1. Introduction
Additive manufacturing (AM) has brought new opportunities to the workflows of individualized treatments in dentistry. Three-dimensional (3D) printing with biocompatible resins offers new competition to the currently dominating subtractive manufacturing workflows. These subtractive manufacturing workflows were often described as computer-aided design (CAD), computer-aided manufacturing (CAM) and Computerized Numerical Control (CNC) systems. One of the well-known representatives from this group is the Cerec system [
1,
2].
The potential of additive manufacturing in medical applications is extensive. It is certain that the field of dentistry will be no exception. Despite dentistry’s strong technological background, it might come as a surprise that the speed of clinical implementation of AM was not as rapid as some might have anticipated. The explanation of what slowed down the implementation of AM brings a better understanding of the likely future development and trajectory of AM applications in dentistry. One of the key factors for the successful individualization of most dental applications in the digital era was efficient and precise intraoral scanning. It must be understood that dental treatments were necessarily highly individualized already, even in the analog era. Therefore, incoming novel technologies, such as AM, during their beginnings, were facing the strong competition of existing and well-established workflows, such as dental impressions techniques or CAD/CAM systems based on subtractive manufacturing, which is the opposite of AM [
3]. It took some time for these technologies, such as 3D face-scanning, 3D printing, cone-beam computer tomography (CBCT), and others, to become more efficient than some analog processes.
Having a 3D model of an exact digital reproduction of the patient anatomy is only the first step. To be able to implement the AM process into the clinical field, and to have it in direct, long-term contact with the patient’s body, the biocompatible materials must be well developed and certified. Only in recent years have wider numbers of class IIa biocompatible dental resins, suitable for long-term dental applications, been approved and EU-certified [
4,
5,
6]. Our early experiences with fused deposition modeling (FDM), digital light processing (DLP), stereolithography laser resin printing (SLA) and laser sintering processes taught us possible clinical applications, which had both the merit of economical affordability, and the sustainability of such processes. Most of our clinical applications currently take advantage of DLP and SLA biocompatible resin printing [
1].
The clinical implementation of AM processes in the orthodontic field today is technologically superior to most of the older existing processes, however, it does require the knowledge and skillsets not regularly taught in medical faculties [
7,
8]. The economical affordability aspect shall not by diminished. In dentistry, the resin 3D printing is used for the manufacturing of surgical guides, splints, long-term provisional crowns and bridges and many other applications. In orthodontics, 3D biocompatible printing is used for individualized retainers, dental splints, biomechanical power-arms (PA) and power-caps (PC), and Carriere Distalizers or surgical dental splints for orthognathic surgeries [
9].
Our experience in 3D printing models for operative planning in situations of complex congenital heart defects [
10] or in ophthalmology for stereotactic radiosurgery planning [
11] taught us about the limits of low-cost FDM printing. For example, our experimental FDM 3D printing of scaffolds for mesenchymal stem cells (MSCs) colonization was not successful due to accuracy issues with PLA 3D printing in 2011. Since then, however significant technological progress has been made, and we are captivated by 3D printing with materials with dynamic properties. As the material properties and shape of 3D printing is changing, it will in time be called 4D printing. For example, shape–memory polymers (SMPs) have the ability to change from an initial shape into a stress-free, temporary shape. For our applications, the current ideal form of AM is DLP (or SLA) in combination with biocompatible resins. In the future, the shape would change, which might be a breakthrough in orthodontics, where clear aligners are used to apply light, continuous forces on the teeth. Such a 3D printed material would take advantage of the sudden change of humidity in oral cavities and from the possibility of different thicknesses, which are now not used in clear aligners. Programable shape transformations of 3D printing is our future. The composites we are using currently could be combined with PLA or with nanomaterials, thus bringing new properties [
12].
Our research was focused on the 3D designing and printing of an individualized biocompatible orthodontic power-arm. The 3D designing was based on stress distribution analysis and finite element modelling. The original and the improved design were evaluated clinically.
The power-arm [
13] is a miniature device, which may have various designs depending on the place of bonding, which can be either tooth surface or the orthodontic wire. Currently, the most frequently used power-arms are prefabricated ones (
Figure 1).
The orthodontic power-arm intended for the tooth surface has a part with a bonding surface for attachment to a tooth. This part is called a base, which could be either prefabricated (slightly curved) or individualized (exactly matching a particular tooth surface). The base is intended for a vestibular or lingual tooth surface. An arm of the power-arm extends gingivally and is intended for engaging a tractive device (usually elastic bands). The length of the arm is different depending on the distance between the place bonded and the center of resistance of that particular tooth. It also provides a leverage to a force vector applied to the end of the power-arm, enabling the preferably orthodontic bodily tooth-movement. After bonding, it must by resilient, aesthetic, and compliant with intraoral anatomy, providing an anchorage for intraoral elastics to control the application of the biomechanical force vector on the tooth.
Oral anatomy varies in everybody. This is the reason for frequent debonding or extreme compromises in applied universal devices (reducing their biomechanical effectiveness). Individualization is the key. The last four years of our clinical experience with 3D printed power-arms revealed that the interference of power-arm with gums, intraoral tissues, or other teeth in the occlusion results in the damage or debonding of the power-arm. Absence of the individual base where tooth-surface curvature is compensated with extra adhesive also reduces the strength of the bond between the power-arm and the tooth surface [
14]. The importance of individualized bases is the fundamental aspect of effectivity for most customized intraoral appliances, such as lingual orthodontic brackets, 3D printed hyrax devices or power-caps, and power-arms [
15].
It is not only existing anatomical objects in the oral cavity that might interfere with a power-arm. For example, food and its processing are possible threats to power-arm retention. To respect the masticatory and articulative functions of oral cavities, the bio-inspired design of the power-arm shall work in harmony with these, whilst at the same time respecting smile aesthetics, and preserving resiliency and biocompatibility. The current topics in biocompatible 3D printing in orthodontics are frequently focused on the direct 3D printing of clear orthodontic aligners or retainers. 3D printed retainers were, until now, preferred to be 3D printed from Remanium (Co-Cr) alloys [
16,
17].
Universal power-arms suffer from frequent debonding, patient discomfort or necessity to reshape or position them in a way that compromises their biomechanical effectivity [
14,
15]. The customized power-arm has details which cannot be properly or economically manufactured in the subtractive manufacturing process. As the power-arms are exposed to the oral cavity for a long time, they must be at least class IIa biocompatible certified. To facilitate a tooth movement in the proper direction, they must translate the force from the intraoral elastic band efficiently. The base of the individualized power-arm matches the exact tooth surface. This provides not only the perfect bonding contact, but also enforces the proper positioning of the power-arm on the specific spot, due to the unique complementarity between tooth and power-arm surface. This is how the individual base of the 3D printed power-arm guides itself to proper positioning, as planned in the virtual setup.
