Protective Effects of Annatto Tocotrienol and Palm Tocotrienol-Rich Fraction on Chondrocytes Exposed to Monosodium Iodoacetate
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Cytotoxicity Assay
2.4. Whole Cell Lysate Preparation
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
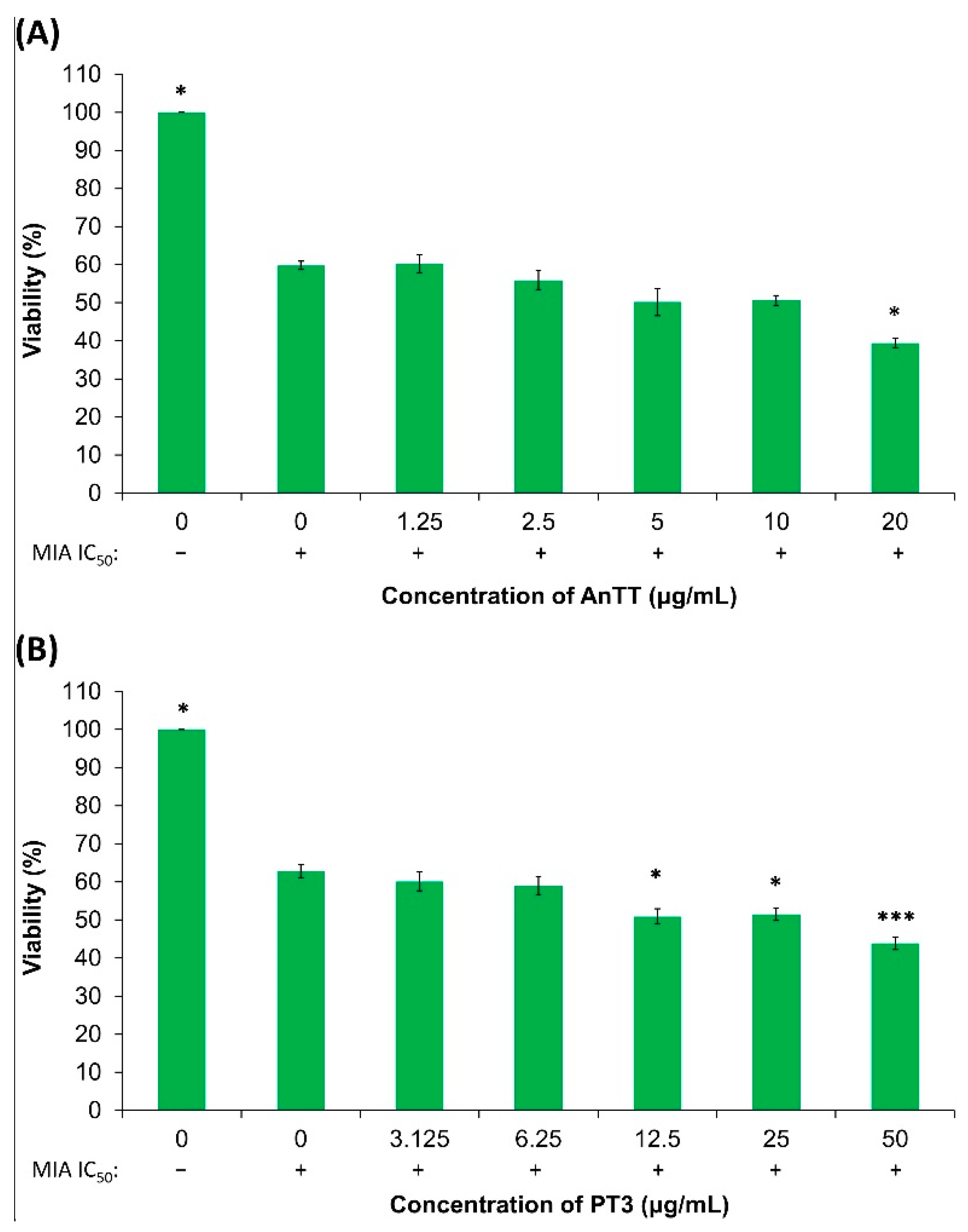
3.1. Cytotoxicity of AnTT and PT3 on SW1353 Chondrocytes
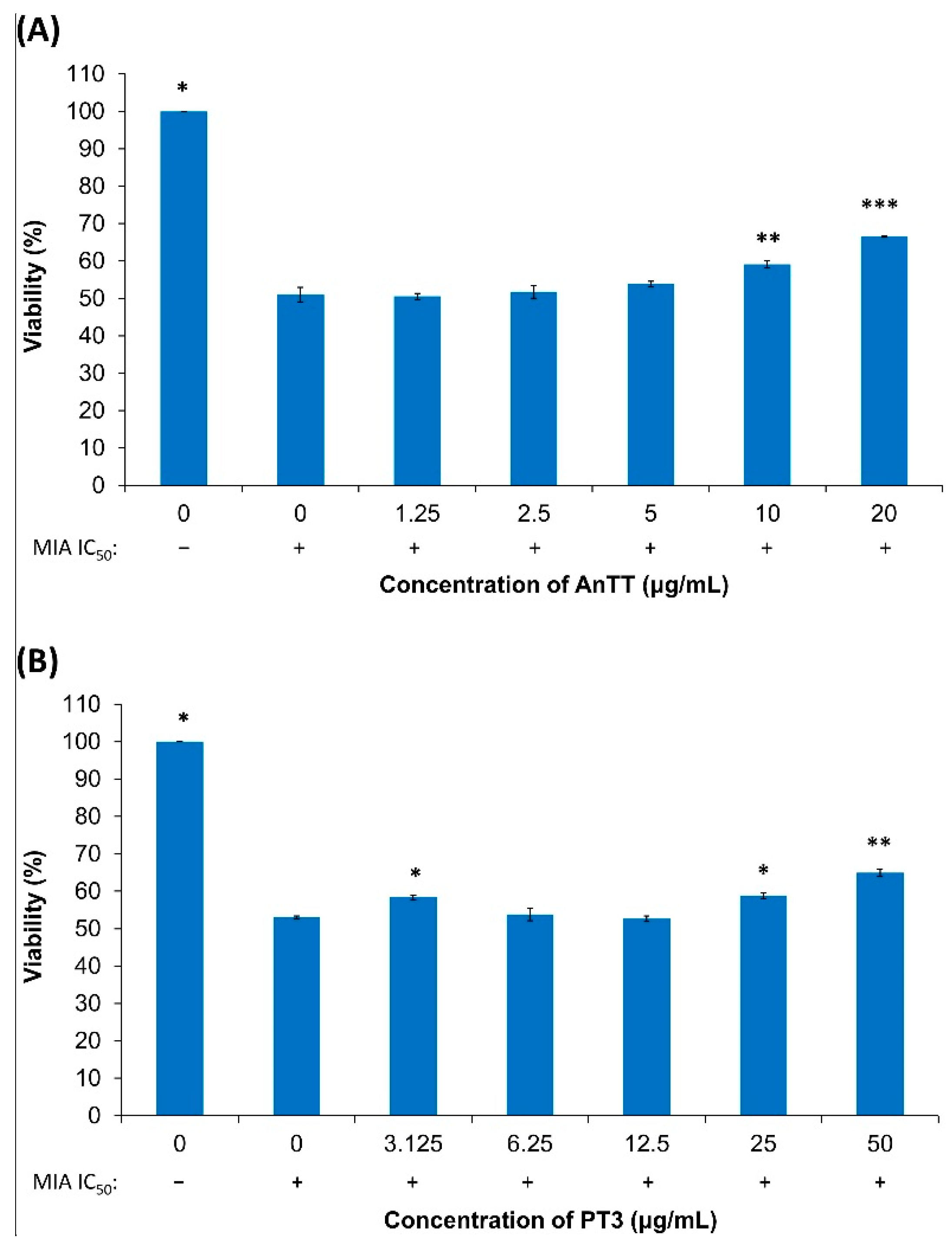
3.2. The Effects of AnTT or PT3 Pre-Treatment on MIA-Induced SW1353 Cell Death
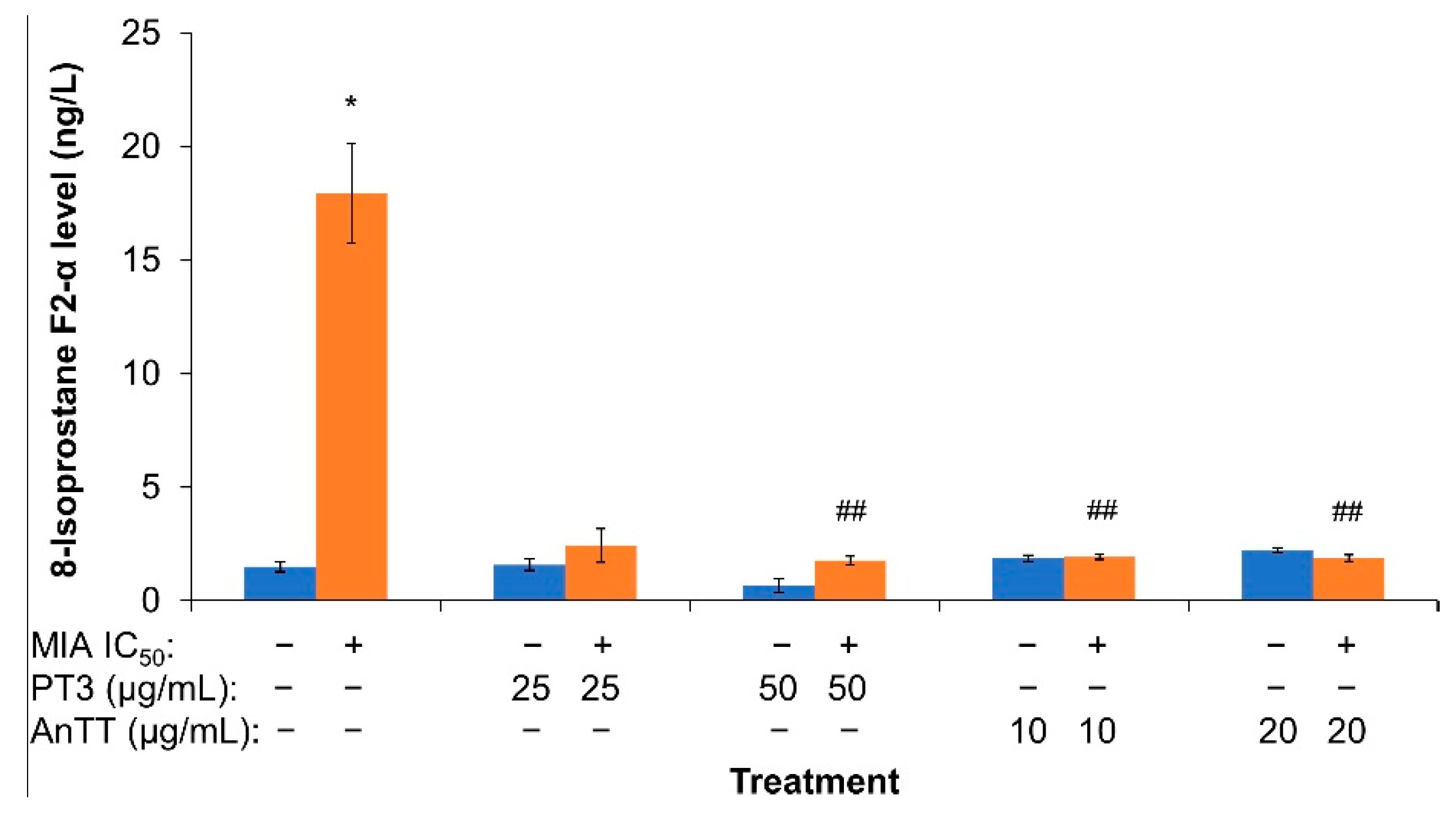
3.3. The Effects of AnTT or PT3 Co-Treatment on 8-Isoprostane F2-α Level
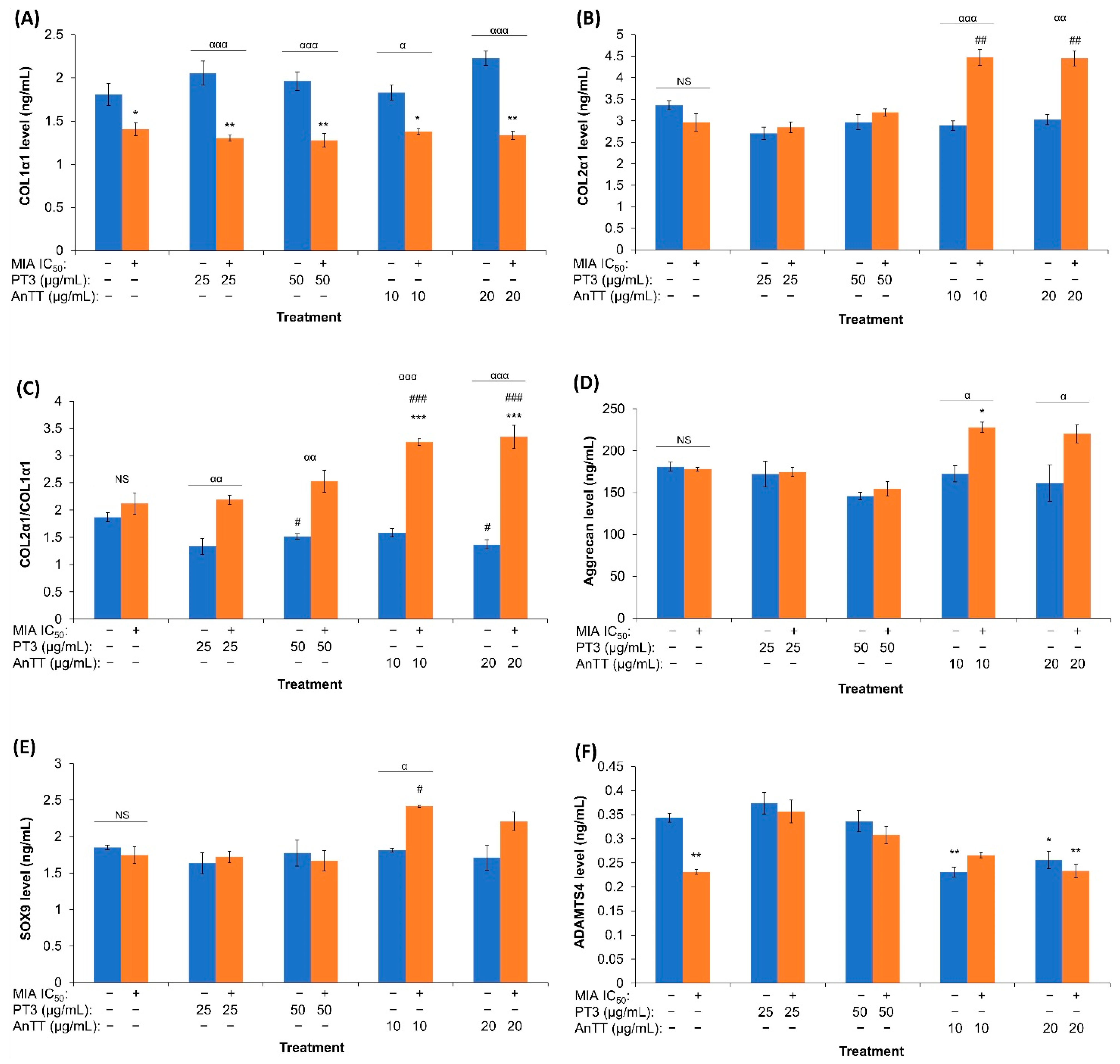
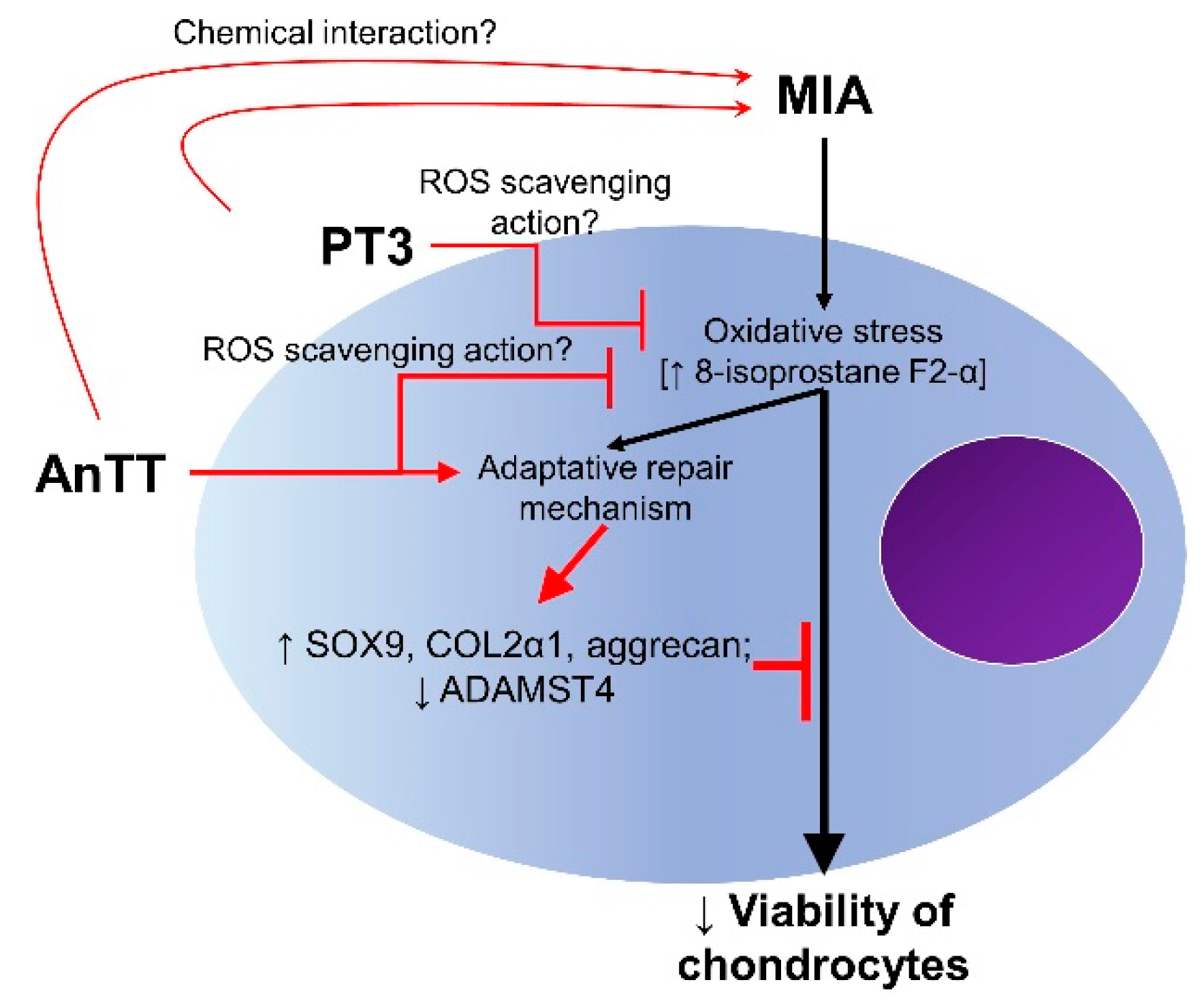
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ebell, M.H. Osteoarthritis: Rapid Evidence Review. Am. Fam. Physician 2018, 97, 523–526. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29-30, 100587. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Osteoarthritis —Level 3 Cause. Available online: http://www.healthdata.org/results/gbd_summaries/2019/osteoarthritis-level-3-cause (accessed on 15 June 2021).
- Wang, H.; Bai, J.; He, B.; Hu, X.; Liu, D. Osteoarthritis and the risk of cardiovascular disease: A meta-analysis of observational studies. Sci. Rep. 2016, 6, 39672. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Maggi, S.; Luchini, C.; Solmi, M.; Smith, T.; Denkinger, M.; Hurley, M.; Thompson, T.; Manzato, E.; et al. Osteoarthritis and mortality: A prospective cohort study and systematic review with meta-analysis. Semin. Arthritis Rheum. 2016, 46, 160–167. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef]
- Zamli, Z.; Sharif, M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 159–166. [Google Scholar] [CrossRef]
- Mobasheri, A. Role of chondrocyte death and hypocellularity in ageing human articular cartilage and the pathogenesis of osteoarthritis. Med. Hypotheses 2002, 58, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef]
