Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief
Abstract
1. Introduction
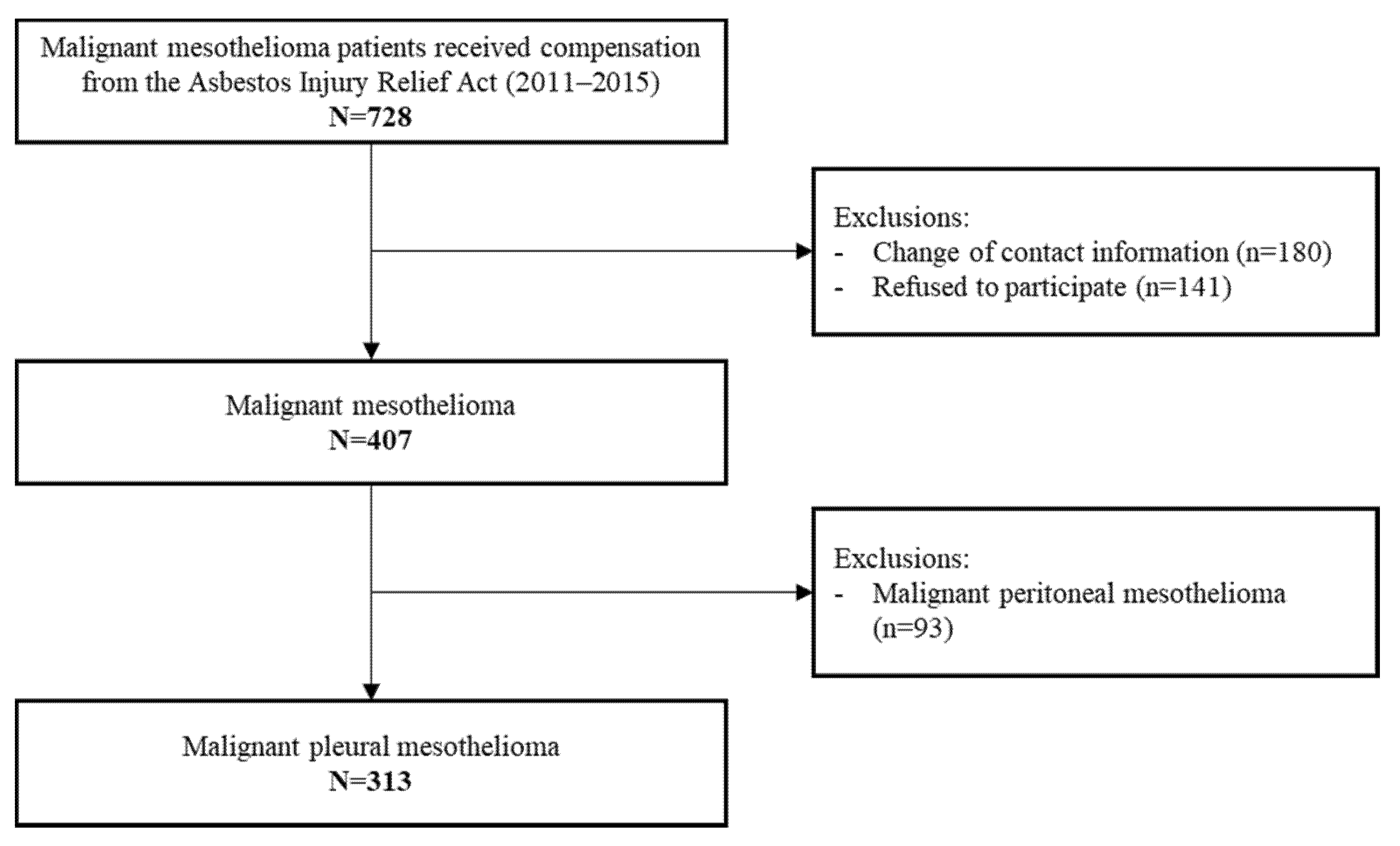
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.R.; Ahn, Y.-S.; Jung, S.-H. Epidemiologic Characteristics of Malignant Mesothelioma in Korea. J. Korean Med. Assoc. 2009, 52, 449. [Google Scholar] [CrossRef][Green Version]
- Milano, M.T.; Zhang, H. Malignant pleural mesothelioma: A population-based study of survival. J. Thorac. Oncol. 2010, 5, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.-W.; Kim, H.R. Occupational and Environmental Asbestos Exposure in Korea. J. Korean Med. Assoc. 2009, 52, 442–448. [Google Scholar] [CrossRef][Green Version]
- Kwak, K.; Cho, S.-I.; Paek, D. Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038. Int. J. Environ. Res. Public Health 2021, 18, 6614. [Google Scholar] [CrossRef] [PubMed]
- An, Y.S.; Kim, H.D.; Kim, H.C.; Jeong, K.S.; Ahn, Y.S. The characteristics of asbestos-related disease claims made to the Korea Workers’ Compensation and Welfare Service (KCOMWEL) from 2011 to 2015. Ann. Occup. Environ. Med. 2018, 30, 45. [Google Scholar] [CrossRef]
- Ahn, Y.-S.; Kang, S.-K. Asbestos-related Occupational Cancers Compensated under the Industrial Accident Compensation Insurance in Korea. Ind. Health 2009, 47, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Taioli, E.; Wolf, A.S.; Flores, R.M. Meta-analysis of survival after pleurectomy decortication versus extra-pleural pneumonectomy in mesothelioma. Ann. Thorac. Surg. 2015, 99, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomized feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- Lee, K.; Godderis, L.; Furuya, S.; Kim, Y.; Kang, D. Comparison of Asbestos Victim Relief Available Outside of Conventional Occupational Compensation Schemes. Int. J. Environ. Res. Public Health 2021, 18, 5236. [Google Scholar] [CrossRef]
- Jung, S.-H.; Kim, H.-R.; Koh, S.-B.; Yong, S.-J.; Chung, M.J.; Lee, C.-H.; Han, J.; Eom, M.-S.; Oh, S.-S. A decade of malignant mesothelioma surveillance in Korea. Am. J. Ind. Med. 2012, 55, 869–875. [Google Scholar] [CrossRef]
