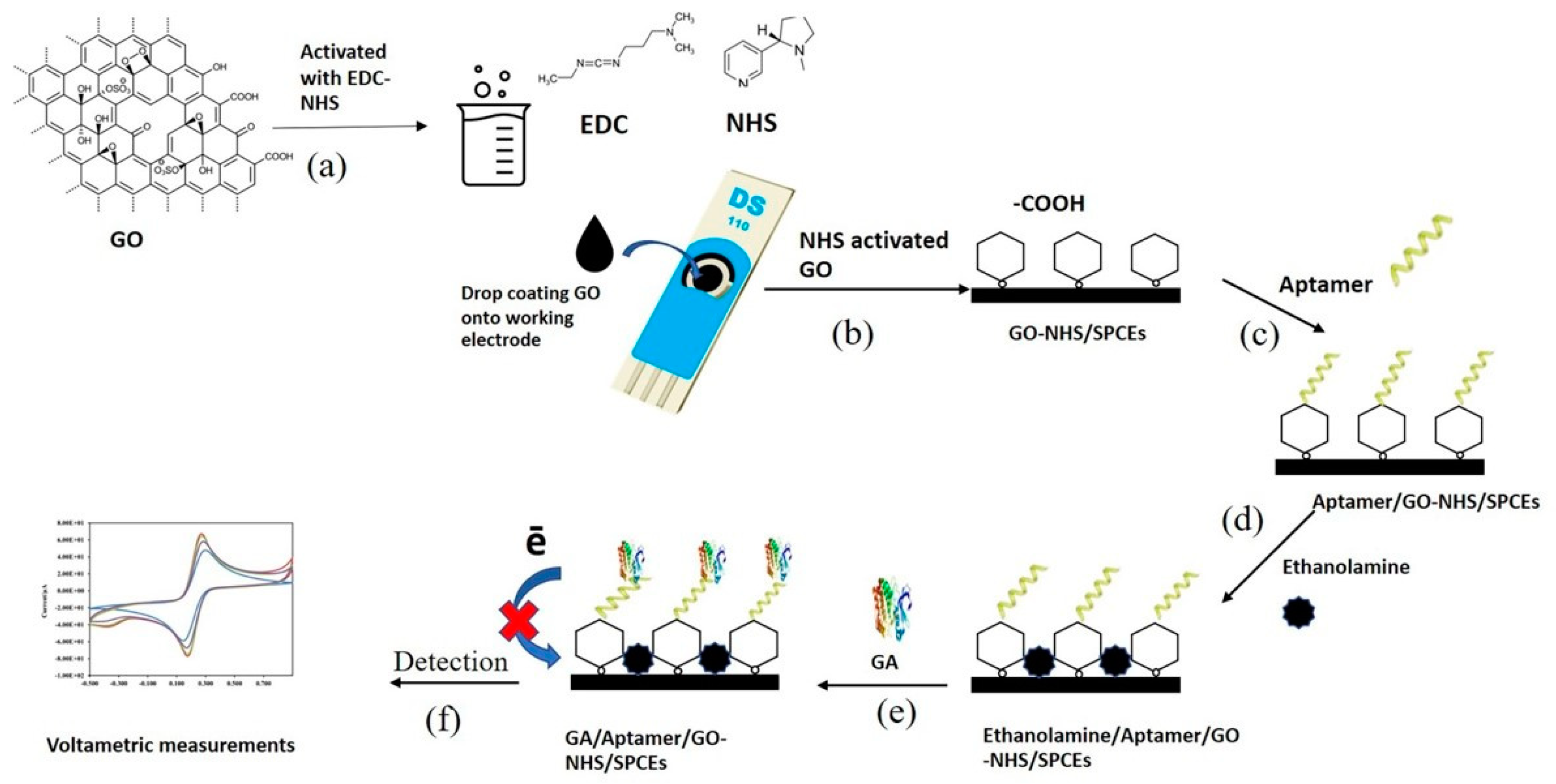
2.3. Functionalization of GO
2.4. Fabrication of the Electrochemical Aptasensor
2.5. Electrochemical Analysis
2.6. Clinical Sample Analysis
2.7. Statistical Analysis
3. Results and Discussion
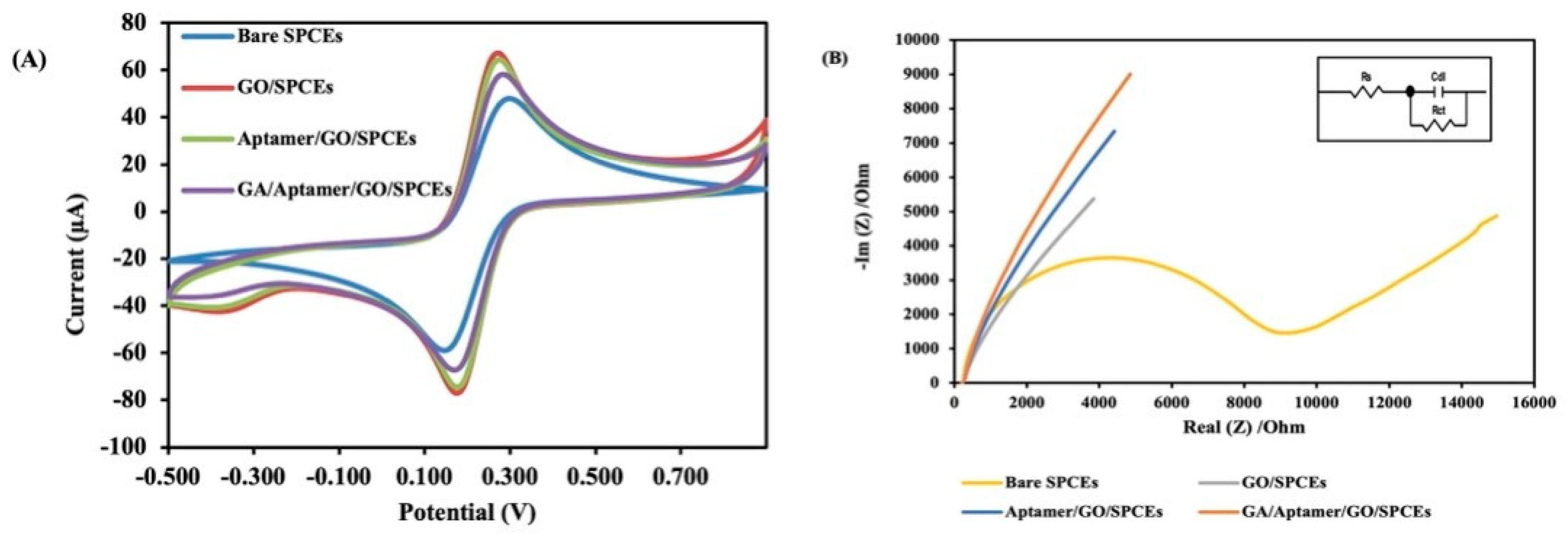
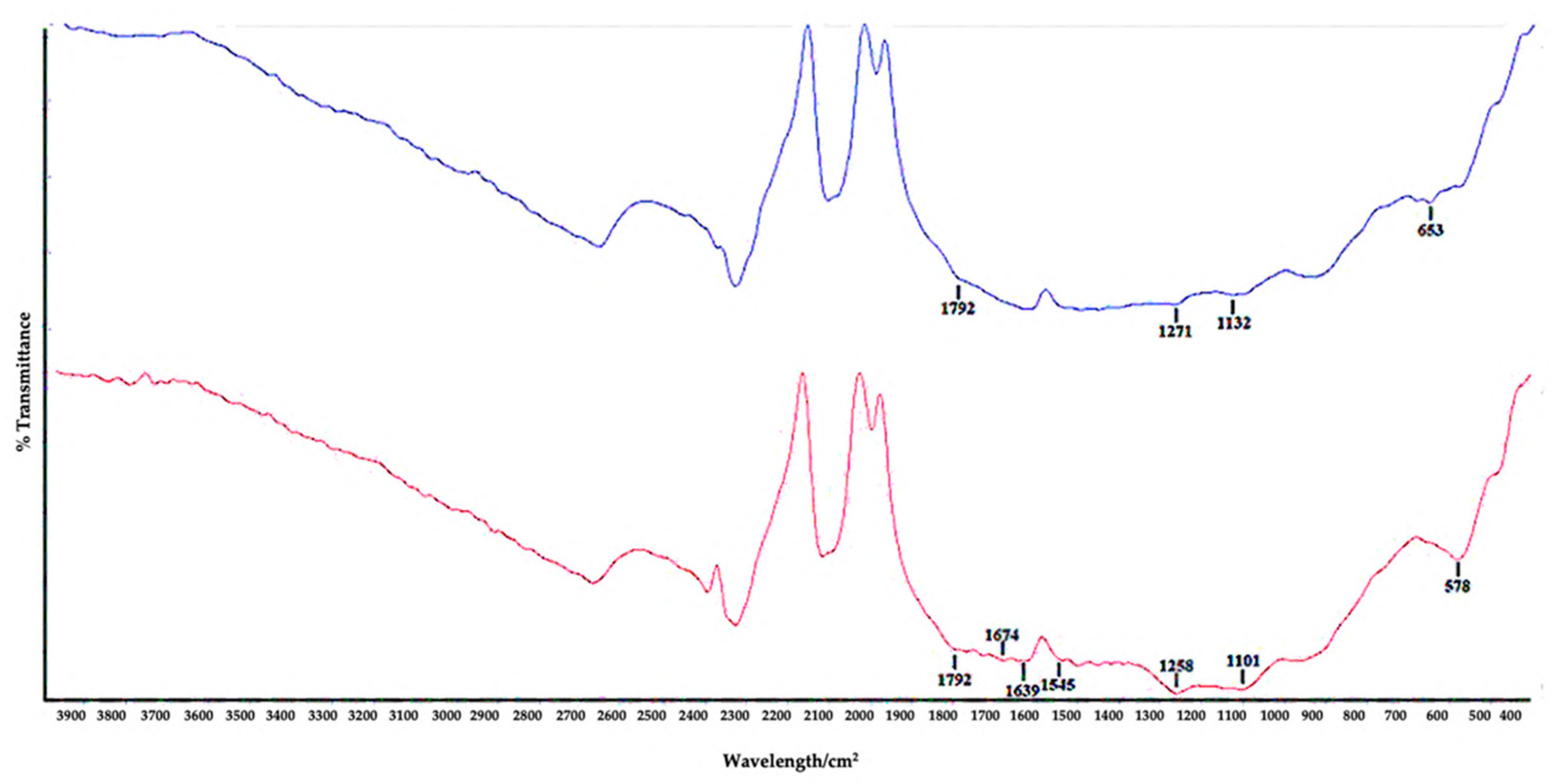
3.1. Characterization of the Electrochemical Aptasensor
3.2. Optimization of the Electrochemical Aptasensor
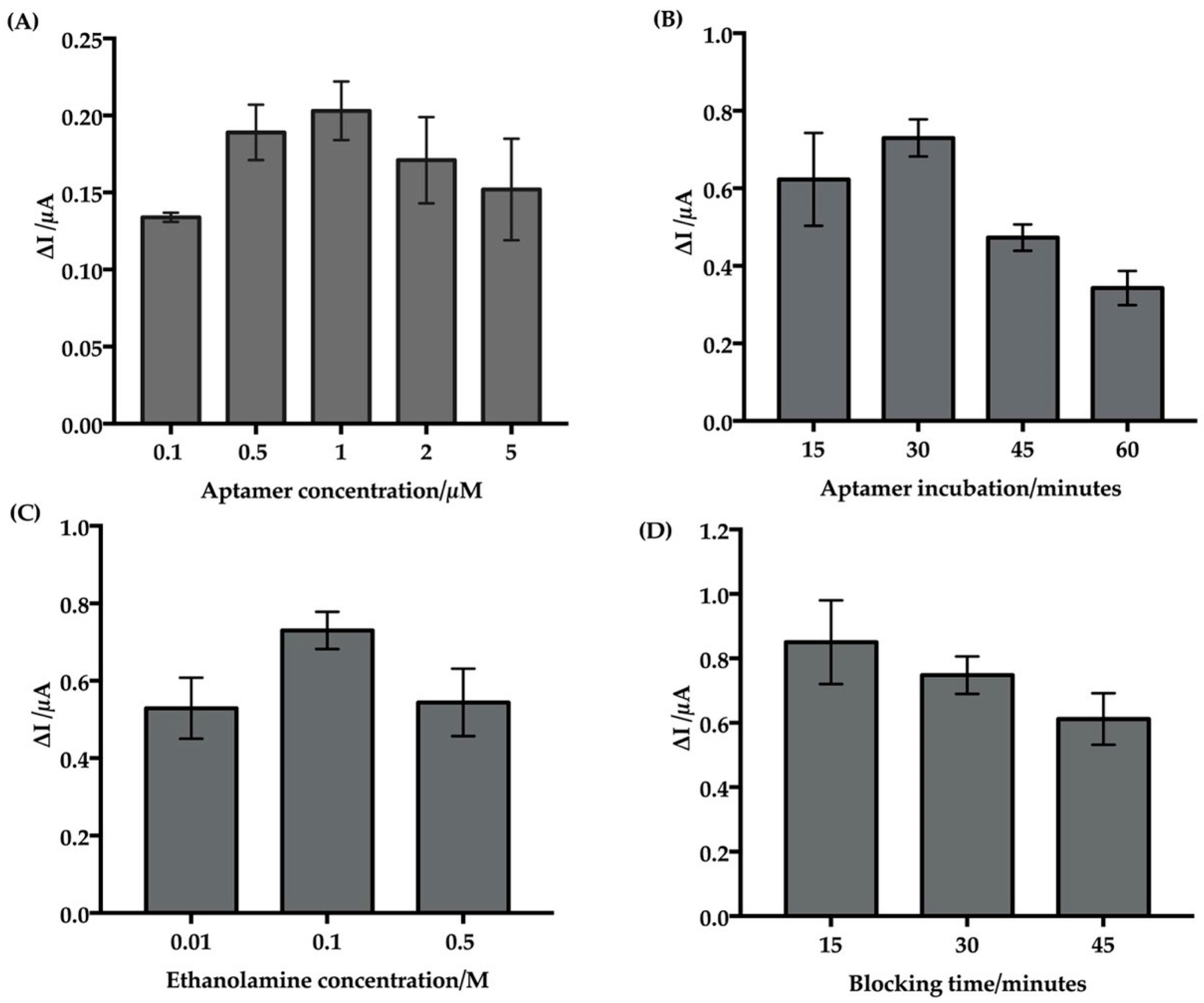
3.2.1. Optimization of Aptamer Immobilization
3.2.2. Optimization of Blocking and Reaction Time
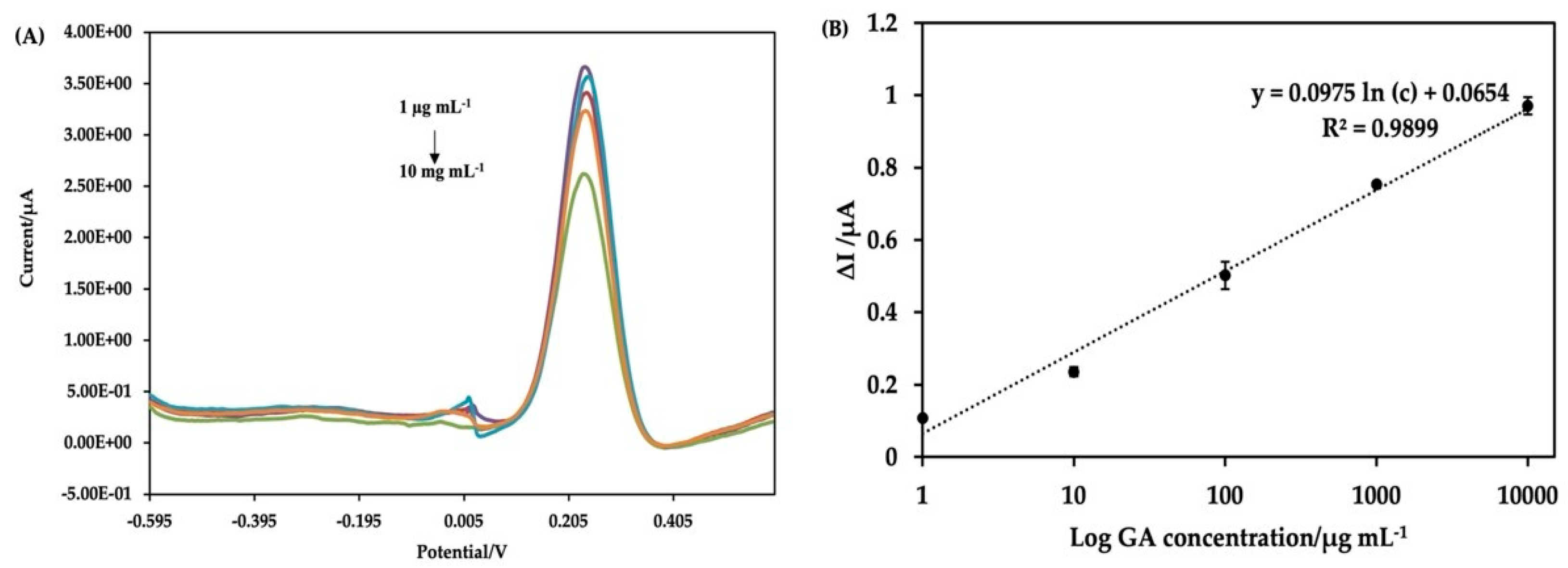
3.3. Analytical Performance of the Electrochemical Aptasensor
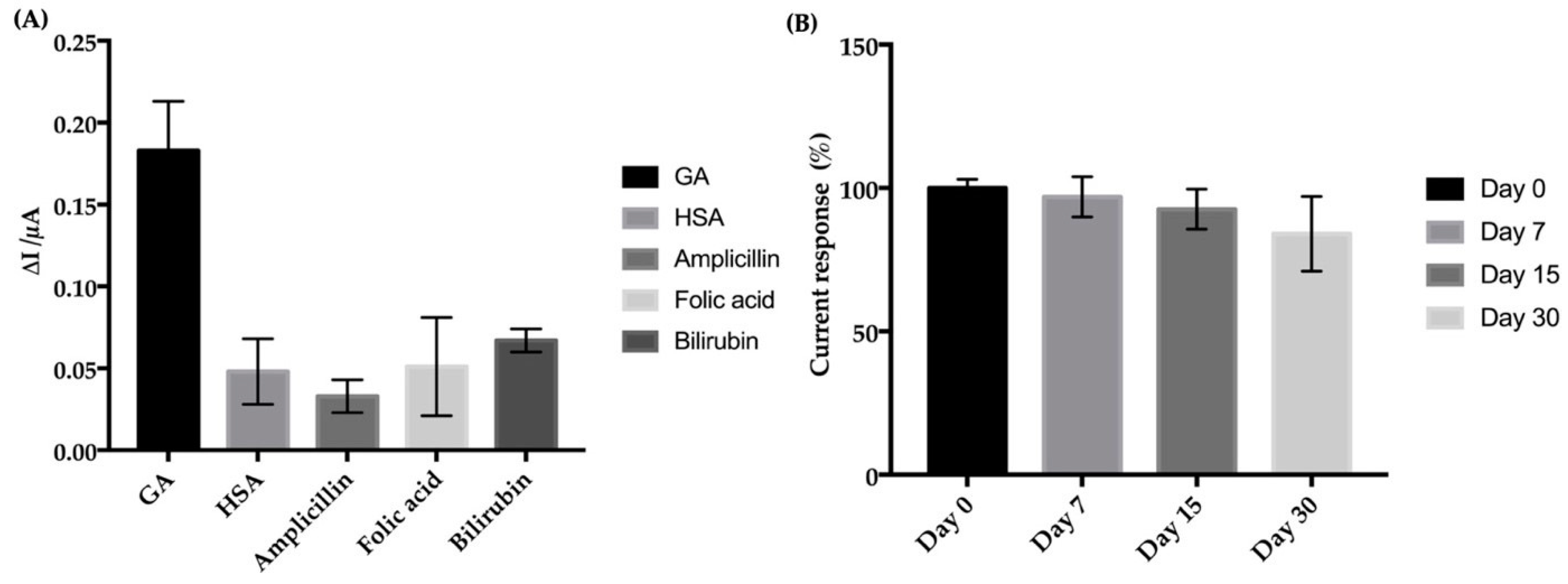
3.4. Specificity of the Electrochemical Aptasensor
3.5. Reproducibility and Stability of the Electrochemical Aptasensor
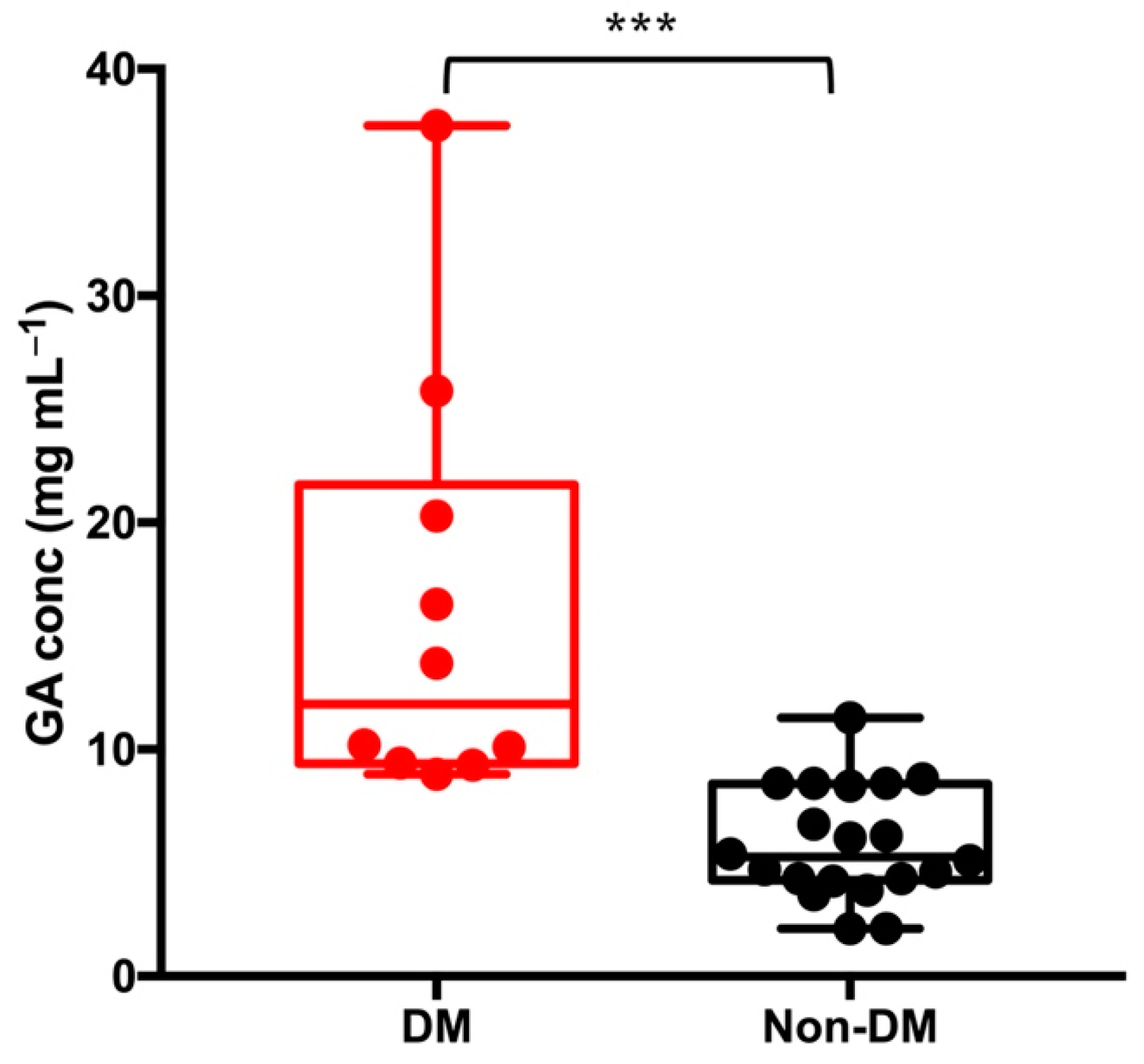
3.6. Clinical Sample Analysis
3.7. Within-Run and Between-Run Coefficients of Variations (CVs)
3.8. Method Comparison with a Commercial ELISA Kit in Clinical Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Hashimoto, K.; Murai, J.; Saito, H.; Mukai, M.; Ikegame, K.; Ogawa, H.; Kasayama, S. Usefulness of Glycated Albumin as an Indicator of Glycemic Control Status in Patients with Hemolytic Anemia. Clin. Chim. Acta 2011, 412, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Nouvenne, A.; Lippi, G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J. Diabetes Sci. Technol. 2015, 9, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Roohk, H.V.; Zaidi, A.R. A Review of Glycated Albumin as an Intermediate Glycation Index for Controlling Diabetes. J. Diabetes Sci. Technol. 2008, 2, 1114–1121. [Google Scholar] [CrossRef] [Green Version]