- Charlier, E.; Relic, B.; Deroyer, C.; Malaise, O.; Neuville, S.; Collée, J.; Malaise, M.G.; De Seny, D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2146. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Lambert, C.; Zappia, J.; Sanchez, C.; Florin, A.; Dubuc, J.-E.; Henrotin, Y. The Damage-Associated Molecular Patterns (DAMPs) as Potential Targets to Treat Osteoarthritis: Perspectives From a Review of the Literature. Front. Med. 2021, 7, 607186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.L.; Pearsall, A.W.; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Chow, Y.Y.; Leong, L.M.; Law, J.X.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Phys. Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Farooq, M. Unicompartmental Knee Arthroplasty: Indications, Outcomes, and Complications. Conn. Med. 2017, 81, 87–90. [Google Scholar]
- Gui, Q.; Zhang, X.; Liu, L.; Zhao, F.; Cheng, W.; Zhang, Y. Cost-utility analysis of total knee arthroplasty for osteoarthritis in a regional medical center in China. Health Econ. Rev. 2019, 9, 15. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Swaminathan, M.; Chakravarthi, S.; Radhakrishnan, A. Therapeutic efficacy of vitamin E δ-tocotrienol in collagen-induced rat model of arthritis. Biomed Res. Int. 2014, 2014, 539540. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Tudawe, D.; Chakravarthi, S.; Chiew, G.S.; Haleagrahara, N. Effect of γ-tocotrienol in counteracting oxidative stress and joint damage in collagen-induced arthritis in rats. Exp. Ther. Med. 2014, 7, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Zainal, Z.; Rahim, A.A.; Radhakrishnan, A.K.; Chang, S.K.; Khaza’ai, H. Investigation of the curative effects of palm vitamin E tocotrienols on autoimmune arthritis disease in vivo. Sci. Rep. 2019, 9, 16793. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Wong, S.K.; Japar Sidik, F.Z.; Abdul Hamid, J.; Abas, N.H.; Mohd Ramli, E.S.; Afian Mokhtar, S.; Rajalingham, S.; Ima Nirwana, S. The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate. Int. J. Environ. Res. Public Health 2019, 16, 2897. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, H.M.; Chin, K.-Y.; Ahmad, F.; Mohd Ramli, E.S.; Arlamsyah, A.M.; Japar Sidik, F.Z.; Abdul Hamid, J.; Soelaiman, I.N. Effects of Palm Tocotrienol-Rich Fraction Alone or in Combination with Glucosamine Sulphate on Grip Strength, Cartilage Structure and Joint Remodelling Markers in a Rat Model of Osteoarthritis. Appl. Sci. 2021, 11, 8577. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis—A Review of the Current Evidence. Front. Pharm. 2018, 9, 946. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Bocci, F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 1998, 75, 1723–1727. [Google Scholar] [CrossRef]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr 2003, 12, 355–362. [Google Scholar] [PubMed]
- Wan Hasan, W.N.; Abd Ghafar, N.; Chin, K.-Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: A temporal sequential study. Drug Des. Dev. Ther. 2018, 12, 1715–1726. [Google Scholar] [CrossRef]
- Wan Hasan, W.N.; Chin, K.Y.; Abd Ghafar, N.; Soelaiman, I.N. Annatto-Derived Tocotrienol Promotes Mineralization of MC3T3-E1 Cells by Enhancing BMP-2 Protein Expression via Inhibiting RhoA Activation and HMG-CoA Reductase Gene Expression. Drug Des. Dev. Ther. 2020, 14, 969–976. [Google Scholar] [CrossRef]
- Fauzi, S.; Rajab, N.; Leong, L.; Pang, K.; Nawi, N.; Nasir, N.; Lorin, F.; Yusof, F. Apoptosis and cell cycle effect of Lignosus rhinocerus extract on HCT 116 human colorectal cancer cells. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 13–17. [Google Scholar]
- Osborne, J. Improving your data transformations: Applying the Box-Cox transformation. Pract. Assess. Res. Eval. 2010, 15, 12. [Google Scholar] [CrossRef]