- Moore, A.J.; Parker, R.J.; Wiggins, J. Malignant mesothelioma. Orphanet J. Rare Dis. 2008, 3, 1–11. [Google Scholar] [CrossRef]
- Montanaro, F.; Rosato, R.; Gangemi, M.; Roberti, S.; Ricceri, F.; Merler, E.; Gennaro, V.; Romanelli, A.; Chellini, E.; Pascucci, C.; et al. Survival of pleural malignant mesothelioma in Italy: A population-based study. Int. J. Cancer 2009, 124, 201–207. [Google Scholar] [CrossRef]
- Beckett PEdwards, J.; Fennell, D.; Hubbarde, R.; Woolhouse, I. Demographics, management and survival of patients with malignant pleural mesothelioma in the National Lung Cancer Audit in England and Wales. Lung Cancer 2015, 88, 344–348. [Google Scholar] [CrossRef]
- Enewold, L.; Sharon, E.; Thomas, A. Patterns of care and survival among patients with malignant mesothelioma in the United States. Lung Cancer 2017, 112, 102–108. [Google Scholar] [CrossRef]
- Edwards, J.G.; Abrams, K.; Leverment, J.N.; Spyt, T.J.; Waller, D.A.; O’Byrne, K.J. Prognostic factors for malignant mesothelioma in 142 patients: Validation of CALGB and EORTC prognostic scoring systems. Thorax 2000, 55, 731–735. [Google Scholar] [CrossRef]
- Kanazawa, N.; Ioka, A.; Tsukuma, H.; Ajiki, W.; Oshima, A. Incidence and Survival of Mesothelioma in Osaka, Japan. Jpn. J. Clin. Oncol. 2006, 36, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Chouaid, C.; Assié, J.B.; Andujar, P.; Blein, C.; Tournier, C.; Vainchtock, A.; Scherpereel, A.; Monnet, I.; Pairon, J.C. Determinants of malignant pleural mesothelioma survival and burden of disease in France: A national cohort analysis. Cancer Med. 2018, 7, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Lee, L.J.-H.; Yuan, C.-T.; Chen, T.W.-W.; Yang, J.C.-H. Prognostic factors and treatment outcomes of malignant pleural mesothelioma in Eastern Asian patients—A Taiwanese study. J. Formos. Med. Assoc. 2018, 118 Pt 2, 230–236. [Google Scholar] [CrossRef]