- Rondeau, P.; Bourdon, E. The Glycation of Albumin: Structural and Functional Impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.J.S.; Ames, J.M. Analysis of Glycated Protein by Capillary Electrophoresis. Int. Congr. Ser. 2002, 1245, 471–474. [Google Scholar] [CrossRef]
- Zhernovaya, O.S.; Tuchin, V.V.; Meglinski, I.V. Monitoring of Blood Proteins Glycation by Refractive Index and Spectral Measurements. Laser Phys. Lett. 2008, 5, 460. [Google Scholar] [CrossRef]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman Spectroscopy Provides a Powerful Diagnostic Tool for Accurate Determination of Albumin Glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef] [Green Version]
- Apiwat, C.; Luksirikul, P.; Kankla, P.; Pongprayoon, P.; Treerattrakoon, K.; Paiboonsukwong, K.; Fucharoen, S.; Dharakul, T.; Japrung, D. Graphene Based Aptasensor for Glycated Albumin in Diabetes Mellitus Diagnosis and Monitoring. Biosens. Bioelectron. 2016, 82, 140–145. [Google Scholar] [CrossRef]
- Sasar, M.; Farzadfard, A.; Abdi, Y.; Habibi-Rezaei, M. Detection of Glycated Albumin Using a Novel Field Effect Aptasensor. IEEE Sens. J. 2020, 20, 10387–10392. [Google Scholar] [CrossRef]
- Bunyarataphan, S.; Dharakul, T.; Fucharoen, S.; Paiboonsukwong, K.; Japrung, D. Glycated Albumin Measurement Using an Electrochemical Aptasensor for Screening and Monitoring of Diabetes Mellitus. Electroanalysis 2019, 31, 2254–2261. [Google Scholar] [CrossRef]
- Farzadfard, A.; Shayeh, J.S.; Habibi-Rezaei, M.; Omidi, M. Modification of Reduced Graphene/Au-Aptamer to Develop an Electrochemical Based Aptasensor for Measurement of Glycated Albumin. Talanta 2020, 211, 120722. [Google Scholar] [CrossRef]