- Huang, T.C.; Chang, W.T.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Yen, J.H.; Chiu, P.R.; Chang, K.L. Zinc Protects Articular Chondrocytes through Changes in Nrf2-Mediated Antioxidants, Cytokines and Matrix Metalloproteinases. Nutrients 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; Geng, C.; Gong, D.; Jiang, L.; Ishikawa, N.; Kajima, K.; Zhong, L. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J. Orthop. Res. 2013, 31, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-W.; Huang, T.-C.; Hu, Y.-C.; Hsieh, B.-S.; Chiu, P.-R.; Cheng, H.-L.; Chang, K.-L. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem. Biophys. Res. Commun. 2020, 521, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Dringen, R. Differential Effects of Iodoacetamide and Iodoacetate on Glycolysis and Glutathione Metabolism of Cultured Astrocytes. Front. Neuroenergetics 2009, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.N.; Bin-Jaliah, I.; Haidara, M.A.; Eid, R.A.; Heidar, E.H.A.; Dallak, M.; Al-Ani, B. Vitamin E ameliorates alterations to the articular cartilage of knee joints induced by monoiodoacetate and diabetes mellitus in rats. Ultrastruct Pathol. 2019, 43, 126–134. [Google Scholar] [CrossRef]
- Beecher, B.R.; Martin, J.A.; Pedersen, D.R.; Heiner, A.D.; Buckwalter, J.A. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop. J. 2007, 27, 1–8. [Google Scholar]
- Bhatti, F.U.R.; Mehmood, A.; Wajid, N.; Rauf, M.; Khan, S.N.; Riazuddin, S. Vitamin e protects chondrocytes against hydrogen peroxide-induced oxidative stress in vitro. Inflamm. Res. 2013, 62, 781–789. [Google Scholar] [CrossRef]
- Xu, W.; Mi, Y.; He, P.; He, S.; Niu, L. γ-Tocotrienol Inhibits Proliferation and Induces Apoptosis Via the Mitochondrial Pathway in Human Cervical Cancer HeLa Cells. Molecules 2017, 22, 1299. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Audano, M.; Beretta, G.; Procacci, P.; Sartori, P.; Mitro, N.; Limonta, P. Mitochondrial functional and structural impairment is involved in the antitumor activity of δ-tocotrienol in prostate cancer cells. Free Radic. Biol. Med. 2020, 160, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef]