- Faig, J.; Howard, S.; Levine, E.A.; Casselman, G.; Hesdorffer, M.; Ohar, J.A. Changing Pattern in Malignant Mesothelioma Survival. Transl. Oncol. 2015, 8, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Carioli, G.; Bonifazi, M.; Rossi, M.; Zambelli, A.; Franchi, M.; Zocchetti, C.; Gasparini, S.; Corrao, G.; La Vecchia, C.; Negri, E. Management and Survival of Pleural Mesothelioma: A Record Linkage Study. Respiration 2018, 95, 405–413. [Google Scholar] [CrossRef]
- Iyoda, A.; Yusa, T.; Kadoyama, C.; Sasaki, K.; Kimura, H.; Yamakawa, H.; Shiba, M.; Fujisawa, T.; Yoshino, I. Diffuse malignant pleural mesothelioma: A multi-institutional clinicopathological study. Surg. Today 2008, 38, 993–998. [Google Scholar] [CrossRef]
- Alpert, N.; van Gerwen, M.; Flores, R.; Taioli, E. Gender differences in outcomes of patients with mesothelioma. Am. J. Clin. Oncol. 2020, 43, 792–797. [Google Scholar] [CrossRef]
- Van Gerwen, M.; Alpert, N.; Wolf, A.; Ohri, N.; Lewis, E.; Rosenzweig, K.E.; Flores, R.; Taioli, E. Prognostic factors of survival in patients with malignant pleural mesothelioma: An analysis of the National Cancer Database. Carcinogenesis 2019, 40, 529–536. [Google Scholar] [CrossRef]
- Flores, R.M.; Zakowski, M.; Venkatraman, E.; Krug, L.; Rosenzweig, K.; Dycoco, J.; Lee, C.; Yeoh, C.; Bains, M.; Rusch, V. Prognostic Factors in the Treatment of Malignant Pleural Mesothelioma at a Large Tertiary Referral Center. J. Thorac. Oncol. 2007, 2, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.M.; Riedel, E.; Donington, J.S.; Alago, W.; Ihekweazu, U.; Krug, L.; Rosenzweig, K.; Adusumilli, P.S.; Carbone, M.; Pass, H.I. Frequency of Use and Predictors of Cancer-Directed Surgery in the Management of Malignant Pleural Mesothelioma in a Community-Based (Surveillance, Epidemiology, and End Results [SEER]) Population. J. Thorac. Oncol. 2010, 5, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Taioli, E.; Wolf, A.S.; Camacho-Rivera, M.; Flores, R.M. Women With Malignant Pleural Mesothelioma Have a Threefold Better Survival Rate Than Men. Ann. Thorac. Surg. 2014, 98, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.S.; Richards, W.G.; Tilleman, T.R.; Chirieac, L.; Hurwitz, S.; Bueno, R.; Sugarbaker, D.J. Characteristics of Malignant Pleural Mesothelioma in Women. Ann. Thorac. Surg. 2010, 90, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Popa, E.; Brun, E.A.; Cerruto, C.A.; Sugarbaker, P.H. Sex difference in diffuse malignant peritoneal mesothelioma. BJS 2006, 93, 1536–1542. [Google Scholar] [CrossRef]