The purpose of this work was also to inspire that such AM workflow is available for the designing or redesigning of any customized intraoral biomedical appliance. Moreover, that process can be completed upon the combination of technical and clinical research, as evidenced in
Scheme 1.
The power-arm is not an essential feature of every orthodontic treatment. However, in difficult tooth movements, it is a crucial tool. Especially in biomechanics, where forces must be applied close to the tooth’s center of resistance to avoid tooth tipping. The power-arm-enabled biomechanical approach offers a critical instrument for treatment efficiency.
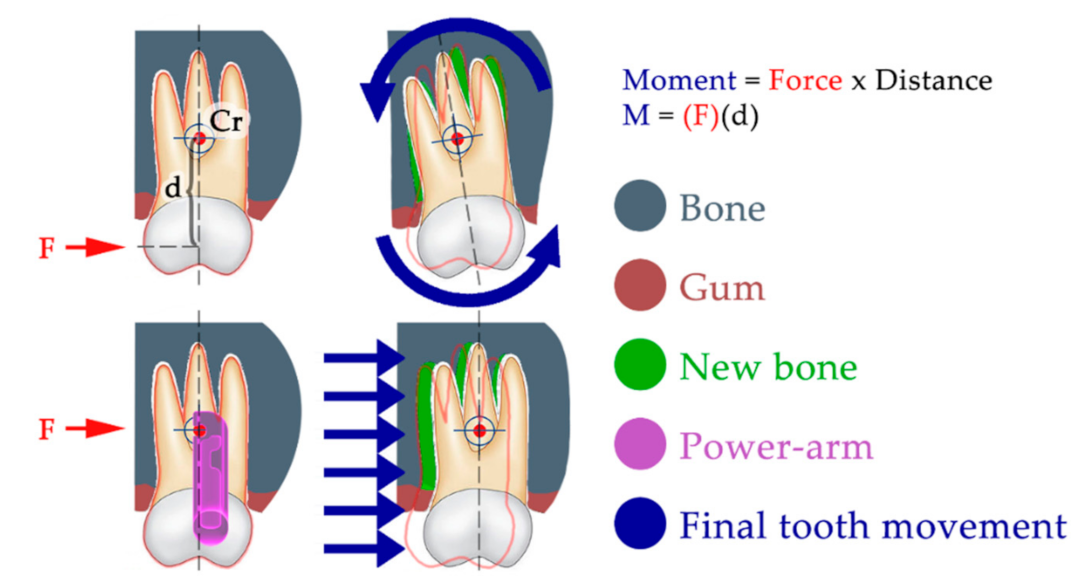
Figure 2 explains the importance of controlled force application in regard to the center of tooth resistance, which can result in significantly different clinical outcomes. The center of tooth resistance (Cr), also called the center of tooth rotation, is a virtual point around which the tooth rotates when force is applied to its crown. This point is not approachable directly, as it is usually located in two-thirds of the length of the root. Its location always differs, as it strongly depends on the level of surrounding bone, as well as the root length and shape. When force is applied to a tooth crown (above the Cr), the tooth movement is described as tipping, which is frequently undesirable. When force is applied at the level of Cr, the resulting tooth movement is described as bodily movement. This is the orthodontically preferred movement. This type of tooth movement is crucial to treatment predictability and efficiency.
Our aim with this research was also to inspire new ideas for manufacturing individualized devices not just for orthodontic tooth movement. The use of mathematical modeling and simulations of material stress upon load is a useful step to improving the intraoral resiliency of any 3D printed intraoral appliance. Our clinical observation of the resiliency of AM appliances provides further information about the material’s long-term behavior in hostile environments, such as in oral cavities [
9].
The correction of malocclusion is performed by moving the teeth to ideal positions and inclinations. One of the main problems associated with moving teeth is that the roots of the teeth need to be moved together with the tooth crown. The roots are deep in the alveolar bone and are inaccessible to common orthodontic biomechanics. For this reason, it is necessary to apply gentle and continuous forces of orthodontic leverage to the roots, and these can be applied only on visible and accessible parts of the tooth—the tooth crown. To be effective in orthodontic treatment, we need effective biomechanics. To achieve effective biomechanics, we need to translate the biomechanical vector of the force as close to the center of resistance of the tooth as possible. This center of resistance of the tooth is located approximately in two thirds of the length of the root. To have this leverage, we use the so-called power-arms. These arms have a body which includes a connecting surface for connection to the surface of the tooth crown, which we call the base. The arm is blade-shaped and is also provided with a plurality of recesses in the medial or distal edges of the arm, which serve to connect the traction device—the elastics. Power-arms are not new to orthodontics [
8]. The possibility to create them with AM is exciting, and the opportunity to have them fully individualized is inspiring.
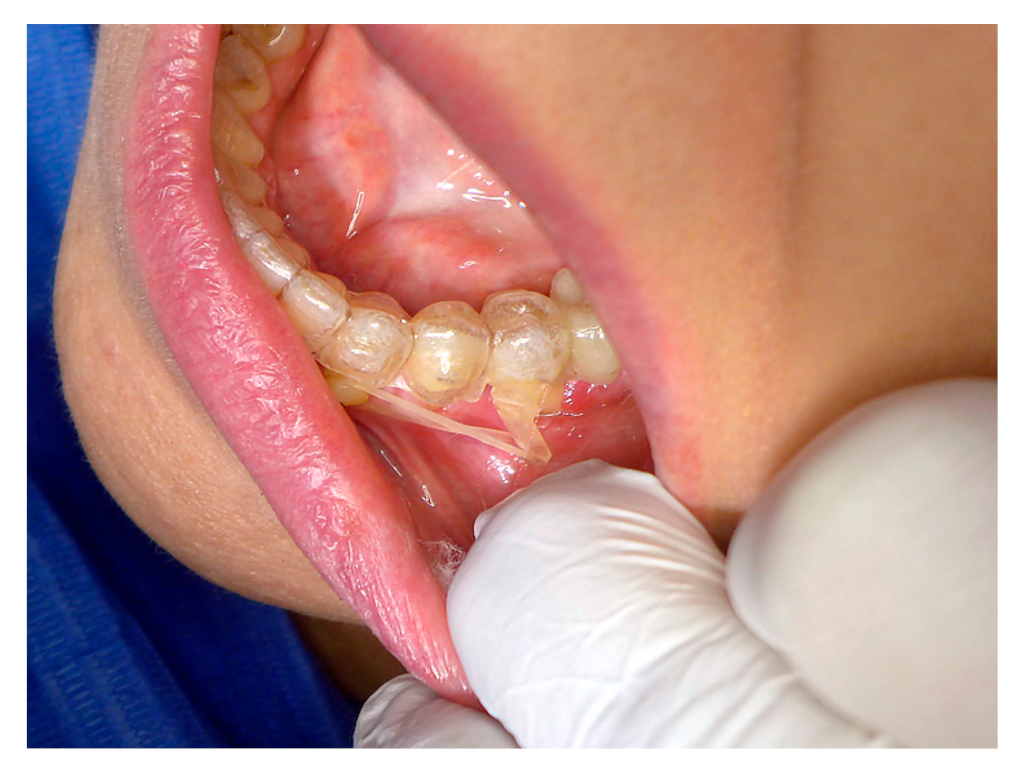
In our work, we have focused first on gaining knowledge about the strength and durability of currently used prefabricated metallic power-arms. In the past, the power-arms were bent from orthodontic wire and, as “power” arms reference, were a commonly used features with the Straight Wire appliances. An article from 1986 describes their employment to advantage in the old orthodontic Begg technique, supporting the extraction space closure from behind [
18]. For the last two decades, power-arms have been frequently used as prefabricated, mostly metallic, orthodontic features, [
19,
20]. The clinical experience (
Figure 3A,B) of our orthodontist with metallic hand-crafted and prefabricated power-arms (
Figure 1) references the following frequent impediments:
As there was no other type of orthodontic power-arm until now, the existing varieties of hand-crafted and prefabricated were used. There is no mention in the literature about any 3D printed orthodontic power-arms with an individual base. Comparing the developed approach to the current state-of-the art power-arms produces promising results. The current state-of-the-art interdisciplinary research concerning power-arms employs finite element analysis to focus the research more on the ideal power-arm placement on the tooth surface or the power-arm length [
21,
22]. Most of the research is not concerning the power-arm shape. This paper is reporting the current, state-of-the-art approach to designing new 3D printed power-arms, upon technical and also clinical analysis. The new design addresses the current clinical limitations of hand-crafted or prefabricated power-arms. The presented workflow, based on AM, is applicable to other intraorally applied medical devices and shall be encouraging for other innovators [
23].
