A Short S-Equol Exposure Has a Long-Term Inhibitory Effect on Adipogenesis in Mouse 3T3-L1 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assays
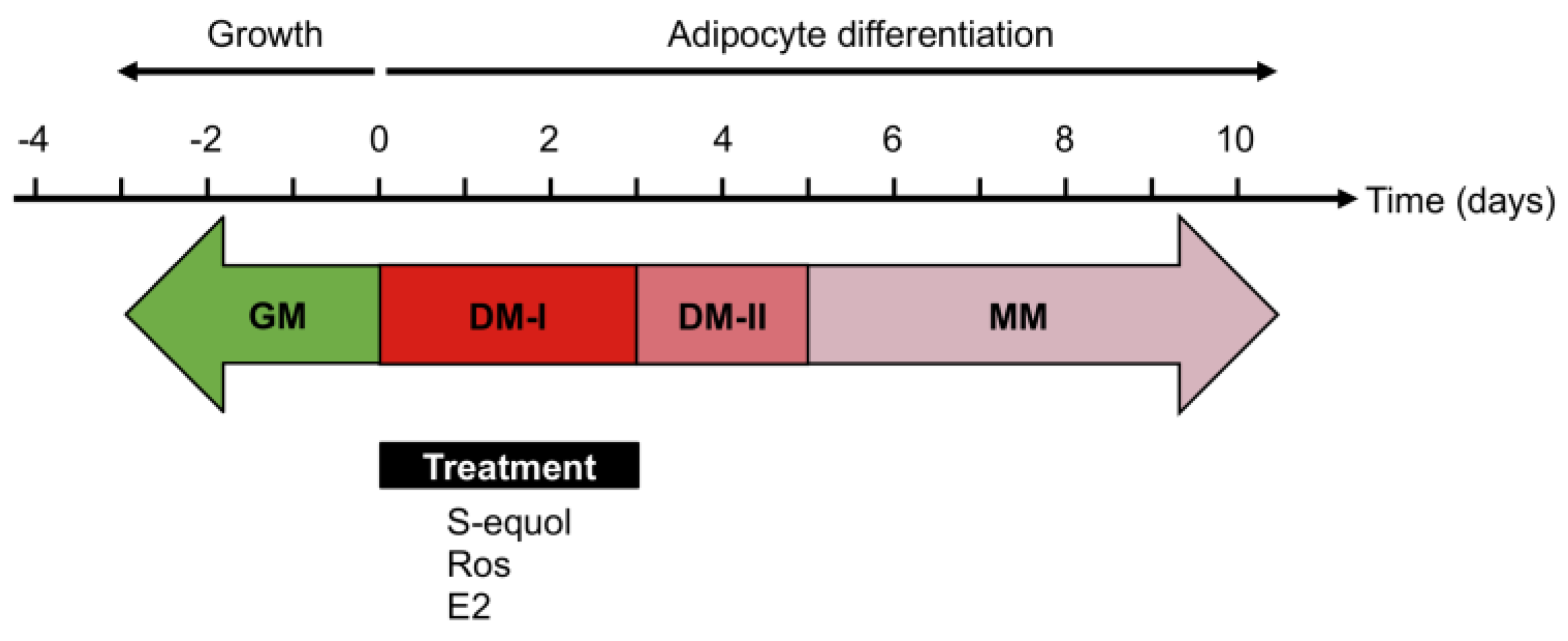
2.3. Adipocyte Differentiation
2.4. Oil Red O Staining
2.5. Real-Time qRT-PCR (Real-Time Quantitative Reverse Transcription-PCR)
2.6. Determination of Adipokine Secretion
2.7. Statistical Analyses
3. Results
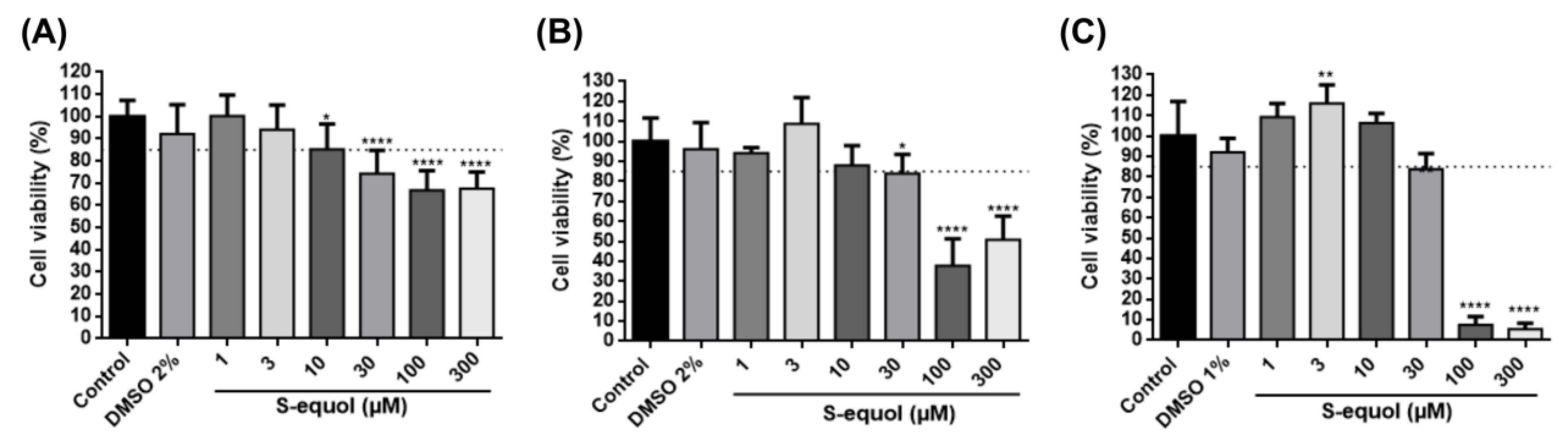
3.1. S-Equol at 1, 3, and 10 µM Does Not Affect 3T3-L1 Cell Viability
3.2. S-Equol Inhibits Adipocyte Differentiation of Cells 3T3-L1
3.3. S-Equol Inhibits Lipid Accumulation
3.4. S-Equol Affects the Expression of Pro-Adipogenic Markers
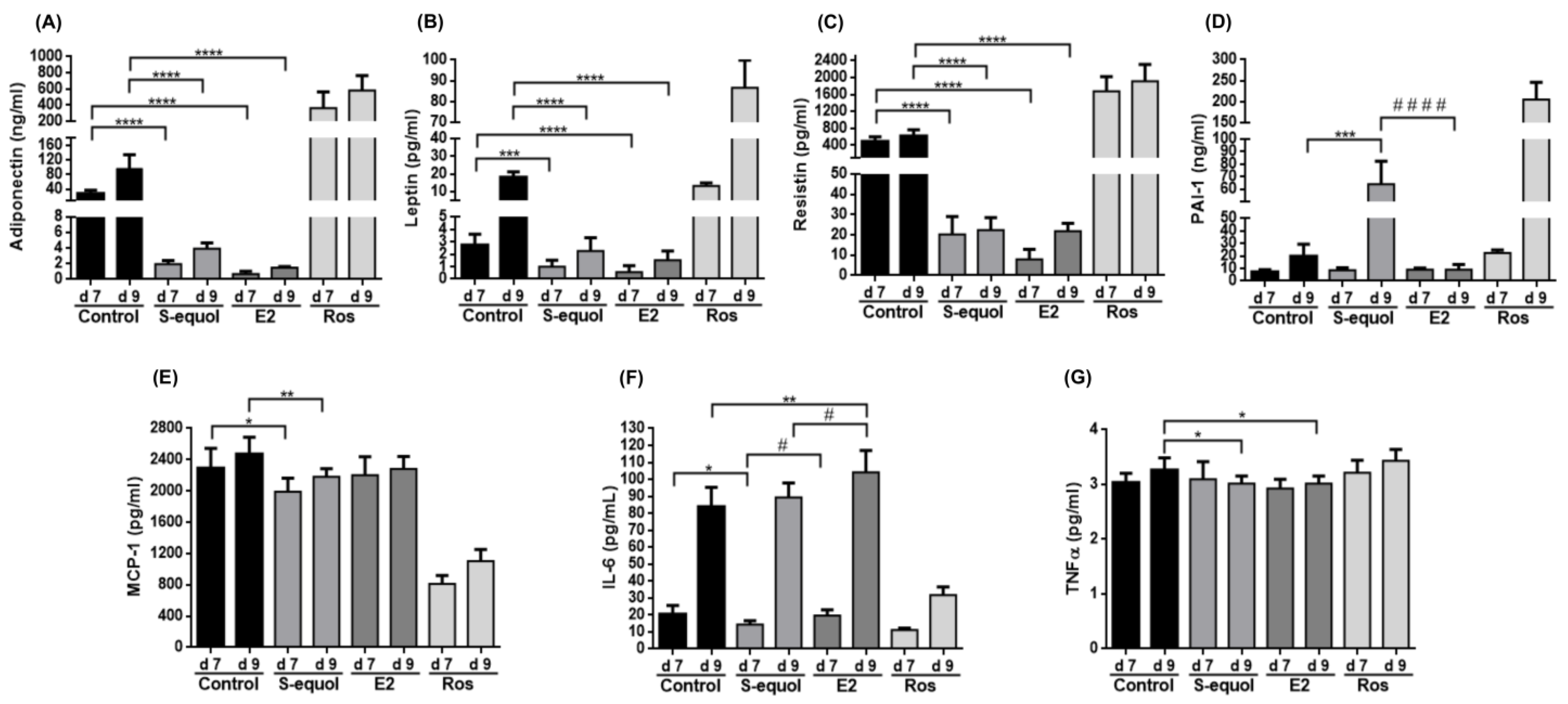
3.5. S-Equol Reduces Adipokine Secretion
3.6. S-Equol Reduces ERα Expression and Attenuates the Subexpression of ERβ
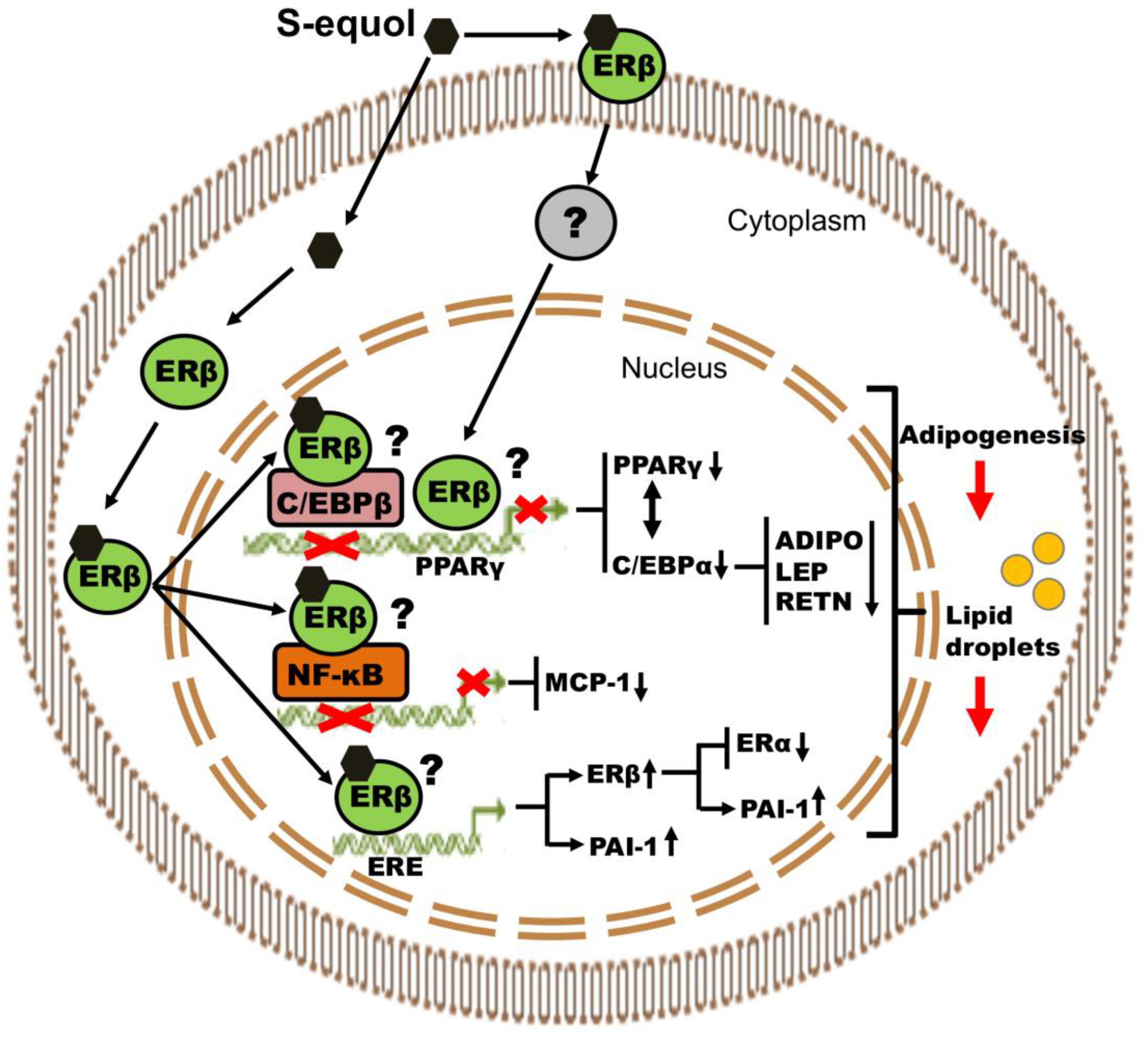
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 906, 1–17. [Google Scholar] [CrossRef]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.; Guillermier, C.; Patwari, P.; Steinhauser, M.L. Loss of White Adipose Hyperplastic Potential Is Associated with Enhanced Susceptibility to Insulin Resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Abranches, M.V.; de Oliveira, F.C.E.; da Conceição, L.L.; Peluzio, M.D.C.G. Obesity and diabetes: The link between adipose tissue dysfunction and glucose homeostasis. Nutr. Res. Rev. 2015, 28, 121–132. [Google Scholar] [CrossRef]
- Jeffery, E.; Church, C.D.; Holtrup, B.; Colman, L.; Rodeheffer, M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 2015, 17, 376–385. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Updat. 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Foryst-Ludwig, A.; Clemenz, M.; Hohmann, S.; Hartge, M.; Sprang, C.; Frost, N.; Krikov, M.; Bhanot, S.; Barros, R.; Morani, A.; et al. Metabolic Actions of Estrogen Receptor Beta (ERβ) are Mediated by a Negative Cross-Talk with PPARγ. PLoS Genet. 2008, 4, e1000108. [Google Scholar] [CrossRef]