- Hillerdal, G. Mesothelioma: Cases associated with non-occupational and low dose exposures. Occup. Environ. Med. 1999, 56, 505–513. [Google Scholar] [CrossRef]
- Pinton, G.; Brunelli, E.; Murer, B.; Puntoni, R.; Puntoni, M.; Fennell, D.A.; Gaudino, G.; Mutti, L.; Moro, L. Estrogen receptor-beta affects the prognosis of human malignant mesothelioma. Cancer Res. 2009, 69, 4598–4604. [Google Scholar] [CrossRef]
- Amin, W.; Linkov, F.; Landsittel, D.P.; Silverstein, J.C.; Bashara, W.; Gaudioso, C.; Feldman, M.D.; Pass, H.I.; Melamed, J.; Friedberg, J.S.; et al. Factors influencing malignant mesothelioma survival: A retrospective review of the National Mesothelioma Virtual Bank cohort. F1000Research 2018, 7, 1184. [Google Scholar] [CrossRef]
- Kwak, K.M.; Paek, D.; Hwang, S.-S.; Ju, Y.-S. Estimated future incidence of malignant mesothelioma in South Korea: Projection from 2014 to 2033. PLoS ONE 2017, 12, e0183404. [Google Scholar] [CrossRef]
- Metintas, S.; Metintas, M.; Ucgun, I.; Oner, U. Malignant mesothelioma due to environmental exposure to asbestos: Follow-up of a Turkish cohort living in a rural area. Chest 2002, 122, 2224–2229. [Google Scholar] [CrossRef]
- Lacourt, A.; Gramond, C.; Rolland, P.; Ducamp, S.; Audignon, S.; Astoul, P.; Chamming’S, S.; Ilg, A.G.S.; Rinaldo, M.; Raherison, C.; et al. Occupational and non-occupational attributable risk of asbestos exposure for malignant pleural mesothelioma. Thorax 2014, 69, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.W. Environmental asbestos exposure and risk of mesothelioma. Ann. Transl. Med. 2017, 5, 234. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Rice, D.C.; Niu, J.; Atay, S.; Vaporciyan, A.A.; Antonoff, M.; Hofstetter, W.L.; Walsh, G.L.; Swisher, S.G.; Roth, J.A.; et al. Long-Term Survival Outcomes of Cancer-Directed Surgery for Malignant Pleural Mesothelioma: Propensity Score Matching Analysis. J. Clin. Oncol. 2017, 35, 3354–3362. [Google Scholar] [CrossRef]
