Chemical Bond Formation between Vertically Aligned Carbon Nanotubes and Metal Substrates at Low Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
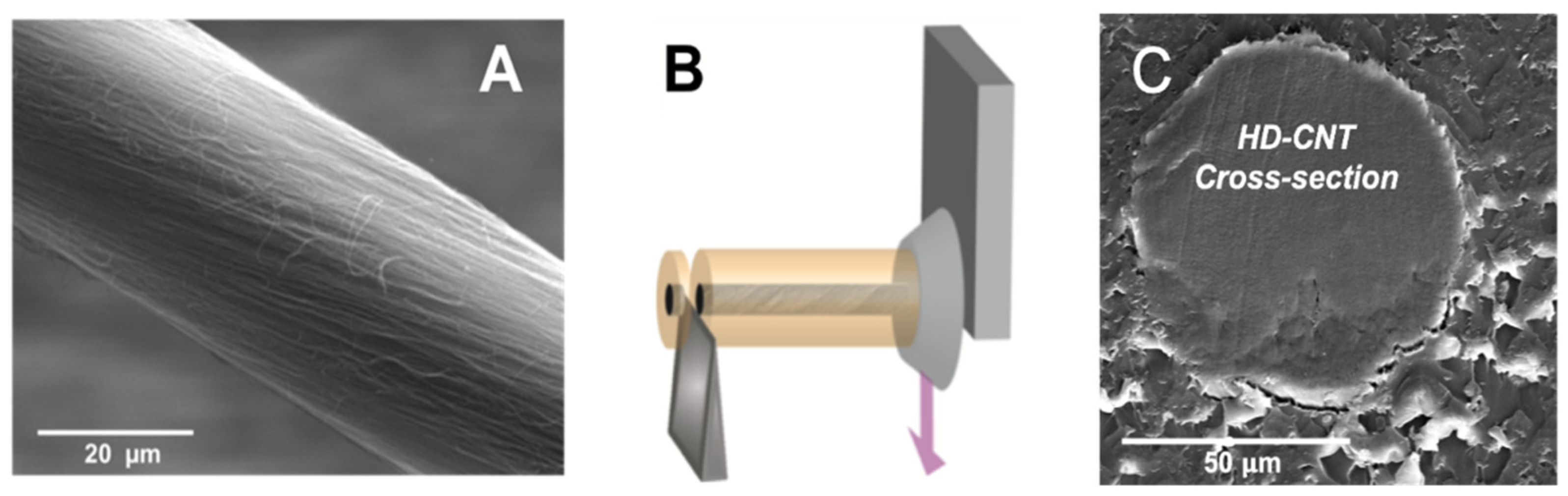
2.2. Carbon Nanotube Fiber Cross-Section