Photocatalytic Degradation of Methylene Blue Dye by Electrospun Binary and Ternary Zinc and Titanium Oxide Nanofibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Synthesis of the Photocatalysts
2.3. Characterization
2.4. Evaluation of the Photocatalytic Activity and Assessment of the Optimal Load
3. Results and discussion
3.1. Morphological and Textural Properties of the Photocatalysts
3.2. Crystalline Phase, Spatial Homogeneity, and Crystallinity Degree of the Oxide
3.3. Effect of the Variation of the Ti:Zn Molar Ratio in the Precursor Solution on the Photocatalytic Activity of the Resulting Electrospun Oxide NFs
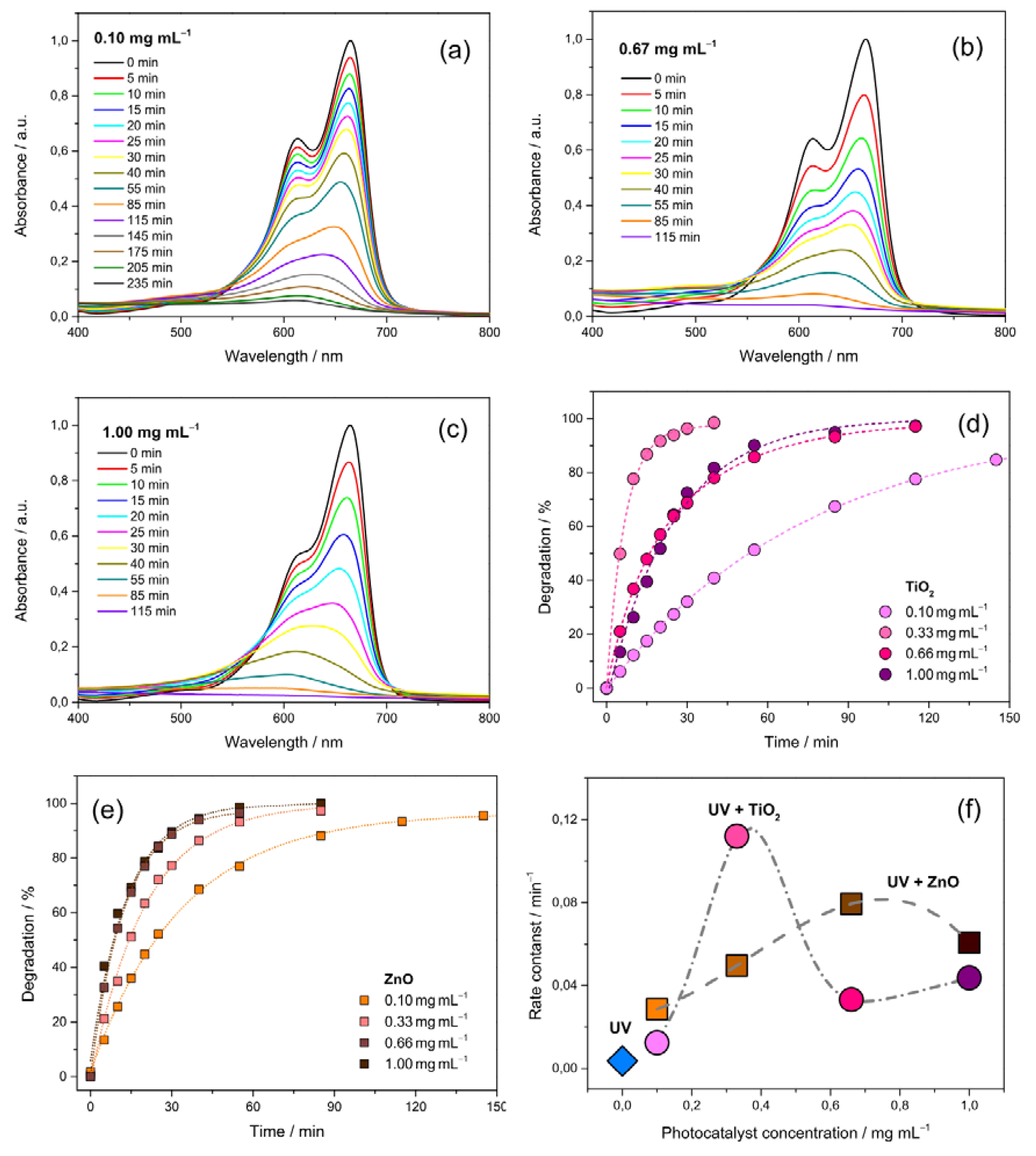
3.4. Effect of the Photocatalyst Load
3.5. On the Best Performing Oxide NFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UN. Historic New Sustainable Development Agenda Unanimously Adopted by 193 UN Members. 2015. Available online: https://www.un.org/sustainabledevelopment/blog/2015/09/historic-new-sustainable-development-agenda-unanimously-adopted-by-193-un-members (accessed on 1 September 2021).
