Engineering Properties of New-Age (Nano) Modified Emulsion (NME) Stabilised Naturally Available Granular Road Pavement Materials Explained Using Basic Chemistry
Abstract
:Featured Application
Abstract
1. Introduction
2. Background
3. Traditional Use of Nanotechnology Products in Road Pavement Engineering
4. Basic Chemistry Applicable to the Understanding of the Successful Introduction of Material Compatible Nanotechnology Solutions for the Treatment, Improvement and/or Stabilisation of Granular Materials for Use in Road Pavement Layers below the Surfacing
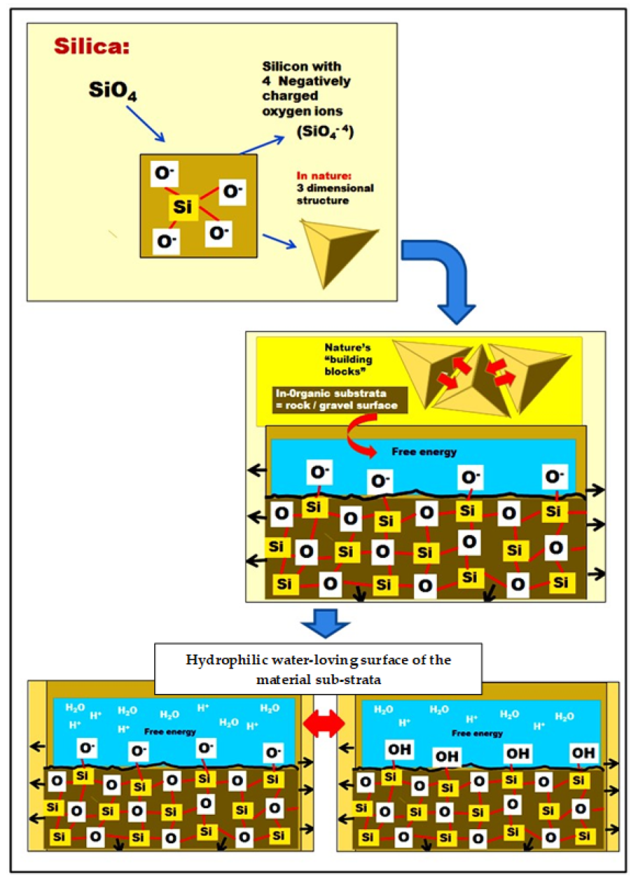
4.1. Silicon Characteristics in the Material Sub-Strata and Applications within the Built Environment
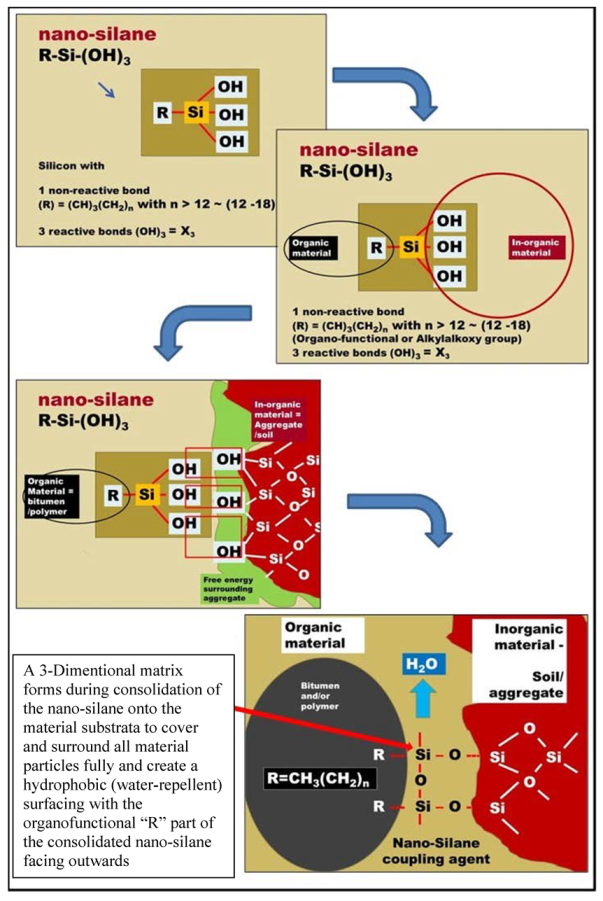
4.2. Chemistry of Organofunctional Nano-Silanes for Application in Pavement Engineering
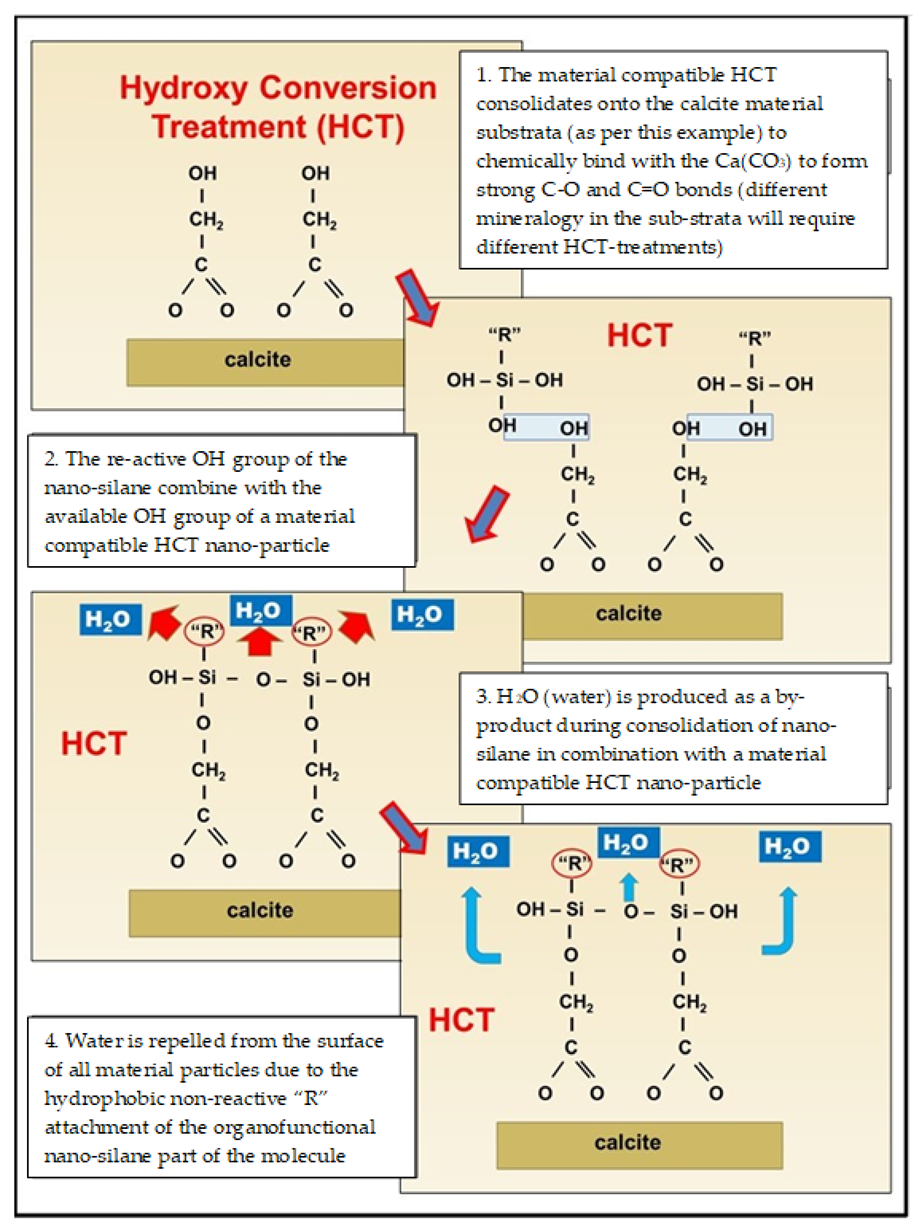
4.3. Modification of Nano-Silanes through Hydroxy Conversion Treatments (HCT) Based on the Mineralogy of the Granular Material Sub-Strata