- Berzenji, L.; Van Schil, P. Multimodality treatment of malignant pleural mesothelioma. F1000Research 2018, 7, 1681. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, R.R.; Yang, C.-F.J.; Speicher, P.J.; Gulack, B.C.; Hartwig, M.G.; D’Amico, T.A.; Harpole, D.H.; Berry, M.F. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J. Surg. Res. 2015, 196, 23–32. [Google Scholar] [CrossRef] [PubMed]
| Variable | Malignant Pleural Mesothelioma N (%) |
|---|---|
| Sex | |
| Male | 216 (69.0) |
| Female | 97 (31.0) |
| Age(years) | |
| Mean ± SD 1 | 63.1 ± 12.0 |
| Median (95% CI 2) | 64.0 (61.7, 64.4) |
| <50 | 42 (13.4) |
| 50–59 | 71 (22.7) |
| 60–69 | 92 (29.4) |
| 70–79 | 94 (30.0) |
| ≥80 | 14 (4.5) |
| Survival duration(months) | |
| Mean ± SD 1 | 21.7 ± 33.0 |
| Median (95% CI 2) | 8.0 (6.2, 9.8) |
| Overall survival (% (95% CI 2)) | |
| 1 year | 43.5 (37.9, 49.0) |
| 2 year | 23.6 (18.9, 28.4) |
| 5 year | 12.5 (8.9, 16.1) |
| Smoking history | |
| No | 154 (49.2) |
| Yes | 159 (50.8) |
| Surgery | |
| No | 218 (69.6) |
| Yes | 95 (30.4) |
| Chemotherapy | |
| No | 89 (28.4) |
| Yes | 224 (71.6) |
| Year of diagnosis | |
| 1997–2004 | 61 (19.5) |
| 2005–2007 | 67 (21.4) |
| 2008–2010 | 73 (23.3) |
| 2011–2013 | 80 (25.6) |
| 2014–2015 | 32 (10.2) |
| Occupational exposure | |
| No | 133 (42.5) |
| Yes | 180 (57.5) |
| Histological subtype | |
| Epiththelioid | 86 (58.9) |
| Sarcomatoid | 15 (10.3) |
| Biphasic | 20 (13.7) |
| NOS | 25 (17.1) |
| Variable | Malignant Pleural Mesothelioma (n = 313) | ||
|---|---|---|---|
| n | 5-Year Survival (Months) Median (95% CI 1) | p-Value 2 | |
| Total subjects | 313 | 8.0 (6.2, 9.8) | |
| Sex | |||
| Male | 216 | 8.0 (6.2, 9.8) | 0.225 |
| Female | 97 | 9.0 (4.9, 13.1) | |
| Age(years) | |||
| <60 | 113 | 12.0 (8.3, 15.7) | <0.001 |
| 60–69 | 92 | 11.0 (7.3, 15.7) | |
| ≥70 | 108 | 6.0 (4.7, 7.2) | |
| Smoking history | |||