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of methylene blue from textile industrial wastewater onto activated carbon of Parthenium hysterophorus. Int. J. Environ. Res. 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Ghanbarian, M.; Nasseri, S.; Khairi, A. Mineralization and discoloration of textile wastewater by TiO2 nanoparticles. Desalination 2009, 239, 309–316. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef] [PubMed]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Methylene Blue Market Size, Industry Analysis Report, Regional Outlook, Application Development Potential, Price Trends, Competitive Market Share & Forecast, 2021–2027. Available online: https://www.gminsights.com/industry-analysis/methylene-blue-market (accessed on 30 July 2021).
- Singh, J.; Chang, Y.Y.; Koduru, J.R.; Yang, J.K. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, V.; Iervolino, G.; Rizzo, L.; Sannino, D. Advanced oxidation processes for the removal of food dyes in wastewater. Curr. Org. Chem. 2017, 21, 1068–1073. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Sales, H.B.; Menezes, R.R.; Neves, G.A.; de Souza, J.J.; Ferreira, J.M.; Chantelle, L.; Lira, H.D.L. Development of sustainable heterogeneous catalysts for the photocatalytic treatment of effluents. Sustainability 2020, 12, 7393. [Google Scholar] [CrossRef]
- Zheng, F.; Zhu, Z. Flexible, freestanding, and functional SiO2 nanofibrous mat for dye-sensitized solar cell and photocatalytic dye degradation. ACS Appl. Nano Mater. 2018, 1, 1141–1149. [Google Scholar] [CrossRef]
- Ali, G.; Jazib Abbas Zaidi, S.; Abdul Basit, M.; Joo Park, T. Synergetic Performance of Systematically Designed g-C3N4/rGO/SnO2 Nanocomposite for Photodegradation of Rhodamine-B Dye. Appl. Surf. Sci. 2021, 570, 151140. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, K.; Wang, J.; Guo, Y.; Yoshida, A.; Abudula, A.; Guan, G. Cu2O Nanoparticle Hyper-Cross-Linked Polymer Composites for the Visible-Light Photocatalytic Degradation of Methyl Orange. ACS Appl. Nano Mater. 2019, 2, 2706–2712. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Ravichandran, K.; Gracia, F.; Agarwal, S.; Gupta, V.K. Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photo catalysts for degradation of textile dyes. J. Photochem. Photobiol. B 2017, 173, 43–49. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, Y.; Zhang, X.Q.; Li, J.F.; Li, X.; Wang, C.W. Design and fabrication of NiS/LaFeO3 heterostructures for high efficient photodegradation of organic dyes. Appl. Surf. Sci. 2020, 504, 144363. [Google Scholar] [CrossRef]