- Santen, R.J.; Allred, D.C.; Ardoin, S.P.; Archer, D.F.; Boyd, N.; Braunstein, G.D.; Burger, H.G.; Colditz, G.A.; Davis, S.R.; Gambacciani, M.; et al. Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2010, 95, s1–s66. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.; Korach-André, M. Estrogen Receptor beta (ERβ) Regulation of Lipid Homeostasis—Does Sex Matter? Metabolites 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Yepuru, M.; Eswaraka, J.; Kearbey, J.D.; Barrett, C.M.; Raghow, S.; Veverka, K.A.; Miller, D.D.; Dalton, J.T.; Narayanan, R. Estrogen Receptor-β-selective Ligands Alleviate High-fat Diet- and Ovariectomy-induced Obesity in Mice. J. Biol. Chem. 2010, 285, 31292–31303. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kasparovska, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Setchell, K.D. The history and basic science development of soy isoflavones. Menopause 2017, 24, 1338–1350. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: History, Chemistry, and Formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Setchell, K.D.; Borriello, S.P.; Hulme, P.; Kirk, D.N.; Axelson, M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984, 40, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Greiwe, J.S.; Schwen, R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr. Rev. 2011, 69, 432–448. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of naturalS-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2012, 78, 365–372. [Google Scholar] [CrossRef]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Antihyperglycemic effect of equol, a daidzein derivative, in cultured L6 myocytes andob/obmice. Mol. Nutr. Food Res. 2014, 58, 267–277. [Google Scholar] [CrossRef]
- Blake, C.; Fabick, K.M.; Setchell, K.D.; Lund, T.D.; Lephart, E.D. Neuromodulation by soy diets or equol: Anti-depressive & anti-obesity-like influences, age- & hormone-dependent effects. BMC Neurosci. 2011, 12, 28. [Google Scholar] [CrossRef]
- Rachoń, D.; Vortherms, T.; Seidlovä-Wuttke, D.; Wuttke, W. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague-Dawley rats. Menopause 2007, 14, 925–932. [Google Scholar] [CrossRef]