The aim of this study was to design and materialize the functional shape of a comfortable and transparent orthodontic power-arm by means of biocompatible 3D printing. The shape needs to withstand the environment of the oral cavity, as well as not only the forces applied by the elastics during orthodontic treatment, but also chewing forces.
3. Results
3.1. Tensile Test Results
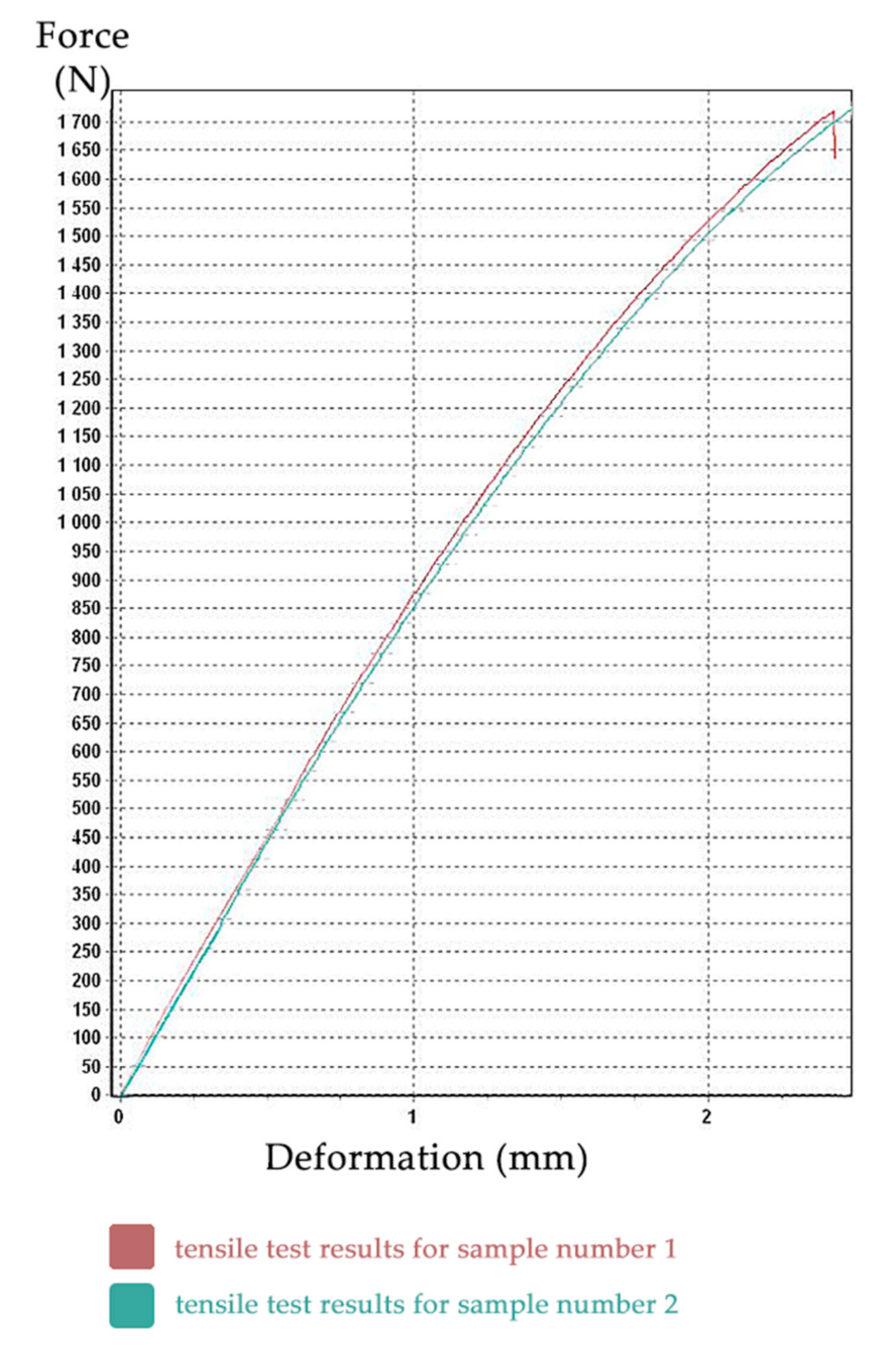
The tensile test was performed for all samples, but the last three samples were incorrectly clamped, so the results from these three specimens were unusable. Full probing dataset is available. The resulting tensile test diagrams of specimens No. 1 (red) and No. 2 (aquamarine) are shown in
Figure 9. The
X-axis shows the deformation (in mm), the
Y-axis shows the force (in N).
Other outputs of tensile tests were also text files with measured values, such as: time (s), force (N), travel (mm), elongation (mm), tensile stress (MPa), and relative elongation (%), etc.
These values were processed using Microsoft Excel 2016 and subsequently, using Hooke’s law, the modulus of elasticity for the first two samples was calculated. As seen in
Figure 9, the curves are slightly different and are shifted in the Y axis. The values of the modulus of elasticity of sample No. 2 were trivially lower. This slight difference is not clinically relevant for medical application. However, as the clinical objective was to design a more resilient power-arm, we have conservatively chosen this second (lower) sample as representative.