- Waiwinya, W.; Putnin, T.; Pimalai, D.; Chawjiraphan, W.; Sathirapongsasuti, N.; Japrung, D. Immobilization-Free Electrochemical Sensor Coupled with a Graphene-Oxide-Based Aptasensor for Glycated Albumin Detection. Biosensors 2021, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Pereira Morais, M.P.; Kassaar, O.; Flower, S.E.; Williams, R.J.; James, T.D.; van den Elsen, J.M.H. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. In Electrophoretic Separation of Proteins: Methods and Protocols; Kurien, B.T., Scofield, R.H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 161–175. ISBN 978-1-4939-8793-1. [Google Scholar]
- Gopinath, S.C.B. Aptamers. In Encyclopedia of Analytical Chemistry; American Cancer Society: Newark, NJ, USA, 2016; pp. 1–33. ISBN 978-0-470-02731-8. [Google Scholar]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.-H. Aptamers as a Replacement for Antibodies in Enzyme-Linked Immunosorbent Assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Tamiya, E. Electrochemical Biosensors for Medical and Food Applications. Electroanalysis 2008, 20, 616–626. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Aptamer-Based Assay of Biomolecules: Recent Advances in Electro-Analytical Approach. TrAC Trends Anal. Chem. 2017, 89, 119–132. [Google Scholar] [CrossRef]