- Bax, E.N.; Cochran, K.E.; Mao, J.; Wiedmeyer, C.E.; Rosenfeld, C.S. Opposing effects of S-equol supplementation on metabolic and behavioral parameters in mice fed a high-fat diet. Nutr. Res. 2018, 64, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Lee, O.-H.; Banz, W.J.; Moustaid-Moussa, N.; Shay, N.F.; Kim, Y.-C. Daidzein and the daidzein metabolite, equol, enhance adipocyte differentiation and PPARγ transcriptional activity. J. Nutr. Biochem. 2010, 21, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Tousen, Y.; Inada, M.; Miyaura, C.; Ishimi, Y. Bi-Phasic Effect of Equol on Adipocyte Differentiation of MC3T3-L1 Cells. Biosci. Biotechnol. Biochem. 2013, 77, 201–204. [Google Scholar] [CrossRef][Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Ojima, K.; Oe, M.; Nakajima, I.; Muroya, S.; Nishimura, T. Dynamics of protein secretion during adipocyte differentiation. FEBS Open Bio. 2016, 6, 816–826. [Google Scholar] [CrossRef]
- Zhang, W.; Schmull, S.; Du, M.; Liu, J.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Estrogen Receptor α and β in Mouse: Adipose-Derived Stem Cell Proliferation, Migration, and Brown Adipogenesis In Vitro. Cell. Physiol. Biochem. 2016, 38, 2285–2299. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors α and β in human adipose cells: Regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 2004, 286, C655–C661. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Bruun, J.; Hube, F.; Kristensen, K.; Hauner, H.; Richelsen, B. Demonstration of estrogen receptor subtypes α and β in human adipose tissue: Influences of adipose cell differentiation and fat depot localization. Mol. Cell. Endocrinol. 2001, 182, 27–37. [Google Scholar] [CrossRef]
- Kawano, J.; Arora, R. The Role of Adiponectin in Obesity, Diabetes, and Cardiovascular Disease. J. CardioMetabolic Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More Than Just Another Fat Cell Hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin, leptin receptors, and the control of body weight. Nutr. Rev. 1998, 56, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Lazar, M.A. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 2002, 13, 18–23. [Google Scholar] [CrossRef]