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259. [Google Scholar] [CrossRef]
- Gruszka, J.; Pawlak, A.; Kruk, J. Tocochromanols, plastoquinol, and other biological prenyllipids as singlet oxygen quenchers—determination of singlet oxygen quenching rate constants and oxidation products. Free Radic. Biol. Med. 2008, 45, 920–928. [Google Scholar] [CrossRef]
- Housam, H.; Warid, K.; Zaid, A. Estimating the antioxidant activity for natural antioxidants (tocochromanol) and synthetic one by DPPH. Int. J. Pharm. Pharm. Sci. 2014, 5, 441–444. [Google Scholar]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic science of articular cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vecsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg Br. 2004, 86, 286–295. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, T.; Sun, H.; Wang, S.; Liu, M. Protective effects of dioscin against cartilage destruction in a monosodium iodoacetate (MIA)-indcued osteoarthritis rat model. Biomed. Pharm. 2018, 108, 1029–1038. [Google Scholar] [CrossRef]
- Lee, H.; Choi, H.-S.; Park, Y.; Ahn, C.W.; Jung, S.U.; Park, S.H.; Suh, H.J. Effects of deer bone extract on the expression of pro-inflammatory cytokine and cartilage-related genes in monosodium iodoacetate-induced osteoarthritic rats. Biosci. Biotechnol. Biochem. 2014, 78, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lim, K.S.; Choe, M.S.; Lee, M.Y.; Kim, C.; Kim, J.-S. Effects of Dipsacus asperoides Extract on Monosodium Iodoacetate–Induced Osteoarthritis in Rats Based on Gene Expression Profiling. Front. Pharm. 2021, 12, 615157. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Anti-Osteoarthritic Effects of Terminalia Chebula Fruit Extract (AyuFlex®) in Interleukin-1β-Induced Human Chondrocytes and in Rat Models of Monosodium Iodoacetate (MIA)-Induced Osteoarthritis. Appl. Sci. 2020, 10, 8698. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.; Park, S.-J.; Yun, J.m.; Oh, D.H.; Lee, J. Antarctic Krill Oil Ameliorates Monosodium Iodoacetate-Induced Irregularities in Articular Cartilage and Inflammatory Response in the Rat Models of Osteoarthritis. Nutrients 2020, 12, 3550. [Google Scholar] [CrossRef]
- Ruszymah, B.; Aminuddin, B.; Gapor, A.; Fuzina, H. The Effects of Palmvitee on Human Nasal Septal Chondrocytes Culture Expansion and Cartilage Reconstruction. ASEAN J. Sci. Technol. Dev. 2005, 22, 211–222. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Song, H.; Park, K.-H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Kothari, P.; Tripathi, A.K.; Singh, S.; Adhikary, S.; Ahmad, N.; Kumar, S.; Dev, K.; Mishra, V.K.; Shukla, S.; et al. Spinacia oleracea extract attenuates disease progression and sub-chondral bone changes in monosodium iodoacetate-induced osteoarthritis in rats. BMC Complement. Altern. Med. 2018, 18, 69. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Li, W.; Du, C.; Wang, H.; Zhang, C. Increased serum ADAMTS-4 in knee osteoarthritis: A potential indicator for the diagnosis of osteoarthritis in early stages. Genet. Mol. Res. 2014, 13, 9642–9649. [Google Scholar] [CrossRef]
- Zhang, E.; Yan, X.; Zhang, M.; Chang, X.; Bai, Z.; He, Y.; Yuan, Z. Aggrecanases in the human synovial fluid at different stages of osteoarthritis. Clin. Rheumatol. 2013, 32, 797–803. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lee, M.Y.; Choe, M.S.; Lim, K.S.; Kim, C.; Kim, J.-S. Protective effects of Phlomis umbrosa extract on a monosodium iodoacetate–induced osteoarthritis model and prediction of molecular mechanisms using transcriptomics. Phytomedicine 2021, 81, 153429. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Qi, Y.; Li, H.; Zhang, R.; Lian, Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp. Ther. Med. 2018, 16, 4737–4744. [Google Scholar] [CrossRef]