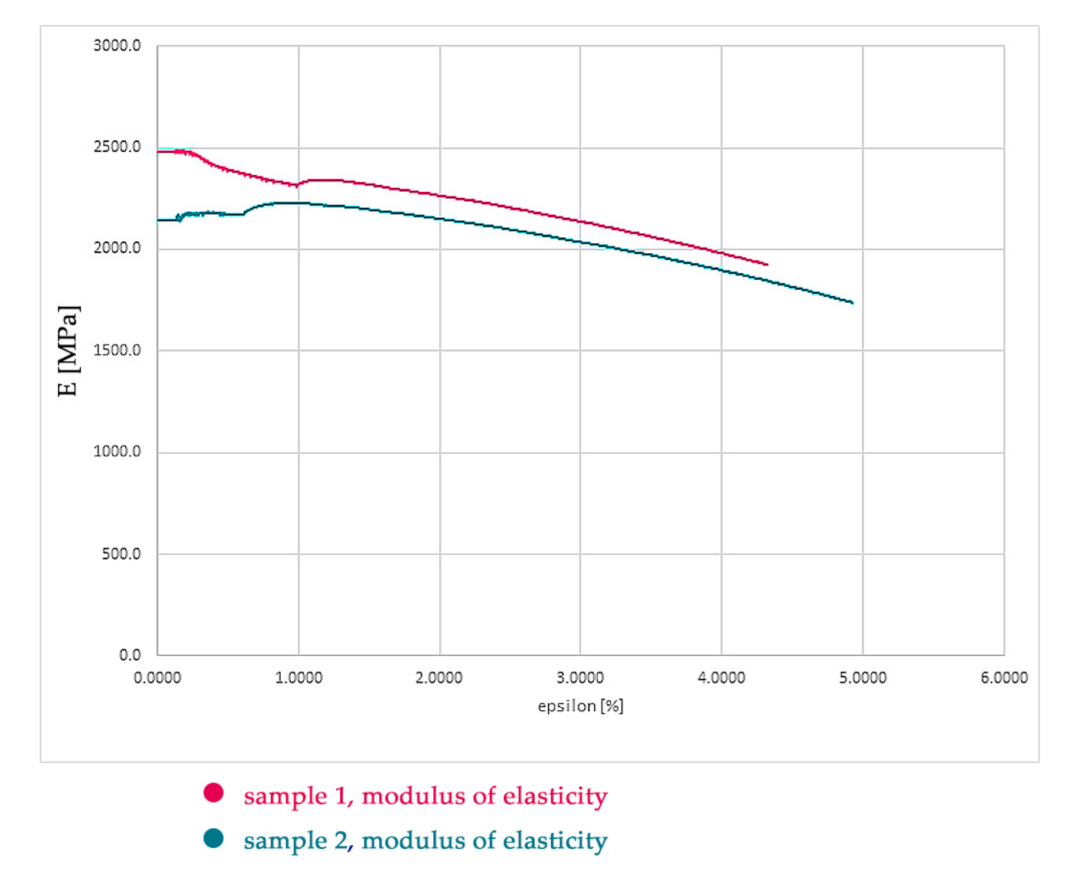
The modulus of elasticity (
Figure 10) was calculated as the arithmetic mean of all values of the modulus of elasticity from test sample No. 2 and its value is 2089 MPa (see
Figure 9). During the testing, the transverse deformations of the sample were not recorded, so it was not possible to determine the Poisson’s number, and therefore we chose the value 0.29 in the calculation. The international standard that specifies the general principles for determining the tensile properties of plastics and plastic composites under defined conditions is: ISO 527-1:2019 [
27]. ISO 527 standard, belonging to the family of plastics standards, specifies the test conditions for the determination of the elastic limit, the deformation, the permissible loads, and the type of breakage of the materials of plastic composite, isotropic or orthotropic, and fiber-reinforced, when subjected to tensile stress. These results were not researched upon the test conditions defined in ISO 527. This norm, updated in 2019, is appropriate for the determination of the tensile properties of plastic composites of unknown composition and mechanical properties.
The step of researching material properties in this research is shown only for information purpose for clinicians experimenting with unknown materials. This paper is intended for medical clinicians to increase their awareness about possible approaches to designing their personalized intraoral appliances. The authors of this paper want to emphasize that testing two specimens is too little for proper material design. As the material properties of Dental LT Clear Biocompatible Resin (version 1) have been confirmed by the manufacturer during the research process, there was no need to print more specimens. This would be otherwise compulsory in research of material mechanical properties. Clinical use of unknown materials can be allowed only under special strict circumstances. Dental LT Clear v1 was tested at NAMSA, Chasse sur Rhône in France, and is certified biocompatible per EN-ISO 10993-1:2009/AC:2010. However, the production of this material is already discontinued, and its version 2 is now commercially available with full material properties’ data available (link on the technical sheet: [
24]).
Researching material properties requires interdisciplinary cooperation and is feasible for medical clinicians. However, it is probably not necessary anymore, as every biocompatible resin, certified for clinical use, is required to publish full material properties suitable directly for FEM.
3.2. Calculation Program and Model Preparation
A software package from ALTAIR company was chosen as a calculation program, which contains a preprocessor (HyperMesh = preparation, discretization of the model, assignment of mechanical properties and boundary conditions). The calculation itself was performed in the program Optistruct, linear solver (sol. 101) and, subsequently, postprocessing was conducted in the program HyperView.