- Reich, P.; Preuß, J.-A.; Bahner, N.; Bahnemann, J. Impedimetric aptamer-based biosensors: Principles and techniques. In Aptamers in Biotechnology; Urmann, K., Walter, J.-G., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–41. ISBN 978-3-030-54061-6. [Google Scholar]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Settu, K.; Liu, J.-T.; Chen, C.-J.; Tsai, J.-Z. Development of Carbon−graphene-Based Aptamer Biosensor for EN2 Protein Detection. Anal. Biochem. 2017, 534, 99–107. [Google Scholar] [CrossRef]
- Hermanson, G.T. (Ed.) Chapter 3—The reactions of bioconjugation. In Bioconjugate Techniques, 3rd ed.; Academic Press: Boston, MA, USA, 2013; pp. 229–258. ISBN 978-0-12-382239-0. [Google Scholar]
- Staros, J.V.; Wright, R.W.; Swingle, D.M. Enhancement by N-Hydroxysulfosuccinimide of Water-Soluble Carbodiimide-Mediated Coupling Reactions. Anal. Biochem. 1986, 156, 220–222. [Google Scholar] [CrossRef]
- Janolino, V.G.; Swaisgood, H.E. Analysis and Optimization of Methods Using Water-Soluble Carbodiimide for Immobilization of Biochemicals to Porous Glass. Biotechnol. Bioeng. 1982, 24, 1069–1080. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luong, J.H. Recent Advances in Electrochemical Biosensing Schemes Using Graphene and Graphene-Based Nanocomposites. Carbon 2015, 84, 519–550. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. A Voltammetric Sensor Based on Chemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrode for the Simultaneous Analysis of Uric Acid, Ascorbic Acid and Dopamine. Chemosensors 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A. Polydopamine-Functionalized Graphene Nanoplatelet Smart Conducting Electrode for Bio-Sensing Applications. Arab. J. Chem. 2020, 13, 1669–1677. [Google Scholar] [CrossRef]