- Chen, H.; Motuzas, J.; Martens, W.; da Costa, J.C.D. Degradation of orange II dye under dark ambient conditions by MeSrCuO (Me = Mg and Ce) metal oxides. Sep. Purif. Technol. 2018, 205, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Mageshwari, K.; Sathyamoorthy, R.; Lee, J.Y.; Park, J. Novel CuCr2O4 embedded CuO nanocomposites for efficient photodegradation of organic dyes. Appl. Surf. Sci. 2015, 353, 95–102. [Google Scholar] [CrossRef]
- Ramar, V.; Balasubramanian, K. Reduced Graphene Oxide/WO3 Nanorod Composites for Photocatalytic Degradation of Methylene Blue under Sunlight Irradiation. ACS Appl. Nano Mater. 2021. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Aytac, Z.; Uyar, T. Grain boundary engineering in electrospun ZnO nanostructures as promising photocatalysts. CrystEngComm 2016, 18, 6341–6351. [Google Scholar] [CrossRef] [Green Version]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic degradation of methylene blue dye by porous zinc oxide nanofibers prepared via electrospinning: When defects become merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis of hexagonal ZnO nanoplates used for photodegradation of methylene blue. Optik 2021, 226, 165949. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Ishchenko, O.M.; Lamblin, G.; Guillot, J.; Infante, I.C.; Guennou, M.; Adjeroud, N.; Lenoble, D. Mesoporous TiO2 anatase films for enhanced photocatalytic activity under UV and visible light. RSC Adv. 2020, 10, 38233–38243. [Google Scholar] [CrossRef]
- Chekir, N.; Benhabiles, O.; Tassalit, D.; Laoufi, N.A.; Bentahar, F. Photocatalytic degradation of methylene blue in aqueous suspensions using TiO2 and ZnO. Desalin. Water Treat. 2016, 57, 6141–6147. [Google Scholar] [CrossRef]
- Lee, C.G.; Na, K.H.; Kim, W.T.; Park, D.C.; Yang, W.H.; Choi, W.Y. TiO2/ZnO nanofibers prepared by electrospinning and their photocatalytic degradation of methylene blue compared with TiO2 nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.T.; Na, K.H.; Park, D.C.; Yang, W.H.; Choi, W.Y. Photocatalytic Methylene Blue Degradation of Electrospun Ti–Zn Complex Oxide Nanofibers. Nanomaterials 2020, 10, 1311. [Google Scholar] [CrossRef]
- Someswararao, M.V.; Dubey, R.S.; Subbarao, P.S.V. Electrospun composite nanofibers prepared by varying concentrations of TiO2/ZnO solutions for photocatalytic applications. J. Photochem. Photobiol. A 2021, 6, 100016. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Manna, A.K.; Soni, R.K. Fabrication of ZnO–TiO2 nanohybrids for rapid sunlight driven photodegradation of textile dyes and antibiotic residue molecules. Opt. Mater. 