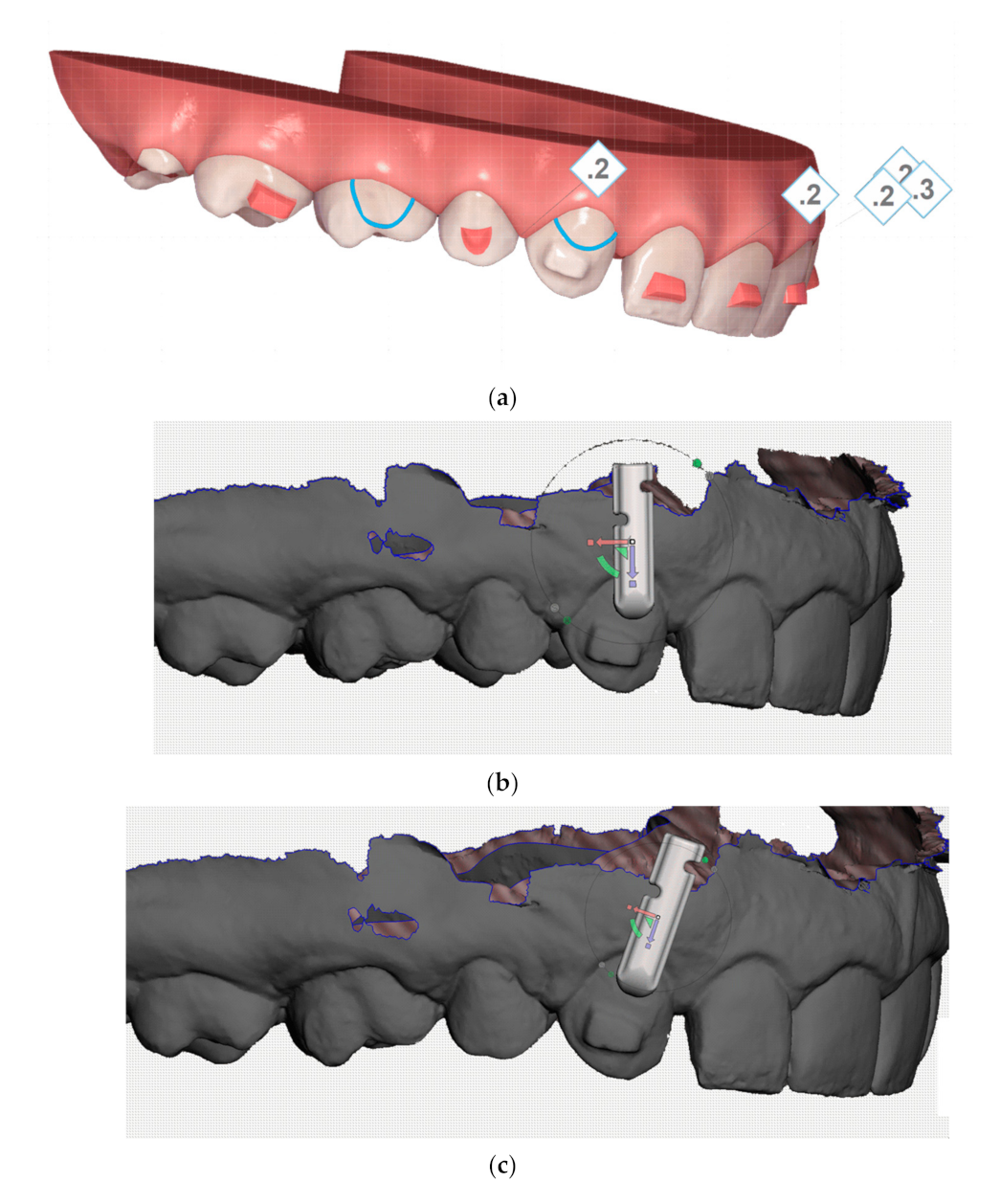
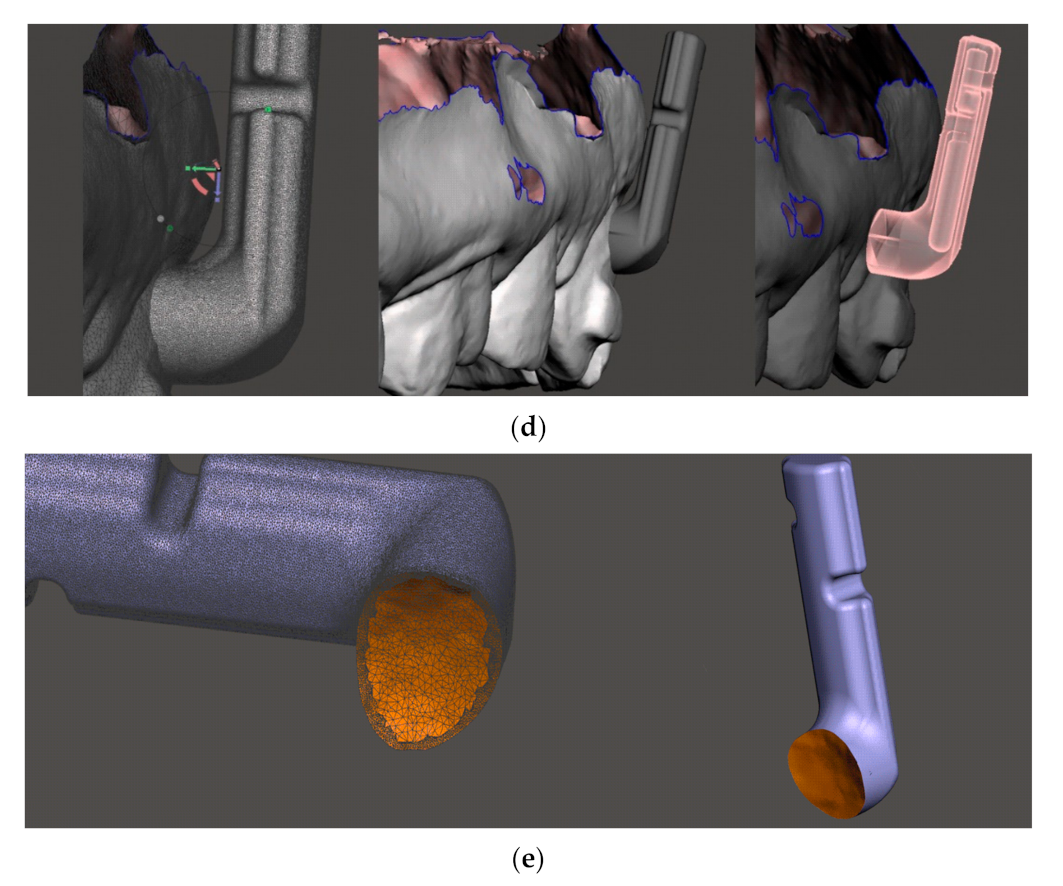
3.3. Input Geometry
Figure 11 on the left shows the original geometry of the power-arm (variant I). This variant, as already described earlier, frequently broke at the top notch in the arm due to insufficient material thickness in the area and the effect of lateral tensile forces. The SolidWorks program, which we used to modify the original geometry to a new one (hereafter referred to as variant II), with the following:
- -
a notch was added to hold the rubber band on both sides (better usability), the notch was widened, and the internal angles/edges were more rounded.
- -
the edges have been rounded, so the food retention shall be less frequent, and variant II shall be less mechanically irritating when in contact with the tongue, oral mucosa and the lips.