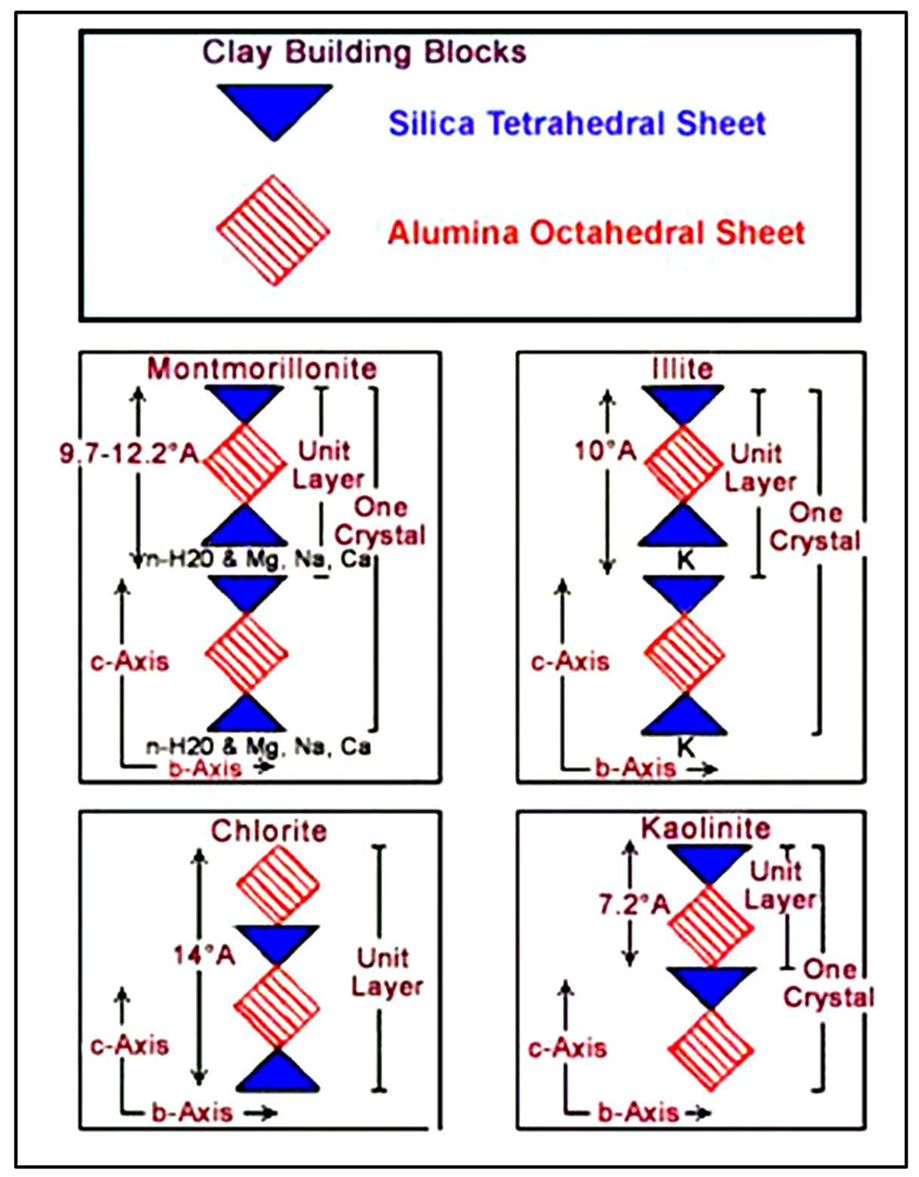
5. The Clay Problem
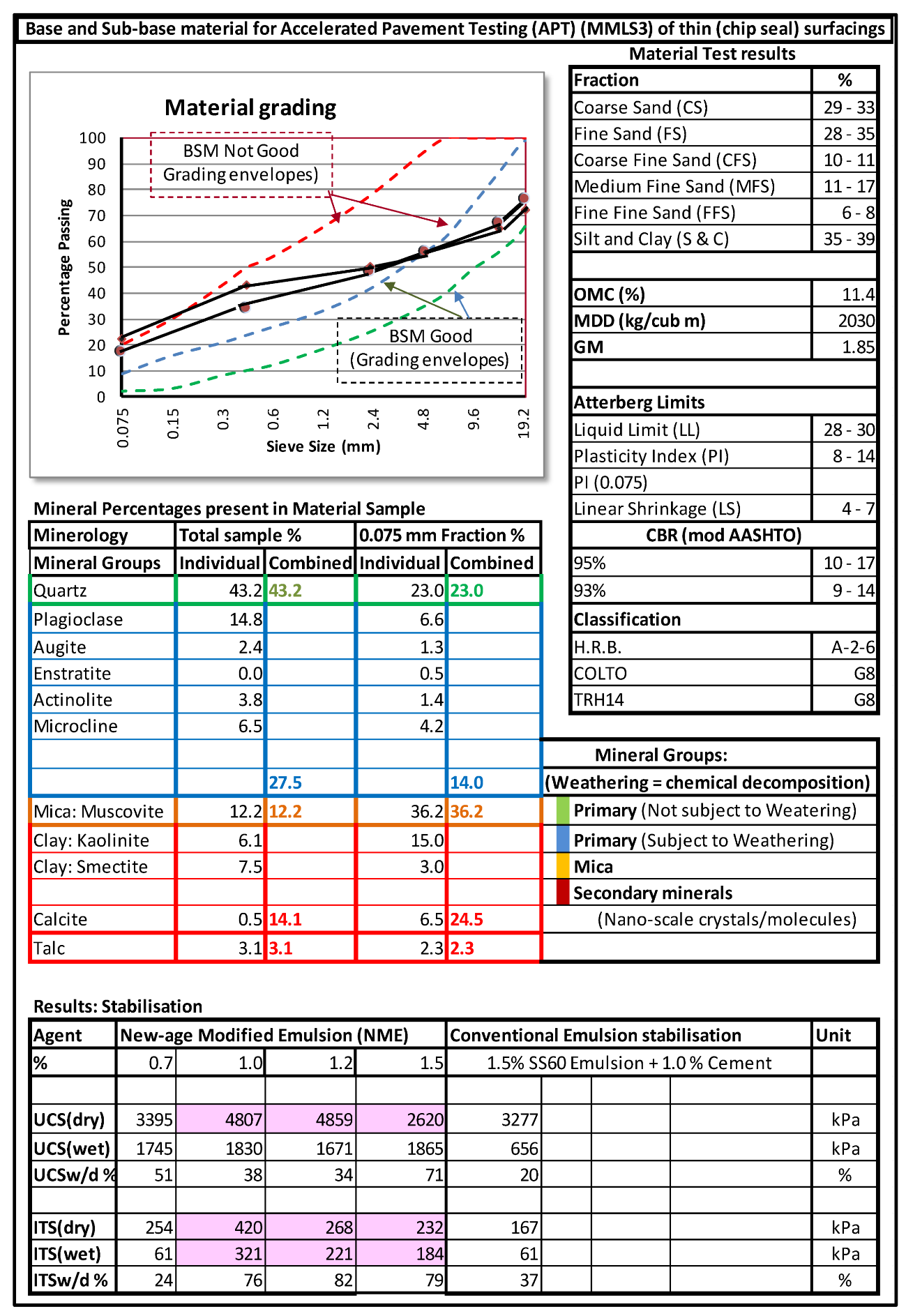
6. Material Compatible Anionic NME Nanotechnology Stabilising Agents Enabling the Use of Unsuitable Granular Materials to Become a Valuable Asset in Road Construction
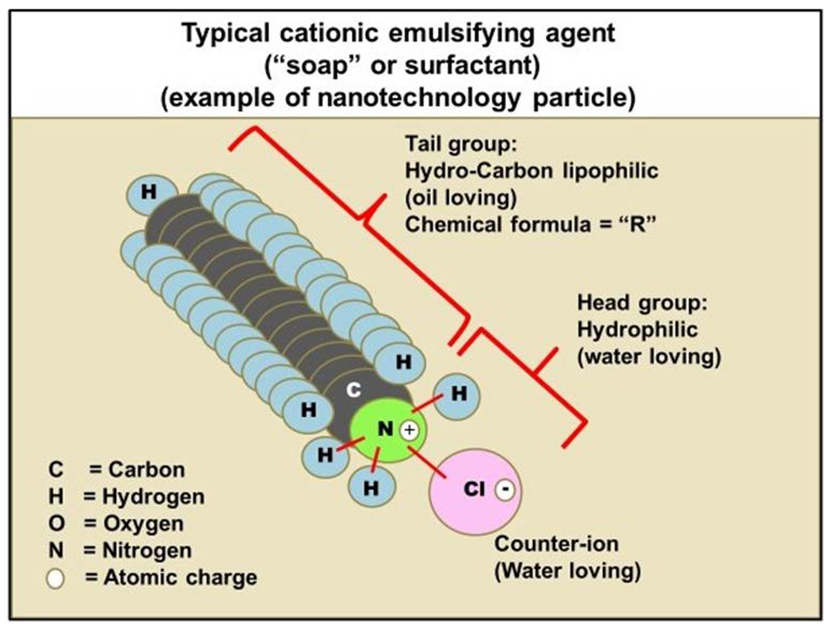
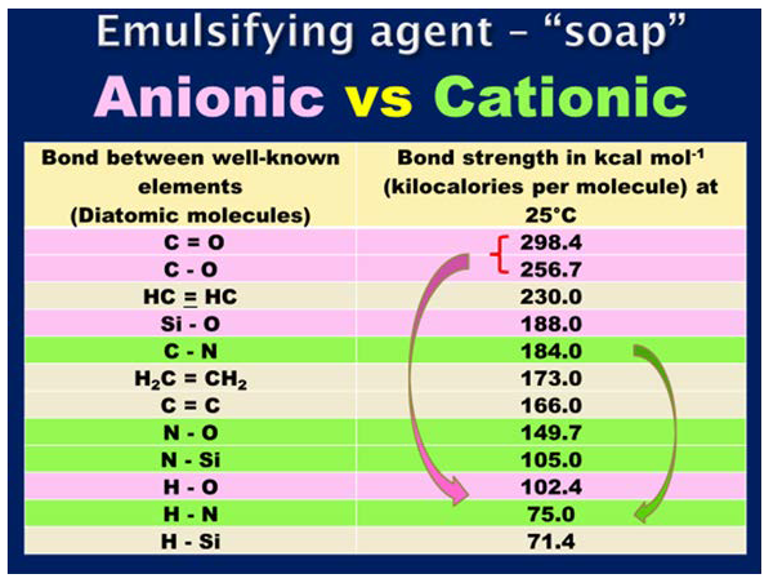
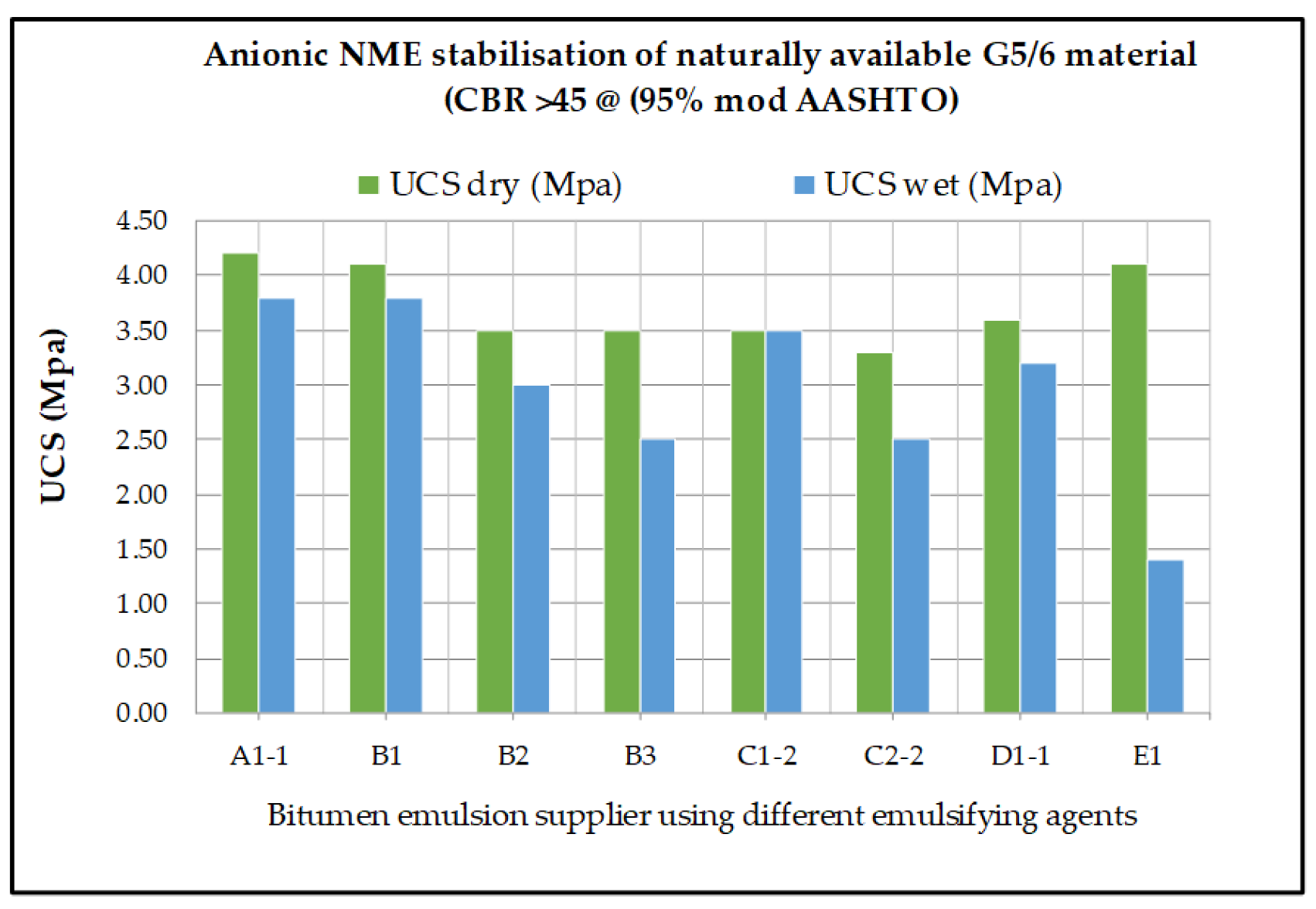
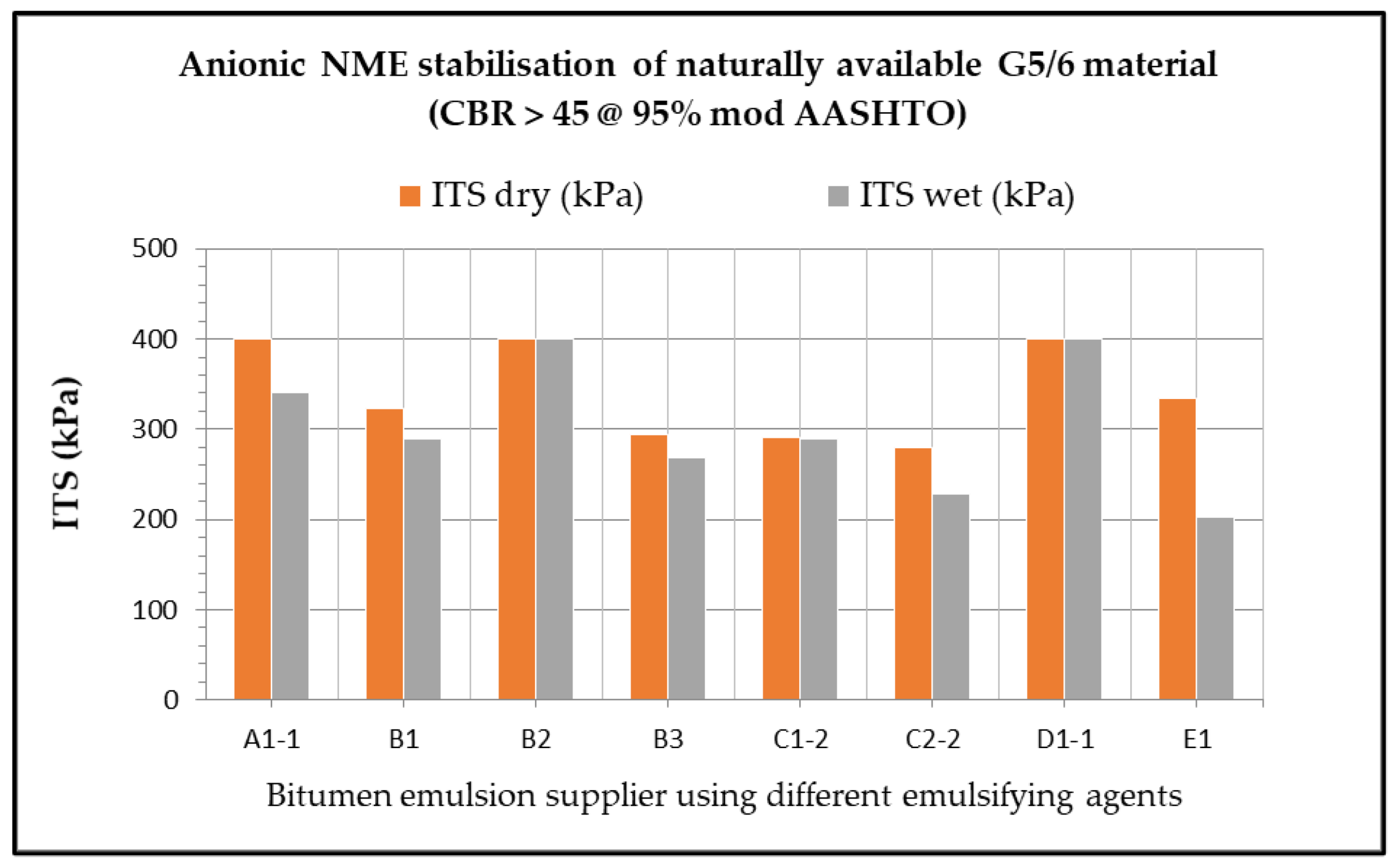
7. Influence of the Chemical Characteristics of the Emulsifying Agent on the Potential Engineering Properties Achievable
8. Conclusions
- Ensure that a high strength chemical bond is established between the stabilising agent and the naturally available granular materials;
- Achieve a high level of hydrophobicity of each particle within the granular materials layer through a 3-dimensional consolidation of the nano-silane, covering the total of surface area of each particle, and