2020, 107, 110138. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, H.; Hu, S.; Li, J. Preparation and enhanced photoelectrochemical performance of coupled bicomponent ZnO−TiO2 nanocomposites. J. Phys. Chem. C 2008, 112, 117–122. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.Y.; Chen, R.Y.; Chen, Z.; Han, H.C. Coaxial nanofibers of ZnO-TiO2 heterojunction with high photocatalytic activity by electrospinning technique. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 449–453. [Google Scholar] [CrossRef]
- Kumar, K.; Chitkara, M.; Sandhu, I.S.; Mehta, D.; Kumar, S. Photocatalytic, optical and magnetic properties of Fe-doped ZnO nanoparticles prepared by chemical route. J. Alloys Compd. 2014, 588, 681–689. [Google Scholar] [CrossRef]
- Habba, Y.G. Capochichi-Gnambodoe, M.; Leprince-Wang, Y. Enhanced photocatalytic activity of iron-doped ZnO nanowires for water purification. Appl. Sci. 2017, 7, 1185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Enhanced photocatalytic activity of iron doped zinc oxide nanowires for water decontamination. Surf. Coat. Technol. 2013, 217, 119–123. [Google Scholar] [CrossRef]
- Wahab, R.; Hwang, I.H.; Kim, Y.S.; Shin, H.S. Photocatalytic activity of zinc oxide micro-flowers synthesized via solution method. Chem. Eng. J. 2011, 168, 359–366. [Google Scholar] [CrossRef]
- Triolo, C.; Fazio, E.; Neri, F.; Mezzasalma, A.M.; Trusso, S.; Patanè, S. Correlation between structural and electrical properties of PLD prepared ZnO thin films used as a photodetector material. Appl. Surf. Sci. 2015, 359, 266–271. [Google Scholar] [CrossRef]
- Santangelo, S. Electrospun nanomaterials for energy applications: Recent advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Sekar, A.D.; Manickam, M. Current trends of electrospun nanofibers in water and wastewater treatment. In Water and Wastewater Treatment Technologies; Springer: Singapore, 2019; pp. 469–485. [Google Scholar] [CrossRef]
- Malara, A.; Frontera, P.; Bonaccorsi, L.; Antonucci, P.L. Hybrid zeolite SAPO-34 fibres made by electrospinning. Materials 2018, 11, 2555. [Google Scholar] [CrossRef] [Green Version]
- Busacca, C.; Donato, A.; Faro, M.L.; Malara, A.; Neri, G.; Trocino, S. CO gas sensing performance of electrospun Co3O4 nanostructures at low operating temperature. Sens. Actuators B Chem. 2020, 303, 127193. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shi, X.; Gautrot, J.E.; Peijs, T. Nanoengineered electrospun fibers and their biomedical applications: A review. Nanocomposites 2021, 7, 1–34. [Google Scholar] [CrossRef]