3.4. Discretization (Meshing), Boundary Conditions and Material Properties
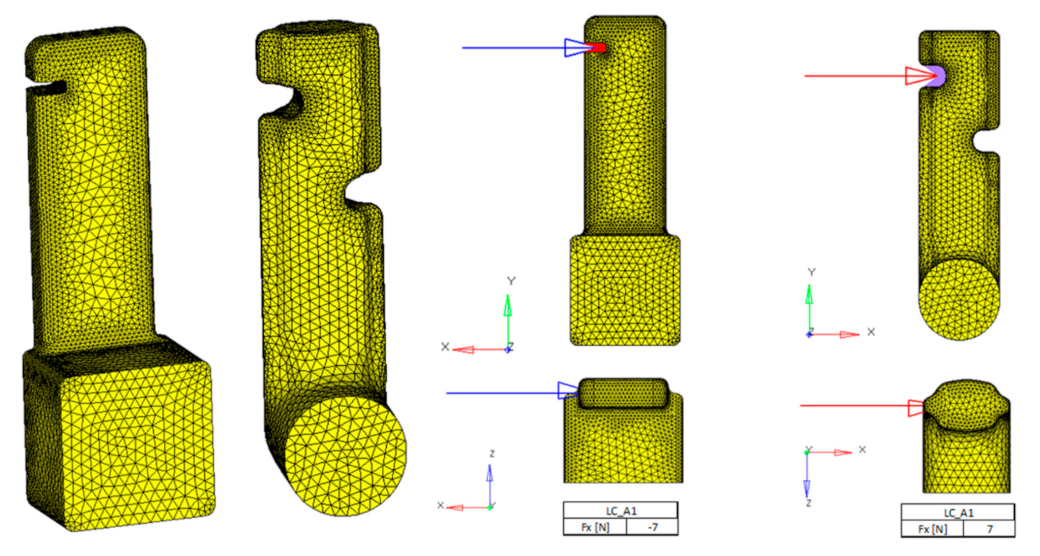
Figure 12 shows finite element models. CTETRA elements (4-node tetrahedron) were used. The number of which, in the critical places of the component (edges, notches, radii), is smaller, in order to better absorb the stress.
We used three loading conditions. The loading conditions were chosen upon the clinical aspect and intended use of the power-arm. The spot of elastic-band insertion is precisely recognized, as is the base pad, which is intended for adhesive attachment to the tooth surface. We tried to load both variants with identical forces. In each load case, the resultant of the forces was 7 N. In the second (LC_B1) and third (LC_C1) load cases, the direction of the force vector differed.
We have used the material properties of dental biocompatible resin measured by us, namely:
Modulus of elasticity in tension E = 2089 MPa;
Poisson’s number ν = 0.29;
Ultimate strength Rm = 83 MPa;
For sample No.1 Rm = 83.09 MPa, for sample No.2 Rm = 85.5 MPa.
The calculation was performed in Optistruct, linear solution 101 (Solution 101). Due to the size (39,525 degrees of freedom for variant I and 26,802 degrees of freedom for variant II) and type of the task, for today’s computer performance, it was calculated in a matter of seconds. A linear elastic material is a mathematical model used to analyze the deformation of solid bodies. When the loads are removed, the material naturally returns to its original, undeformed shape. In other words, the material stress level doesn’t reach the ultimate strength limit. The linear elastic FE analysis is an efficient modelling approach for our power-arm virtual model modeling. Oral masticatory functions can deform PA, which would result in an undesired clinical effect. As this paper is aimed at medical clinicians, it shall be considered what masticatory pressures will affect the intraoral appliance, their intensities, directions and other characteristics. This is different for each intraoral personalized device.
3.5. Postprocessing
The results were loaded into the HyperView program, which offers a wide range of assessments of various stresses (von Mises, mainly stress, and maximum shear stress).
3.6. Deformation Stress in the Arm
Figure 14 and
Figure 15 describe stress under various loading conditions. The loading conditions were chosen upon the clinical aspect and intended use of the power-arm, as the spot of the elastic-band insertion is known, as well as the base attachment to the tooth surface.
3.7. Analysis of Technical Aspects of the Results
As mentioned above, for the brittle material (our case), the von Mises method was chosen as the method for stress evaluation. The following applies to the given power-arms:
The major difference between variants in deformations is for the load LC_A1, however, the probability of its realization is very small (the force acts only in the direction of the X-axis).
The probability of loading LC_B1 and LC_C1 is very high, but here the difference between the deformations is negligible. The stresses in contrast to the deformations are considerably higher for variant I, which means that variant II is stiffer and shall be more resilient. Clinical aspects of PA loads are known;
Table 3. summarizes the differences between both PA variants under various loads.
In the case of load LC_B1, variant II is 11% more flexible (less rigid) compared to variant I, but the stresses for variant I are 76% higher than for variant II.
In the case of load LC_C1, variant II shows overall higher stiffness and lower stresses than variant I, namely:
Variant I is 7% more flexible and shows higher stresses of up to 82% compared to variant II.
3.8. Analysis of Clinical Aspects of the Results
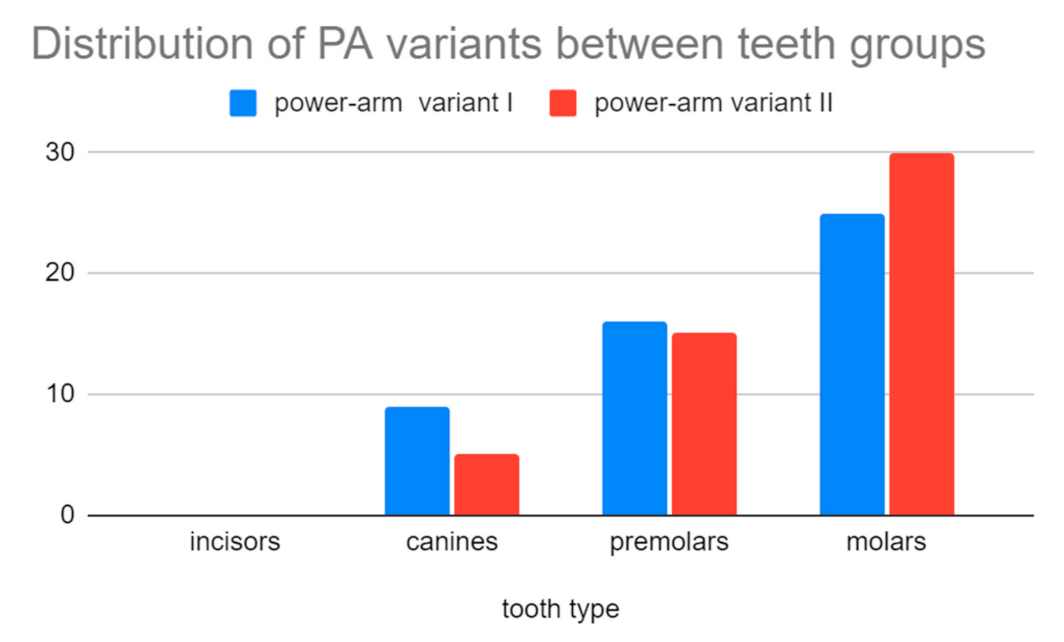
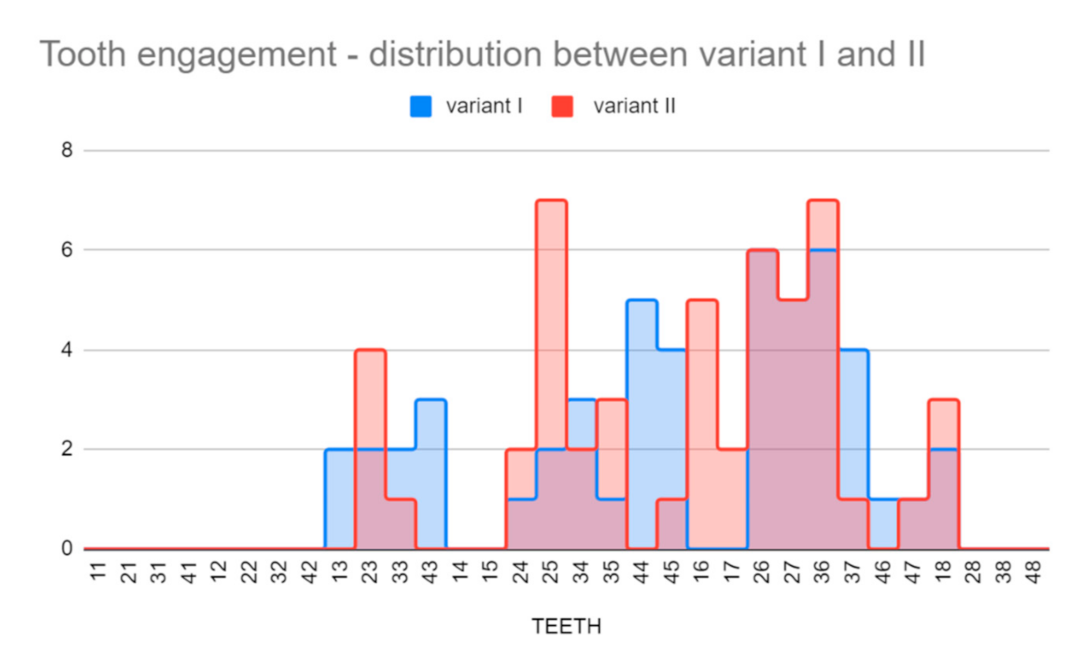
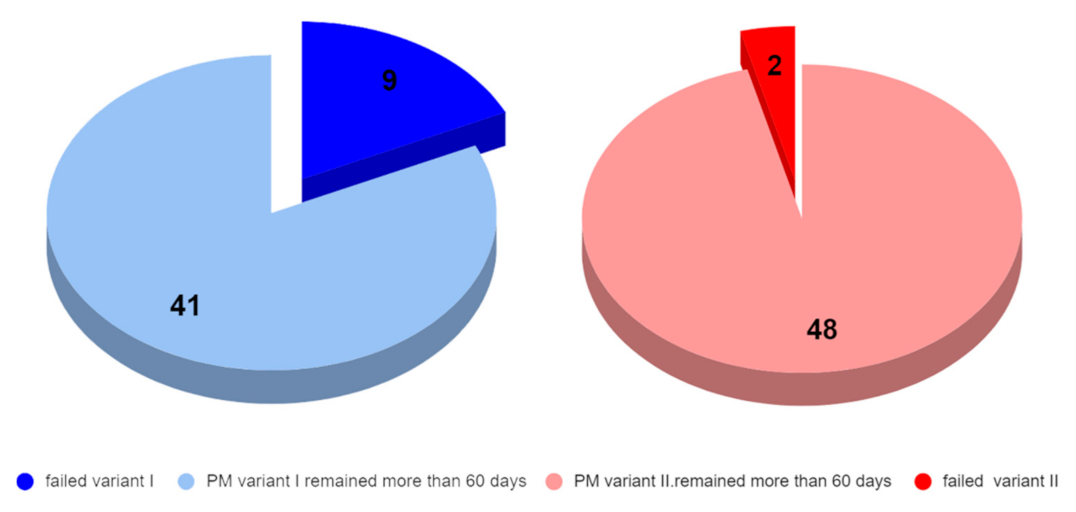