- Filkova, M.; Haluzik, M.; Gay, S.; Senolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Niwa, T.; Yokoyama, S.-I.; Ito, T.; Osawa, T. Reduction of leptin secretion by soy isoflavonoids in murine adipocytes in vitro. Phytochem. Lett. 2010, 3, 122–125. [Google Scholar] [CrossRef]
- Wagner, J.D.; Zhang, L.; Shadoan, M.K.; Kavanagh, K.; Chen, H.; Tresnasari, K.; Kaplan, J.R.; Adams, M.R. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism 2008, 57, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary Soy Protein Isolate Ameliorates Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice Potentially by Inhibiting Monocyte Chemoattractant Protein-1 Expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, C.; Luo, J.; Wei, Y.; Wang, W.; Zhong, Y. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol. Med. Rep. 2017, 17, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ekmen, N.; Helvaci, A.; Gunaldi, M.; Sasani, H.; Yildirmak, S.T. Leptin as an important link between obesity and cardiovascular risk factors in men with acute myocardial infarction. Indian Hear. J. 2016, 68, 132–137. [Google Scholar] [CrossRef]
- Liuzzi, A.; Savia, G.; Tagliaferri, M.; Lucantoni, R.; Berselli, M.; Petroni, M.; De Medici, C.; Viberti, G. Serum leptin concentration in moderate and severe obesity: Relationship with clinical, anthropometric and metabolic factors. Int. J. Obes. 1999, 23, 1066–1073. [Google Scholar] [CrossRef]
- Palhinha, L.; Liechocki, S.; Hottz, E.D.; Pereira, J.A.D.S.; De Almeida, C.J.; Vieira, P.; Bozza, P.T.; Maya-Monteiro, C.M. Leptin Induces Proadipogenic and Proinflammatory Signaling in Adipocytes. Front. Endocrinol. 2019, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Tsuchiya, H.; Hama, S.; Kajimoto, K.; Kogure, K. Resistin affects lipid metabolism during adipocyte maturation of 3T3-L1 cells. FEBS J. 2013, 280, 5884–5895. [Google Scholar] [CrossRef]
- Liang, X.; Kanjanabuch, T.; Mao, S.-L.; Hao, C.-M.; Tang, Y.-W.; Declerck, P.; Hasty, A.; Wasserman, D.H.; Fogo, A.B.; Ma, L.-J. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E103–E113. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Jeong, S. Repression of PPARγ Activity on Adipogenesis by 17β-estradiol in Differentiated 3T3-L1 Cell. J. Exp. Bio-Med. Sci. 2009, 15, 179–185. [Google Scholar]