- Christen, S.; Woodall, A.A.; Shigenaga, M.K.; Southwell-Keely, P.T.; Duncan, M.W.; Ames, B.N. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: Physiological implications. Proc. Natl. Acad. Sci. USA 1997, 94, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.V.; Harwood, P.J.; Franke, A.A.; Narala, K.; Sundström, A.-K.; Berggren, P.-O.; Mordan, L.J. Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic. Biol. Med. 1995, 19, 259–269. [Google Scholar] [CrossRef]
- Cooney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-Pigott, V.; Custer, L.J.; Mordan, L.J. Gamma-tocopherol detoxification of nitrogen dioxide: Superiority to alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef]
- Salman Khan, M.; Akhtar, S.; Al-Sagair, O.A.; Arif, J.M. Protective effect of dietary tocotrienols against infection and inflammation-induced hyperlipidemia: An in vivo and in silico study. Phytother. Res. 2011, 25, 1586–1595. [Google Scholar] [CrossRef]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef]
- Chia, L.L.; Jantan, I.; Chua, K.H.; Lam, K.W.; Rullah, K.; Aluwi, M.F. Effects of tocotrienols on insulin secretion-associated genes expression of rat pancreatic islets in a dynamic culture. Front. Pharmacol. 2016, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Bradlow, B.A.; Brace, L.; Manganello, J.; Peterson, D.M.; Pearce, B.C.; Wright, J.J.; Gapor, A.; Elson, C.E. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids 1995, 30, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A.; Peterson, D.M.; Elson, C.E. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J. Nutr. 1996, 126, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, F.; Gkretsi, V.; Malizos, K.; Iliopoulos, D.; Oikonomou, P.; Poultsides, L.; Tsezou, A. Central Role of SREBP-2 in the Pathogenesis of Osteoarthritis. PLoS ONE 2012, 7, e35753. [Google Scholar] [CrossRef] [PubMed]