Clinical application of the power-arms was always incorporated to the complex orthodontic treatment plan. There was a clear benefit for the patient biomechanics and two groups of 50 applications were formed. A total of two designs of customized power-arms were used (variant I—older, variant II—newer). Only one power-arm per individual was observed. The indications of particular variants of PA (variant I/II) were random. A total of 50 older and 50 improved designs were applied and observed. The data were analyzed for normal distribution between both groups. Teeth used for PA placement were grouped to four main groups according to their type (incisors, canines, premolars, and molars). PA fractures, deboning or another damage was evaluated as failure.
Frequency of failures between groups was evaluated by two tailed T-test.
Hypothesis 0 (H0). The Null Hypothesis was that there is no significant difference between the power-arm designs’ clinical performance.
Hypothesis 1 (H1). Alternative Hypothesis was that there is a significant difference between the clinical performance of one of the PA designs. By convention, two-tailed tests are used to determine significance at the 5% level, meaning each side of the distribution is cut at 2.5%. Before comparing both groups, data distribution was evaluated.
We were comparing two groups (PA variant 1 and 2) and have quantified the difference between them with a two-tailed T-test determining significance at the 5% level. This means that each side of the distribution is cut at 2.5%. We have also assumed that the variation of data in both sets were not the same.
Calculated p value of two tailed T-test was 0.0260.
A p-value less than 0.05 (typically ≤ 0.05) is statistically significant. It indicates strong evidence against the null hypothesis, as there is less than a 5% probability that the null is correct.
Clinical application of the power-arms was always incorporated to the complex treatment plan. A total of two groups of 50 applications were chosen randomly. Only one power-arm per individual was observed.
Table 4. summarizes the clinical evaluation.
Figure 18 and
Figure 19 (
Supplementary Materials) visualize the different clinical performances of the older and newer power-arms.
4. Discussion
Designing and manufacturing one’s own patient-customized designs in dentistry is not new. In fact, this is a modus operandi for orthodontics since its beginnings. With the power of AM, together with digital intraoral scanning, we can engage the trajectory of even greater treatment individualization. This will result in higher treatment predictability and efficiency.
The results showed that with proper analysis, the current designs can be improved towards the higher resiliency of biomedical devices applied intraorally. Three-dimensional biocompatible printing can achieve outputs which are not only biomechanically fully functional but are also more effective than handmade devices [
25,
26,
27,
28,
29,
30]. Results of this study revealed that redesigning the shapes in concordance with biocompatible material properties can provide a more resilient device. Orthodontics as well as dentistry are undergoing a technological revolution, with advances in medical imaging, 3D printing and customization of appliances [
31]. Future research directions may cover another customized device. We are already researching the possibilities of how to improve our current biocompatible designs of the biocompatible 3D individualized retainer (“bio3dir”), “power-cap” and “carriere-distalizer”. Digital workflows are currently trending in the orthodontic practice. They have affected every aspect of orthodontics—with transformations in documentation, study casts, analysis of dental malocclusion, smile designing, treatment planning and for fabrication of orthodontic appliances. The three-dimensional imaging of dentition, skeletal components and the face allows treatment planning in 3D and the use of computer aided design (CAD) and computer aided manufacturing (CAM) for customization of orthodontic appliances. Software integration of digital models, 3D facial imaging and cone-beam computed tomography (CBCT) makes it possible to simulate the treatment plan and to attain good communication with the patients. [
31,
32,
33].