- Protect each particle within the granular material against the effect of water and neutralise the presence of any secondary minerals (e.g., clay) within the granular material by ensuring that a material compatible nano-silane (modified where necessary with a HCT nano-particle), is utilised based on the scientifically measured mineralogy of the granular material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Man, G.-M.; Man, M. Challenges in the Fourth Industrial Revolution. Sciendo Land Forces Acad. Rev. 2019, 24, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Republic of South Africa. Report Recommendations & Way Forward; Presidential Commission on the Fourth Industrial Revolution: Pretoria, South Africa, 2020. [Google Scholar]
- Steyn, W.J.v.M. The Upside of Disruptive 4IR Technology and Innovation; University World News—Africa Edition: London, UK, 2021. [Google Scholar]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Von Ebelman, J.J. Untersuchungen über die Verbinddungender Borsäure mit Aetther. Ann. Chem. Pharm. 1846, 57, 319–353. [Google Scholar] [CrossRef]
- Van Hoffman, A.W. Stone-preserving processes: Royal Institute of British Architects. Builder 1861, 1, 103–105. [Google Scholar]
- Jordaan, G.J.; Steyn, W.J.vdM. Fundamental Principles Ensuring Successful Implementation of New-Age (Nano) Modified Emulsions (NME) for the Stabilisation of Naturally Available Materials in Pavement Engineering. Appl. Sci. 2021, 11, 1745. [Google Scholar] [CrossRef]
- Rust, F.C.; Akhalwaya, I.; Jordaan, G.J.; Du Plessis, L. Evaluation of a nano-silane-modified emulsion stabilised base and subbase under HVS traffic. In Proceedings of the 12th Conference on Asphalt Pavements for Southern Africa (CAPSA 2019), Sun City, South Africa, 13–16 October 2019. [Google Scholar]
- Rust, F.C.; Smit, M.A.; Akhalwaya, I.; Jordaan, G.J.; Du Plessis, L. Evaluation of two nano-silane-modified emulsion stabilised pavements using accelerated pavement testing. Int. J. Pavement Eng. 2020, 1–14. [Google Scholar] [CrossRef]