- Folino, A.; Triolo, C.; Petrovičová, B.; Pantò, F.; Zema, D.A.; Santangelo, S. Evaluation of Electrospun Self-Supporting Paper-Like Fibrous Membranes as Oil Sorbents. Membranes 2021, 11, 515. [Google Scholar] [CrossRef]
- Ponti, A.; Raza, M.H.; Pantò, F.; Ferretti, A.M.; Triolo, C.; Patane, S.; Pinna, N.; Santangelo, S. Structure, Defects, and Magnetism of Electrospun Hematite Nanofibers Silica-Coated by Atomic Layer Deposition. Langmuir 2020, 36, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Rafidah, J.; Mohd-Sahaid, K.; Norliza, A.R.; Aidil, A.H.; Mohd-Farid, A. Effect of sodium hydroxide pretreatment on chemical composition of treated acacia mangium using response surface methodology. J. Trop. For. Sci. 2020, 32, 391–401. [Google Scholar] [CrossRef]
- Santangelo, S.; Patanè, S.; Frontera, P.; Pantò, F.; Triolo, C.; Stelitano, S.; Antonucci, P. Effect of calcium-and/or aluminum-incorporation on morphological, structural and photoluminescence properties of electro-spun zinc oxide fibers. Mater. Res. Bull. 2017, 92, 9–18. [Google Scholar] [CrossRef]
- He, G.; Cai, Y.; Zhao, Y.; Wang, X.; Lai, C.; Xi, M.; Fong, H. Electrospun anatase-phase TiO2 nanofibers with different morphological structures and specific surface areas. J. Colloid Interface Sci. 2013, 398, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, K.S.; Uyar, T. Conscientious design of Zn-S/Ti-N layer by transformation of ZnTiO3 on electrospun ZnTiO3@ TiO2 nanofibers: Stability and reusable photocatalytic performance under visible irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12980–12992. [Google Scholar] [CrossRef]
- Mebrek, A.; Alleg, S.; Benayache, S.; Benabdeslem, M. Preparation and characterization of spinel type Zn2TiO4 nanocomposite. Ceram. Int. 2018, 44, 10921–10928. [Google Scholar] [CrossRef]
- Arin, J.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Template synthesis of Zn2TiO4 and Zn2Ti3O8 nanorods by hydrothermal-calcination combined processes. Mater. Lett. 2017, 193, 270–273. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Sheikh, F.A.; Barakat, N.A.; Li, X.; Kim, H.Y.; Chronakis, I.S. Zinc oxide’s hierarchical nanostructure and its photocatalytic properties. Appl. Surf. Sci. 2012, 258, 3695–3702. [Google Scholar] [CrossRef] [Green Version]
- Otieno, F.; Airo, M.; Erasmus, R.M.; Billing, D.G.; Quandt, A.; Wamwangi, D. Structural and spectroscopic analysis of ex-situ annealed RF sputtered aluminium doped zinc oxide thin films. J. Appl. Phys. 2017, 122, 075303. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Du, Y.L.; Deng, Y.; Zhang, M.S. Variable-temperature Raman scattering study on anatase titanium dioxide nanocrystals. J. Phys. Chem. Solids 2006, 67, 2405–2408. [Google Scholar] [CrossRef]