- Cyclic Voltammetry; Simulation and Analysis of Reaction Mechanisms. Synth. React. Inorg. Met.-Org. Chem. 1994, 24, 1237–1238. [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct Electrochemistry of Glucose Oxidase and Biosensing for Glucose Based on Graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable Aqueous Dispersions of Graphitic Nanoplatelets via the Reduction of Exfoliated Graphite Oxide in the Presence of Poly(Sodium 4-Styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-Soluble Graphene Covalently Functionalized by Biocompatible Poly-L-Lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Tertiş, M.; Hosu, O.; Fritea, L.; Farcau, C.; Cernat, A.; Săndulescu, R.; Cristea, C. A Novel Label-Free Immunosensor Based on Activated Graphene Oxide for Acetaminophen Detection. Electroanalysis 2015, 27, 638–647. [Google Scholar] [CrossRef]
- Xu, H.; Mao, X.; Zeng, Q.; Wang, S.; Kawde, A.-N.; Liu, G. Aptamer-Functionalized Gold Nanoparticles as Probes in a Dry-Reagent Strip Biosensor for Protein Analysis. Anal. Chem. 2009, 81, 669–675. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods, and Applications; Oxford Science Publications; Oxford University Press: Oxford, UK; New York, NY, USA, 1993; ISBN 978-0-19-855389-2. [Google Scholar]
- Dijksma, M.; Kamp, B.; Hoogvliet, J.C.; Van Bennekom, W.P. Development of an Electrochemical Immunosensor for Direct Detection of Interferon-γ at the Attomolar Level. Anal. Chem. 2001, 73, 901–907. [Google Scholar] [CrossRef]
- Bardea, A.; Katz, E.; Willner, I. Biosensors with Amperometric Detection of Enzymatically Controlled PH-Changes. Electroanalysis 2000, 12, 731–735. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of Electrochemical Impedance Spectroscopy in Protein Biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Chen, Z.; Mu, X.; Guo, L. Aptamer Biosensor for Label-Free Square-Wave Voltammetry Detection of Angiogenin. Biosens. Bioelectron. 2011, 30, 261–266. [Google Scholar] [CrossRef]