- Kelsall, R.W.; Himley, I.W.; Geoghegan, M. Nanoscale Science and Technology; Wiley: Chisester, UK, 2004. [Google Scholar]
- Zhang, S. Material Characterisation Techniques; CRC Press: Boca Raton, FL, USA, 2008; ISBN 1420042947. [Google Scholar]
- Steyn, W.J.v.d.M. Potential Applications of Nanotechnology in Pavement Engineering. J. Transp. Eng. 2009, 135, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Jordaan, G.J.; Kilian, A.; Muthivelli, N.; Dlamini, D. Practical Application of Nano-Technology in Roads in southern Africa. In Proceedings of the 8th Transportation Technology Transfer (T2) Conference, Lusaka, Zambia, 8–10 May 2017. [Google Scholar]
- Jordaan, G.J.; Kilian, A. The cost-effective upgrading, preservation and rehabilitation of roads—Optimising the use of available technologies. In Proceedings of the 2016 Southern Africa Transportation Conference (SATC 2016), Pretoria, South Africa, 4–7 July 2016. [Google Scholar]
- Weinert, H.H. The Natural Road Construction Materials of Southern Africa; National Book Printers: Cape Town, South Africa, 1980. [Google Scholar]
- Southern Africa Transport and Communications Commission (SATCC). Guideline: Low-Volume Sealed Roads; Southern Africa Development Community (SADC), SADC House: Gaborone, Botswana, 2003; ISBN 99912-0-456-5. [Google Scholar]
- James, A. Overview of Asphalt Emulsion; Transportation Research Circular, Number E-C102; Transportation Research Board: Washington, DC, USA, 2006. [Google Scholar]
- Jordaan, G.J.; Kilian, A.; Du Plessis, L.; Murphy, M. The development of cost-effective pavement design approaches using mineralogy tests with new nano-technology modifications of materials. In Proceedings of the 2017 Southern Africa Transportation Conference (SATC 2017), Pretoria, South Africa, 6–9 July 2017. [Google Scholar]
- Asphalt Academy. Technical Guideline (TG2): Bitumen Stabilised Materials—A Guideline for the Design and Construction of Bitumen Emulsion and Foamed Bitumen Stabilised Materials; Asphalt Academy c/o CSIR Built Environment: Pretoria, South Africa, 2009. [Google Scholar]
- Jordaan, G.J.; Steyn, W.J.vdM. Testing of granular/soil characteristics for the optimisation of pavement designs using reactive stabilising agents including “new-age” nano-technologies. In Proceedings of the 12th Conference of Asphalt Pavements for Southern Africa (CAPSA 2019), Sun City, South Africa, 13–16 October 2019. [Google Scholar]
- Mshali, M.R.S.; Visser, A. Influence of Mica on Unconfined Compressive Strength of a cement-treated weathered granite gravel. In Proceedings of the 2013 Southern African Transportation Conference (SATC), Pretoria, South Africa, 8–11 July 2013. [Google Scholar]
- Netterberg, F. Pedocretes; RR430; NITRR; CSIR: Pretoria, South Africa, 1985. [Google Scholar]
- South African National Standards. SANS 4001-BT3, (2014). Civil Engineering Specifications Part BT3: Anionic Bitumen Road Emulsion; South African Bureau of Standards (SABS): Pretoria, South Africa, 2014.