- Arin, J.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Characterization of ZnO–TiO2 and zinc titanate nanoparticles synthesized by hydrothermal process. Rev. Chem. Intermed. 2017, 43, 3183–3195. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Pizá-Ruiz, P.; Chávez-Flores, D.; Ogaz-Parada, J.; Amézaga-Madrid, P.; Vega-Ríos, A.; Miki-Yoshida, M. On the discoloration of methylene blue by visible light. J. Fluoresc. 2019, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Zalani, N.; Koozegar Kaleji, B.; Mazinani, B. Synthesis and characterisation of the mesoporous ZnO-TiO2 nanocomposite; Taguchi optimisation and photocatalytic methylene blue degradation under visible light. Mater. Technol. 2020, 35, 281–289. [Google Scholar] [CrossRef]
- Choudhury, B.; Bayan, S.; Choudhury, A.; Chakraborty, P. Narrowing of band gap and effective charge carrier separation in oxygen deficient TiO2 nanotubes with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2016, 465, 1–10. [Google Scholar] [CrossRef]
- Montenegro, D.N.; Hortelano, V.; Martínez, O.; Martínez-Tomas, M.C.; Sallet, V.; Muñoz-Sanjosé, V.; Jiménez, J. Non-radiative recombination centres in catalyst-free ZnO nanorods grown by atmospheric-metal organic chemical vapour deposition. J. Phys. D Appl. Phys. 2013, 46, 235302. [Google Scholar] [CrossRef]
- Sarkar, K.; Braden, E.V.; Fröschl, T.; Hüsing, N.; Müller-Buschbaum, P. Spray-deposited zinc titanate films obtained via sol–gel synthesis for application in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 15008–15014. [Google Scholar] [CrossRef] [Green Version]
- Mekasuwandumrong, O.; Pawinrat, P.; Praserthdam, P.; Panpranot, J. Effects of Synthesis Conditions and Annealing Post-Treatment on the Photocatalytic Activities of ZnO Nanoparticles in the Degradation of Methylene Blue Dye. Chem. Eng. J. 2010, 164, 77–84. [Google Scholar] [CrossRef]
- Al-Shamali, S.S. Photocatalytic degradation of methylene blue in the presence of TiO2 catalyst assisted solar radiation. Aust. J. Basic Appl. Sci. 2013, 7, 172–176. [Google Scholar]
- Ali, A.S.; Khan, I.; Zhang, B.; Nomura, K.; Homonnay, Z.; Kuzmann, E.; Kubuki, S. Photo-Fenton degradation of methylene blue using hematite-enriched slag under visible light. J. Radioanal. Nucl. Chem. 2020, 325, 537–549. [Google Scholar] [CrossRef]