| No | 154 | 9.0 (6.0, 12.0) | 0.790 |
| Yes | 159 | 8.0 (5.7, 10.3) | |
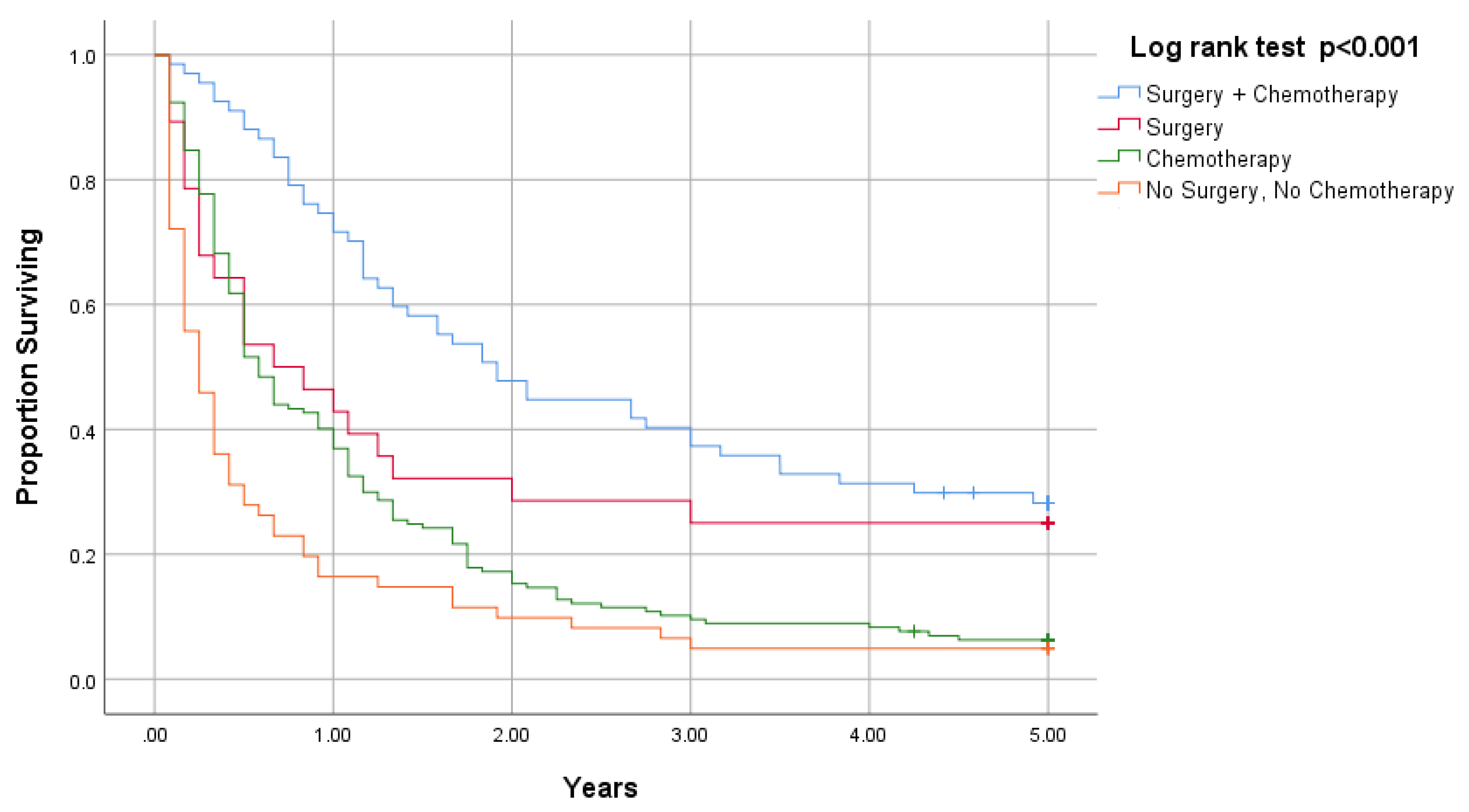
| Treatment | |||
| Surgery and chemotherapy | 67 | 23.0 (16.1, 29.9) | <0.001 |
| Surgery only | 28 | 8.0 (1.1, 14.9) | |
| Chemotherapy only | 157 | 7.0 (5.7, 8.3) | |
| No surgery, no chemotherapy | 61 | 3.0 (1.7, 4.3) | |
| Occupational exposure | |||
| No | 133 | 10.0 (6.5, 13.5) | 0.163 |
| Yes | 180 | 8.0 (5.8, 10.2) | |
| Histological subtype | |||
| Epithelioid | 86 | 10.0 (6.8, 13.3) | 0.342 |
| Nonepithelioid | 60 | 5.0 (2.8, 7.2) | |
| Variables | Adjusted HR (95% CI 1) | p-Value |
|---|---|---|
| Sex (0 = female, 1 = male) | 1.48 (0.86, 2.54) | 0.156 |
| Age | ||
| <60 | Reference | |
| 60–69 | 0.99 (0.61, 1.61) | 0.969 |
| ≥70 | 1.35 (0.85, 2.12) | 0.216 |
| Smoking history (0 = no, 1 = yes) | 0.77 (0.49, 1.20) | 0.244 |
| Occupational exposure (0 = no, 1 = yes) | 1.17 (0.79, 1.76) | 0.456 |
| Treatment | ||
| No surgery, no chemotherapy | Reference | |
| Surgery only | 0.59 (0.29, 1.18) | 0.132 |
| Chemotherapy only | 0.72 (0.44, 1.18) | 0.186 |
| Surgery and chemotherapy | 0.28 (0.15, 0.53) | <0.001 |
| Histological subtype (0 = Nonepithelioid, 1 = Epihelioid) | 0.73 (0.50, 1.06) | 0.102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.-S.; Lee, S.-S.; Kwon, S.-C.; Huh, D.-A.; Lee, Y.-J. Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief. Appl. Sci. 2021, 11, 9713. https://doi.org/10.3390/app11209713
Kang M-S, Lee S-S, Kwon S-C, Huh D-A, Lee Y-J. Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief. Applied Sciences. 2021; 11(20):9713. https://doi.org/10.3390/app11209713
Chicago/Turabian StyleKang, Min-Sung, Sung-Soo Lee, Soon-Chan Kwon, Da-An Huh, and Yong-Jin Lee. 2021. "Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief" Applied Sciences 11, no. 20: 9713. https://doi.org/10.3390/app11209713
APA StyleKang, M.-S., Lee, S.-S., Kwon, S.-C., Huh, D.-A., & Lee, Y.-J. (2021). Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief. Applied Sciences, 11(20), 9713. https://doi.org/10.3390/app11209713