- Simpson, P.L. Overview of Asphalt Emulsion Applications in North America; Transportation Research Circular, Number E-C102; Transportation Research Board: Washington, DC, USA, 2006. [Google Scholar]
- Baumgardner, G.L. Asphalt Emulsion Manufacturing Today and Tomorrow; Transportation Research Circular, Number E-C102; Transportation Research Board: Washington, DC, USA, 2006. [Google Scholar]
- Casagrande, C.; Veyssié, M. “Janus Beads”: Realization and Behaviour at Water/Oil Interfaces. C. R. Acad. Sci. 1988, 306, 1423. [Google Scholar] [CrossRef]
- Solá, N.Q. Highly Concentrated Bitumen Emulsions—A State of the Art, Review of Experimental Results. Màster en Ciències Aplicades a l’ Enginyeria, Universitat de Lleida, Catalonia, Spain, 2013. [Google Scholar]
- The Chemical Rubber Company. Handbook of Chemistry and Physics; A Division of the Chemical Rubber Company; CRC Press: Cleveland, OH, USA, 1973. [Google Scholar]
- Grim, R.E. Clay Minerology, 2nd ed.; McGraw-Hill Book Company: New York, NY, USA, 1968. [Google Scholar]
- Pearch, C.A. Silicon Chemistry and Applications; The Chemistry Society: London, UK, 1972; pp. 28–47. [Google Scholar]
- AkzoNobel Surface Chemistry. Adhesion Promoters; Technical Bulletin; AkzoNobel: Stockholm, Sweden, 2003. [Google Scholar]
- Committee of Land Transport Officials (COLTO). Draft TRH14: Guidelines for Road Construction Materials; National Institute for Transport and Road Research (NITRR); CSIR: Pretoria, South Africa, 1985. [Google Scholar]
- American Association of State and Highway Transportation Officials (AASHTO). M145-91: Standard Specification for Classification of Soils and Soil-Aggregate Mixtures for Highway Construction Purposes; American Association of State Highway and Transportation Officials (AASHTO): Washington, DC, USA, 1995. [Google Scholar]
- American Society for Testing Materials (ASTM). D3282-09: Standard Practice for Classification of Soils and Soil-Aggregate Mixtures for Highway Construction Purposes; The American Society for Testing and Materials: Pennsylvania, PA, USA, 2009. [Google Scholar]
- Moore, J.E. Clay mineralogy problems in oil recovery. Pet. Eng. 1960, 32, 78–101. [Google Scholar]
- Jordaan, G.J. Optimisation of Flexible Road Pavement Rehabilitation Investigations and Design; Department of Civil Engineering, University of Pretoria: Pretoria, South Africa, 2013; ISBN 978-1-77592-036-6. [Google Scholar]
- Highway Research Board (HRB). The AASHO Road Test; National Academy of Science—National Research Council: Washington, DC, USA, 1962. [Google Scholar]
- Jordaan, G.J.; Steyn, W.J.vdM. Cost-Effective Upgrading of Gravel Roads Using Naturally Available Materials with New-Age Modified Emulsion (NME) Stabilisation; Department of Civil Engineering, University of Pretoria: Pretoria, South Africa, 2020; ISBN 978-0-620-91415-4. [Google Scholar]
- Jordaan, G.J. Life-cycle cost analysis—An integral part of pavement rehabilitation design. In Proceedings of the 10th Conference on Asphalt Pavements for Southern Africa (CAPSA 2011), Drakensberg, South Africa, 11–14 September 2011. [Google Scholar]
- Queensland Government. Technical Specification: Transport and Main Road Specifications-MRTS21 Bitumen Emulsion; Department of Transport and Main Roads, The State of Queensland: Brisbane, Australia, 2020.
- Bureau of Indian Standards. Bitumen Emulsion for Roads—Specification (Second Revision); Bureau of Indian Standards: New Delhi, India, 2004.
- Transportation Research Board. Asphalt Emulsion Technology; Transportation Research Board: Washington, DC, USA, 2006. [Google Scholar]
- Murphy, M.R.; Jordaan, G.J.; Modise, T.; Dryburgh, R.C. The influence of the characteristics of emulsifying agents on the stabilisation of granular materials using nano-silane modified bitumen emulsions. In Proceedings of the 12th Conference of Asphalt Pavements for Southern Africa (CAPSA 2019), Sun City, South Africa, 13–16 October 2019. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordaan, G.J.; Steyn, W.J.v. Engineering Properties of New-Age (Nano) Modified Emulsion (NME) Stabilised Naturally Available Granular Road Pavement Materials Explained Using Basic Chemistry. Appl. Sci. 2021, 11, 9699. https://doi.org/10.3390/app11209699
Jordaan GJ, Steyn WJv. Engineering Properties of New-Age (Nano) Modified Emulsion (NME) Stabilised Naturally Available Granular Road Pavement Materials Explained Using Basic Chemistry. Applied Sciences. 2021; 11(20):9699. https://doi.org/10.3390/app11209699
Chicago/Turabian StyleJordaan, Gerrit J., and Wynand J. vdM. Steyn. 2021. "Engineering Properties of New-Age (Nano) Modified Emulsion (NME) Stabilised Naturally Available Granular Road Pavement Materials Explained Using Basic Chemistry" Applied Sciences 11, no. 20: 9699. https://doi.org/10.3390/app11209699
APA StyleJordaan, G. J., & Steyn, W. J. v. (2021). Engineering Properties of New-Age (Nano) Modified Emulsion (NME) Stabilised Naturally Available Granular Road Pavement Materials Explained Using Basic Chemistry. Applied Sciences, 11(20), 9699. https://doi.org/10.3390/app11209699