- Le, T.K.; Flahaut, D.; Martinez, D.D.; Nguyen, H.K.H.; Huynh, T.K.X. Study of the effects of surface modification by thermal shock method on photocatalytic activity of TiO2 P25. Appl. Catal. B. Environ. 2015, 165, 260–268. [Google Scholar] [CrossRef]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Fan, G.; Zhan, J.; Luo, J.; Zhang, J.; Chen, Z.; You, Y. Photocatalytic degradation of naproxen by a H2O2-modified titanate nanomaterial under visible light irradiation. Catal. Sci. Technol. 2019, 9, 4614–4628. [Google Scholar] [CrossRef]
- Zhu, K.R.; Zhang, M.S.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Balaji, S.Y.D.J.R.; Djaoued, Y.; Robichaud, J. Phonon confinement studies in nanocrystalline anatase-TiO2 thin films by micro Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1416–1422. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M. Fabbri, F.; Cappelli, S.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2-and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
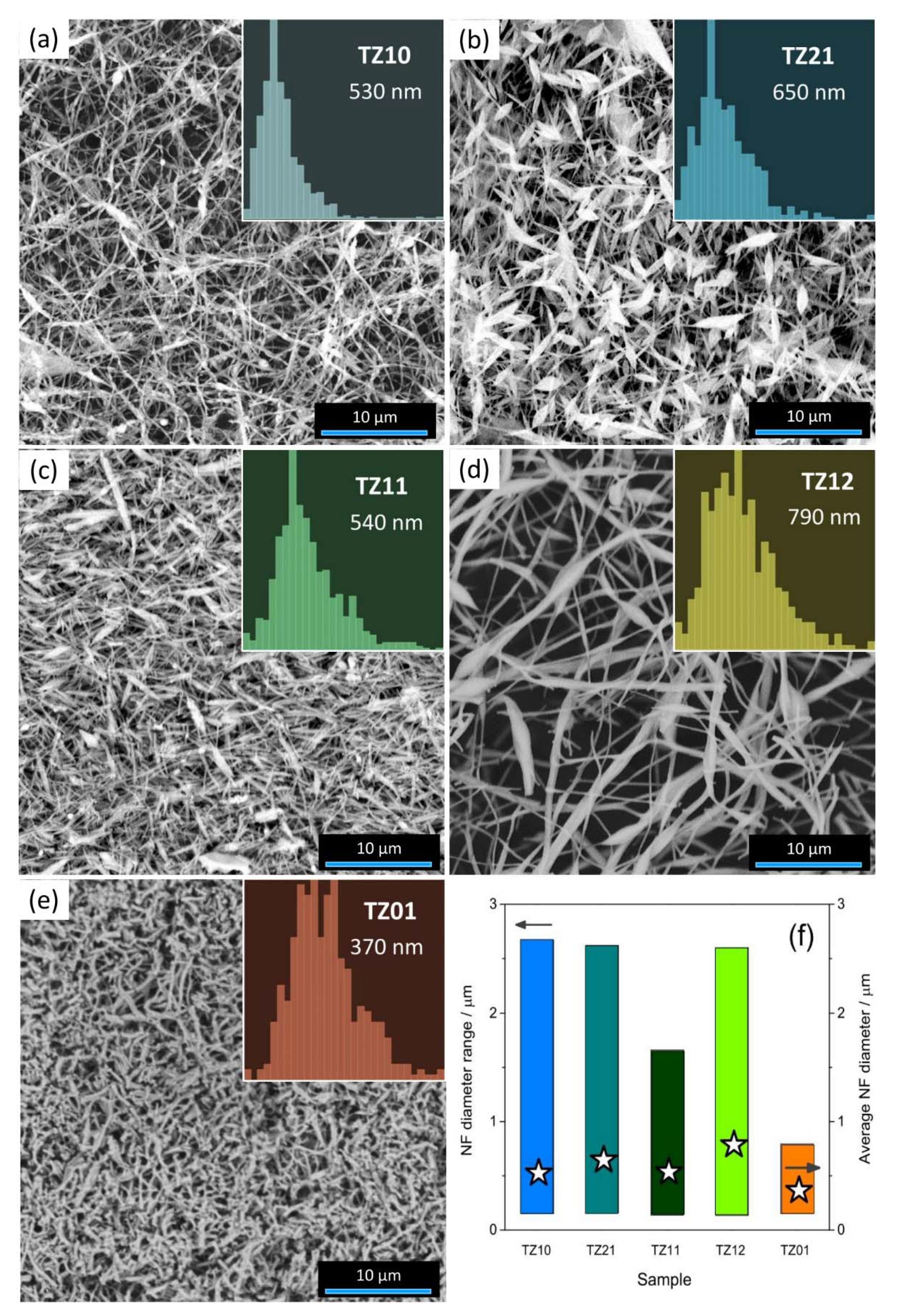

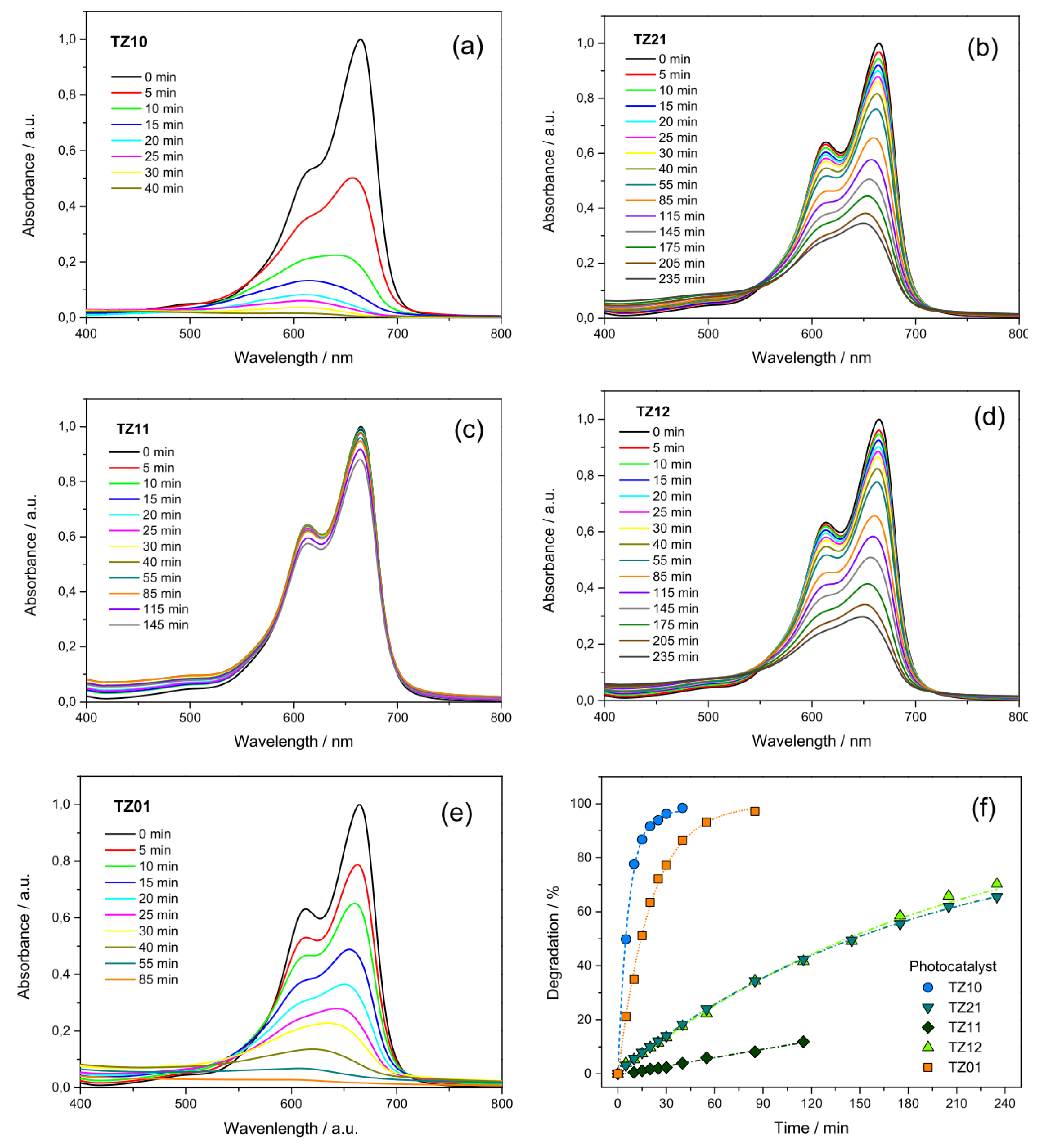

| Sample Code | TiBO/mmol | ZnAc2/mmol | Resulting Phase(s) |
|---|---|---|---|
| TZ10 | 2.60 | 0.00 | a-TiO2 |
| TZ21 | 1.73 | 0.87 | ZnTiO3 (a-TiO2 + r-TiO2) |
| TZ11 | 1.30 | 1.30 | disordered Zn2TiO4 (r-TiO2) |
| TZ12 | 0.87 | 1.73 | Zn2TiO4 (r-TiO2) |
| TZ01 | 0.00 | 2.60 | ZnO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovičová, B.; Dahrouch, Z.; Triolo, C.; Pantò, F.; Malara, A.; Patanè, S.; Allegrini, M.; Santangelo, S. Photocatalytic Degradation of Methylene Blue Dye by Electrospun Binary and Ternary Zinc and Titanium Oxide Nanofibers. Appl. Sci. 2021, 11, 9720. https://doi.org/10.3390/app11209720
Petrovičová B, Dahrouch Z, Triolo C, Pantò F, Malara A, Patanè S, Allegrini M, Santangelo S. Photocatalytic Degradation of Methylene Blue Dye by Electrospun Binary and Ternary Zinc and Titanium Oxide Nanofibers. Applied Sciences. 2021; 11(20):9720. https://doi.org/10.3390/app11209720
Chicago/Turabian StylePetrovičová, Beatrix, Zainab Dahrouch, Claudia Triolo, Fabiola Pantò, Angela Malara, Salvatore Patanè, Maria Allegrini, and Saveria Santangelo. 2021. "Photocatalytic Degradation of Methylene Blue Dye by Electrospun Binary and Ternary Zinc and Titanium Oxide Nanofibers" Applied Sciences 11, no. 20: 9720. https://doi.org/10.3390/app11209720
APA StylePetrovičová, B., Dahrouch, Z., Triolo, C., Pantò, F., Malara, A., Patanè, S., Allegrini, M., & Santangelo, S. (2021). Photocatalytic Degradation of Methylene Blue Dye by Electrospun Binary and Ternary Zinc and Titanium Oxide Nanofibers. Applied Sciences, 11(20), 9720. https://doi.org/10.3390/app11209720