- Kohzuma, T.; Yamamoto, T.; Uematsu, Y.; Shihabi, Z.K.; Freedman, B.I. Basic Performance of an Enzymatic Method for Glycated Albumin and Reference Range Determination. J. Diabetes Sci. Technol. 2011, 5, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Inoue, M.; Saito, M.; Yoshikawa, H.; Tamiya, E. Sensitive Detection of Glycated Albumin in Human Serum Albumin Using Electrochemiluminescence. Anal. Chem. 2017, 89, 5909–5915. [Google Scholar] [CrossRef]
- Ghosh, S.; Datta, D.; Cheema, M.; Dutta, M.; Stroscio, M.A. Aptasensor Based Optical Detection of Glycated Albumin for Diabetes Mellitus Diagnosis. Nanotechnology 2017, 28, 435505. [Google Scholar] [CrossRef]
- Reed, P.; Bhatnagar, D.; Dhar, H.; Winocour, P.H. Precise Measurement of Glycated Serum Albumin by Column Affinity Chromatography and Immunoturbidimetry. Clin. Chim. Acta 1986, 161, 191–199. [Google Scholar] [CrossRef]
- Kim, A.-R.; Choi, Y.; Kim, S.-H.; Moon, H.-S.; Ko, J.-H.; Yoon, M.-Y. Development of a Novel SsDNA Sequence for a Glycated Human Serum Albumin and Construction of a Simple Aptasensor System Based on Reduced Graphene Oxide (RGO). Biosensors 2020, 10, 141. [Google Scholar] [CrossRef]
- Sonntag, O.; Scholer, A. Drug Interference in Clinical Chemistry: Recommendation of Drugs and Their Concentrations to Be Used in Drug Interference Studies. Ann. Clin. Biochem. 2001, 38, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Karnes, H.T.; March, C. Precision, Accuracy, and Data Acceptance Criteria in Biopharmaceutical Analysis. Pharm. Res. 1993, 10, 1420–1426. [Google Scholar] [CrossRef]