- Jeong, S.; Yoon, M. 17β-Estradiol inhibition of PPARγ-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol. Sin. 2011, 32, 230–238. [Google Scholar] [CrossRef]
- Yoon, M.; Jeong, S. 17β-estradiol Prevents the Expression of CEBPα-mediated Adipocyte Marker Genes in Female Ovariecto-mized C57BL/6 Mice. J. Exp. Biomed. Sci. 2008, 14, 131–137. [Google Scholar]
- Naaz, A.; Zakroczymski, M.; Heine, P.; Taylor, J.; Saunders, P.; Lubahn, D.; Cooke, P.S. Effect of Ovariectomy on Adipose Tissue of Mice in the Absence of Estrogen Receptor Alpha (ERα): A Potential Role for Estrogen Receptor Beta (ERβ). Horm. Metab. Res. 2002, 34, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic activation of estrogen receptor α increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J. 2017, 31, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.W.; Shin, J.-H.; Seo, H.S.; Lee, J.K.; Oh, M.-J.; Kim, T.; Saw, H.S.; Kim, S.-H.; Hur, J.-Y. Role of Estrogen Receptor-α and -β in Regulating Leptin Expression in 3T3-L1 Adipocytes. Obesity 2008, 16, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, T.; Zhou, M.; He, G.; Ma, Y.; Shi, Y.; Wang, Y.; Tang, H.; Kang, X.; Yang, M.; et al. Absence of estrogen receptor beta leads to abnormal adipogenesis during early tendon healing by an up-regulation of PPARγ signalling. J. Cell. Mol. Med. 2019, 23, 7406–7416. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Movérare, S.; Skrtic, S.; Gao, H.; Dahlman-Wright, K.; Gustafsson, J.-A.; Ohlsson, C. Estrogen Receptor (ER)-β Reduces ERα-Regulated Gene Transcription, Supporting a “Ying Yang” Relationship between ERα and ERβ in Mice. Mol. Endocrinol. 2003, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.M.; McDonnell, D.P. The Estrogen Receptor β-Isoform (ERβ) of the Human Estrogen Receptor Modulates ERα Transcriptional Activity and Is a Key Regulator of the Cellular Response to Estrogens and Antiestrogens1. Endocrinology 1999, 140, 5566–5578. [Google Scholar] [CrossRef]
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.-A. Estrogen Receptor (ER) β Modulates ERα-Mediated Transcriptional Activation by Altering the Recruitment of c-Fos and c-Jun to Estrogen-Responsive Promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef]