Biocompatible resins are not strongly dominating the AM field of dentistry. Frequently porous titanium alloy structures are more suitable for use in biomedical applications when resilience to forces is necessary [
34]. However, the power is placed visually on the teeth’s surfaces, so its aesthetical aspect is very important, and titanium or Remanium alloys are not suitable from this aspect.
There is an important part of the orthodontic profession focused in care for cleft patients. Biocompatible 3D printing can affordably provide them with a sequence of customized biocompatible appliances to compensate anatomical defects before surgical interventions are performed. There are different time-protocols of cleft lip and palate operations. Especially in the first few months before surgery, compensation with a customized appliance is needed [
35].
Power-arm designing based on stress distribution analysis with finite element modelling research was a specific task. In the past, the use of stress distribution analysis in real components and also in the finite element modelling were challenging procedures that required sophisticated infrastructure. Such research is still inter-disciplinary; however, it is quite accessible. Product analysis is a commonly used production process for metal alloys. The resin we have used did not have its complete technical data published by its manufacturer at the time of this research. We had to discover these data thanks to material analysis in the laboratory. Due to that, we cannot compare our power-arms with commonly manufactured ones, as commonly produced power-arms are made of metal alloys and have completely different material properties. After gaining knowledge about the properties of the material, we redesigned the shape of the prototype power-arms. The strength of each prototype had to be calculated by computer. After complete tuning, the spatial shape was finally modeled with a cavity inside. We managed to model power-arms using 3D printing without the prototype cracking. We managed to achieve our goal, which is confirmed by our calculations and clinically applied arms.
The disinfection of the 3D printed part is an important aspect to be discussed. As described in the Materials and Methods section, our procedures of power-arm disinfection were consisting of UV post-curing and IPA. The 3D printed power-arms are intended to be fixed in the oral cavity, which is not a sterile place. They are not considered to be implants which are required to follow strict rules, for example, prosthesis for hips or knees, dental implants, or spine screws [
36].
Results of the analysis and design of increasing the tensile arm strength showed that deformations of the originally analyzed arm pointed to the strength deficiencies of the original model. The disadvantages were a weak layer of material in the notch area for the tension rubber and an insufficient amount of material for the vertical part of the arm under the application of lateral forces. This was followed by adjusting the shape of the base from square to circular to eliminate tension nodes. The vertical part was adjusted from a rectangular shape to a “lemon” shape. Thanks to this modification, we have achieved the largest increase in strength in our favor. The rounded edges and three-dimensional shape increased the tensile strength, especially in the lateral action of forces. We performed all strength calculations on FEM virtual calculation programs [
37]. The finite element analysis was already used in orthodontic research; however, it was not applied to power-arm 3D design, it had merely been used to research an ideal power-arm location in the oral cavity [
38]. We are not aware of any similar research in the world that has been published in regards to the 3D printing of customized orthodontic power-arms, that utilizes finite element modelling to upgrade an existing design of the individualized 3D printed power-arm.
As a result of the technical part of this research, we have managed to increase the strength by 7% and reduce the stress by up to 82%. The modifications we make increase the service life of the given component several times, which in practice means a lesser load for the attending physician, due to the need to glue a new arm, but also a lesser load for the patient.
The stress at the interface between tooth surface and the power-arm is important. One of the limitations of this study is the lack of analysis of this connection and adhesives [
39].
Other limitations of this study are those characteristics of design and methodology that influenced the interpretation of the findings. For example, the individuality of behavior and eating habits of the patients has not been researched. These might have influence on result interpretations.
Considering other pros and cons of this new approach, another limitation is the technical and computer skills of doctors. Capability to perform technical analysis of the material requires interdisciplinary cooperation, however, the new trend is the online availability of all certified material properties regarding biocompatible resins for 3D printing. So, the advantage of this situation is that only FEM analysis for redesigning current individualized intraoral appliances will be necessary.
The novelty of the proposed approach rests not only in the biocompatible 3D printing of an intraoral appliance never 3D printed before, but also in the way of its individualization based on digital intraoral scans, and its redesign based on FEM analysis.
Today, there are many clinically applied customized intraoral appliances that might benefit from the described novel approach. It is the patients that will enjoy significant improvements in clinical performance of the intraoral appliances, such as 3D printed obturators in cleft patients, 3D printed hyrax and other devices in distraction osteogenesis, and many more.
This article shows medical clinicians, working with 3D printed customized appliances from certified biocompatible resins, that there are methods they can employ to improve their designs. Currently, the technical material datasheet is provided to all certified materials, so the material research is not necessary, and they can start directly with FEM modeling with data provided by the manufacturer. For redesigning an already optimized design, a convergence study should be done on FEM, as the mesh convergence is an important issue that needs to be addressed. It must be remembered that, when performing theoretical redesigning without planned clinical application and whilst experimenting with uncertified unknown materials, the material properties must be researched properly in compliance with ISO standards with significantly more specimens that two. The material research step in this article is for demonstration purposes only and the researched material parameters were confirmed by the manufacturer during this process (2019).
Dental LT Clear Resin (RS-F2-DLCL-01—version 1) used in this research is not produced anymore and was substituted with version 2 with all mechanical properties available (technical data sheet: [
40]). However, this research is not focused on a particular material, it is about the redesigning and clinical evaluation of customized AM biocompatible appliances.
To better simulate all possible forces affecting the power-arm, this research could employ much more sophisticated technical solutions. For example, the modeling of possible combined axial–torsional non-proportional loadings that were used to simulate shape–memory alloys (SMAs) [
40]. Though in our use case, where we are well aware of the force from the elastic band to the power-arm and also with the well-defined bonding area, more sophisticated methods are not clinically indispensable [
41]. Moreover, topology optimization analysis in combination with shape and sizing optimization can be employed. We have also considered using artificial intelligence simulating material properties of the 3D printed object [
42]. All these methods are possible and suitable for high-end engineering tasks, albeit not crucial for this level of guided power-arm redesign. In this clinical application, where every power-arm is positioned virtually by a doctor on the intraoral scan and then its shape is adapted to the clinical condition of the patient, this would also currently be an analytic overkill. Every 3D printed power-arm in every patient will be different and possibly even manually abraded with a dental drill in the oral cavity by an orthodontist. However, for future research, this is an interesting consideration. Possibly, with this new direction of using topology-optimized analysis, some other more sophisticated intraoral devices might be redesigned [
42].