| No. | Method | Limit of Detection (LOD) | Linear Response Range | Reference |
|---|---|---|---|---|
| 1. | Enzymatic assay-based electrochemiluminescence sensor | 6.6 µg mL−1 | 6.6–660 µg mL−1 | [41] |
| 2. | Graphene-based optical aptasensor | 50 µg mL−1 | 0.05–3 mg mL−1 | [10] |
| 3. | Electrochemical aptasensor | 0.003 µg mL−1 | 0.002–16 mg mL−1 | [12] |
| 4. | Optical aptasensor | 0.067 µg mL−1 | 0.067–967 µg mL−1 | [42] |
| 5. | Electrochemical aptasensor | 0.07 µg mL−1 | 8–36 mg mL−1 | [13] |
| 6. | Enzymatic assay-based sensor | 0.36 µg mL−1 | 0–0.6 mg mL−1 | [40] |
| 7. | Ramen spectroscopy-based sensor | 13.7 µM (0.9 mg mL−1) | 7–250 µM | [9] |
| 8. | Affinity chromatography-based immunoturbidimetric sensor | 0.81 mg mL−1 | ND | [43] |
| 9. | Immobilization-free electrochemical aptasensor | 8.7 ng mL−1 | 0.01–50 µg mL−1 | [14] |
| 10. | rGO-based aptasensor | 16.40 µg mL−1 | 0–125 µg mL−1 | [44] |
| 11. | GO-based electrochemical aptasensor | 0.031 µg mL−1 | 0.001–10 mg mL−1 | This study |
| GA Concentration Spiked in Serum (µg mL−1) | Measured Concentration (µg mL−1) | Recovery (%) | RSD (%) (n = 5) |
|---|---|---|---|
| 49.50 | 48.90 ± 0.01 | 98.7 | 2.1 |
| 233.00 | 214.80 ± 0.02 | 92.2 | 2.6 |
| GA (mg mL−1) | |||||
|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error | Min | Max | |
| Diabetic (n = 10) | 16.17 | 9.36 | 2.96 | 8.90 | 37.50 |
| Non-diabetic (n = 20) | 5.86 | 2.47 | 0.55 | 2.10 | 11.40 |