- Stein, B.; Yang, M.X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell. Biol. 1995, 15, 4971–4979. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Y.; Bucher, N.L.; Farmer, S. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev. 1995, 9, 2350–2363. [Google Scholar] [CrossRef]
- Zuo, Y.; Qiang, L.; Farmer, S. Activation of CCAAT/Enhancer-binding Protein (C/EBP) α Expression by C/EBPβ during Adipogenesis Requires a Peroxisome Proliferator-activated Receptor-γ-associated Repression of HDAC1 at the C/ebpα Gene Promoter. J. Biol. Chem. 2006, 281, 7960–7967. [Google Scholar] [CrossRef]
- Hartman, H.B.; Hu, X.; Tyler, K.X.; Dalal, C.K.; Lazar, M.A. Mechanisms Regulating Adipocyte Expression of Resistin. J. Biol. Chem. 2002, 277, 19754–19761. [Google Scholar] [CrossRef]
- Mason, M.M.; He, Y.; Chen, H.; Quon, M.J.; Reitman, M. Regulation of Leptin Promoter Function by Sp1, C/EBP, and a Novel Factor 1. Endocrinology 1998, 139, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; MacLean, P.; Schaack, J.; Orlicky, D.J.; Darimont, C.; Pagliassotti, M.; Friedman, J.E.; Shao, J. C/EBP Regulates Human Adiponectin Gene Transcription Through an Intronic Enhancer. Diabetes 2005, 54, 1744–1754. [Google Scholar] [CrossRef]
- Fox, K.E.; Fankell, D.M.; Erickson, P.F.; Majka, S.M.; Crossno, J.T., Jr.; Klemm, D.J. Depletion of cAMP-response Element-binding Protein/ATF1 Inhibits Adipogenic Conversion of 3T3-L1 Cells Ectopically Expressing CCAAT/Enhancer-binding Protein (C/EBP) α, C/EBP β, or PPARγ2. J. Biol. Chem. 2006, 281, 40341–40353. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Yoshimoto, T.; Hirono, Y.; Tateno, T.; Sugiyama, T.; Hirata, Y. Angiotensin II induces monocyte chemoattractant protein-1 expression via a nuclear factor-κB-dependent pathway in rat preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E771–E778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahluwalia, A.; Hoa, N.; Ge, L.; Blumberg, B.; Levin, E.R. Mechanisms by Which Membrane and Nuclear ER Alpha Inhibit Adipogenesis in Cells Isolated from Female Mice. Endocrinology 2020, 161, bqaa175. [Google Scholar] [CrossRef]