- Tao, K., Sr.; Tang, X.; Wang, B.; Li, J.R.; Jiang, L.; Lin, H.J. Distinct expression of SREBP-2 in normal and osteoarthritic chondrocytes. Osteoarthr. Cartil. 2015, 23, A162. [Google Scholar] [CrossRef][Green Version]
- Kadam, U.T.; Blagojevic, M.; Belcher, J. Statin use and clinical osteoarthritis in the general population: A longitudinal study. J. Gen. Intern. Med. 2013, 28, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, D.J.; Rocha, L.G.; Silva, L.A.; Brito, A.C.; Rueff-Barroso, C.R.; Porto, L.C.; Pinho, R.A. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthr. Cartil. 2010, 18, 1088–1095. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.-L.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.-Y. Protective Effects of Annatto Tocotrienol and Palm Tocotrienol-Rich Fraction on Chondrocytes Exposed to Monosodium Iodoacetate. Appl. Sci. 2021, 11, 9643. https://doi.org/10.3390/app11209643
Pang K-L, Ghafar NA, Soelaiman IN, Chin K-Y. Protective Effects of Annatto Tocotrienol and Palm Tocotrienol-Rich Fraction on Chondrocytes Exposed to Monosodium Iodoacetate. Applied Sciences. 2021; 11(20):9643. https://doi.org/10.3390/app11209643
Chicago/Turabian StylePang, Kok-Lun, Norzana Abd Ghafar, Ima Nirwana Soelaiman, and Kok-Yong Chin. 2021. "Protective Effects of Annatto Tocotrienol and Palm Tocotrienol-Rich Fraction on Chondrocytes Exposed to Monosodium Iodoacetate" Applied Sciences 11, no. 20: 9643. https://doi.org/10.3390/app11209643
APA StylePang, K.-L., Ghafar, N. A., Soelaiman, I. N., & Chin, K.-Y. (2021). Protective Effects of Annatto Tocotrienol and Palm Tocotrienol-Rich Fraction on Chondrocytes Exposed to Monosodium Iodoacetate. Applied Sciences, 11(20), 9643. https://doi.org/10.3390/app11209643