| Sample | ELISA (mg mL −1) | Aptasensor (mg mL −1) | t-Test a | F-Test b |
|---|---|---|---|---|
| 1 | 1.60 ± 0.08 | 2.10 ± 0.04 | 0.10 | 4.90 |
| 2 | 2.30 ± 0.02 | 2.10 ± 0.05 | 0.80 | 0.80 |
| 3 | 8.70 ± 0.02 | 8.50 ± 0.09 | 0.40 | 1.00 |
| 4 | 10.10 ± 0.04 | 10.00 ± 0.06 | 0.60 | 0.25 |
| 5 | 36.90 ± 0.06 | 37.50 ± 0.04 | 0.12 | 3.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aye, N.N.S.; Maraming, P.; Tavichakorntrakool, R.; Chaibunruang, A.; Boonsiri, P.; Daduang, S.; Teawtrakul, N.; Prasongdee, P.; Amornkitbamrung, V.; Daduang, J. A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin. Appl. Sci. 2021, 11, 10315. https://doi.org/10.3390/app112110315
Aye NNS, Maraming P, Tavichakorntrakool R, Chaibunruang A, Boonsiri P, Daduang S, Teawtrakul N, Prasongdee P, Amornkitbamrung V, Daduang J. A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin. Applied Sciences. 2021; 11(21):10315. https://doi.org/10.3390/app112110315
Chicago/Turabian StyleAye, Nang Noon Shean, Pornsuda Maraming, Ratree Tavichakorntrakool, Attawut Chaibunruang, Patcharee Boonsiri, Sakda Daduang, Nattiya Teawtrakul, Prinya Prasongdee, Vittaya Amornkitbamrung, and Jureerut Daduang. 2021. "A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin" Applied Sciences 11, no. 21: 10315. https://doi.org/10.3390/app112110315
APA StyleAye, N. N. S., Maraming, P., Tavichakorntrakool, R., Chaibunruang, A., Boonsiri, P., Daduang, S., Teawtrakul, N., Prasongdee, P., Amornkitbamrung, V., & Daduang, J. (2021). A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Selective Detection of Glycated Albumin. Applied Sciences, 11(21), 10315. https://doi.org/10.3390/app112110315