- Smith, L.H.; Coats, S.R.; Qin, H.; Petrie, M.S.; Covington, J.W.; Su, M.; Eren, M.; Vaughan, D.E. Differential and Opposing Regulation of PAI-1 Promoter Activity by Estrogen Receptor α and Estrogen Receptor β in Endothelial Cells. Circ. Res. 2004, 95, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-C.; Yeh, C.-C.; Nojima, D.; Dahiya, R. Cloning and Characterization of Human Estrogen Receptor β Promoter. Biochem. Biophys. Res. Commun. 2000, 275, 682–689. [Google Scholar] [CrossRef]
- Lee, C.; Huang, C.-H. LASAGNA-Search: An integrated web tool for transcription factor binding site search and visualization. BioTechniques 2013, 54, 141–153. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandujano-Lázaro, G.; Galaviz-Hernández, C.; Reyes-López, C.A.; Almanza-Pérez, J.C.; Giacoman-Martínez, A.; López-Camarillo, C.; Huang, F.; Marchat, L.A. A Short S-Equol Exposure Has a Long-Term Inhibitory Effect on Adipogenesis in Mouse 3T3-L1 Cells. Appl. Sci. 2021, 11, 9657. https://doi.org/10.3390/app11209657
Mandujano-Lázaro G, Galaviz-Hernández C, Reyes-López CA, Almanza-Pérez JC, Giacoman-Martínez A, López-Camarillo C, Huang F, Marchat LA. A Short S-Equol Exposure Has a Long-Term Inhibitory Effect on Adipogenesis in Mouse 3T3-L1 Cells. Applied Sciences. 2021; 11(20):9657. https://doi.org/10.3390/app11209657
Chicago/Turabian StyleMandujano-Lázaro, Gilberto, Carlos Galaviz-Hernández, César A. Reyes-López, Julio C. Almanza-Pérez, Abraham Giacoman-Martínez, César López-Camarillo, Fengyang Huang, and Laurence A. Marchat. 2021. "A Short S-Equol Exposure Has a Long-Term Inhibitory Effect on Adipogenesis in Mouse 3T3-L1 Cells" Applied Sciences 11, no. 20: 9657. https://doi.org/10.3390/app11209657
APA StyleMandujano-Lázaro, G., Galaviz-Hernández, C., Reyes-López, C. A., Almanza-Pérez, J. C., Giacoman-Martínez, A., López-Camarillo, C., Huang, F., & Marchat, L. A. (2021). A Short S-Equol Exposure Has a Long-Term Inhibitory Effect on Adipogenesis in Mouse 3T3-L1 Cells. Applied Sciences, 11(20), 9657. https://doi.org/10.3390/app11209657