Preparation and Bonding to Metal
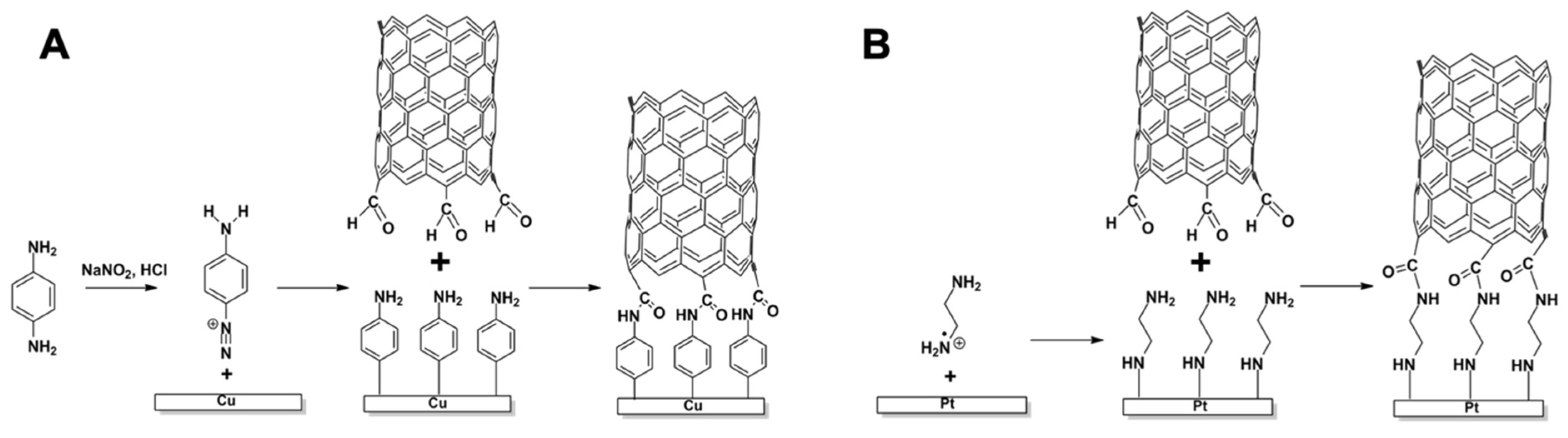
2.3. Modification of the Metal Surface
2.4. CNTs Bonded to Metal as a Working Electrode
2.5. Instrumentation
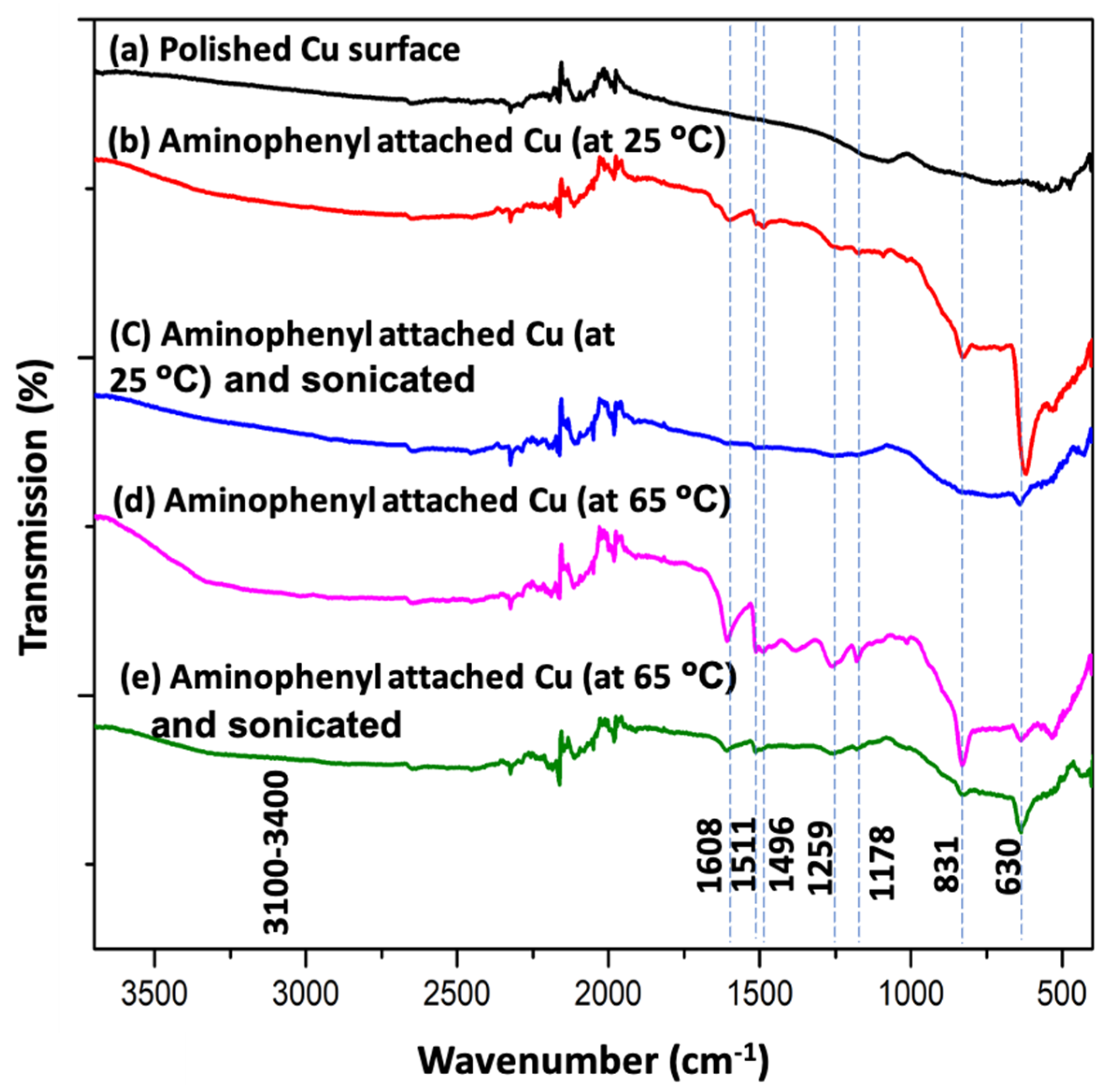
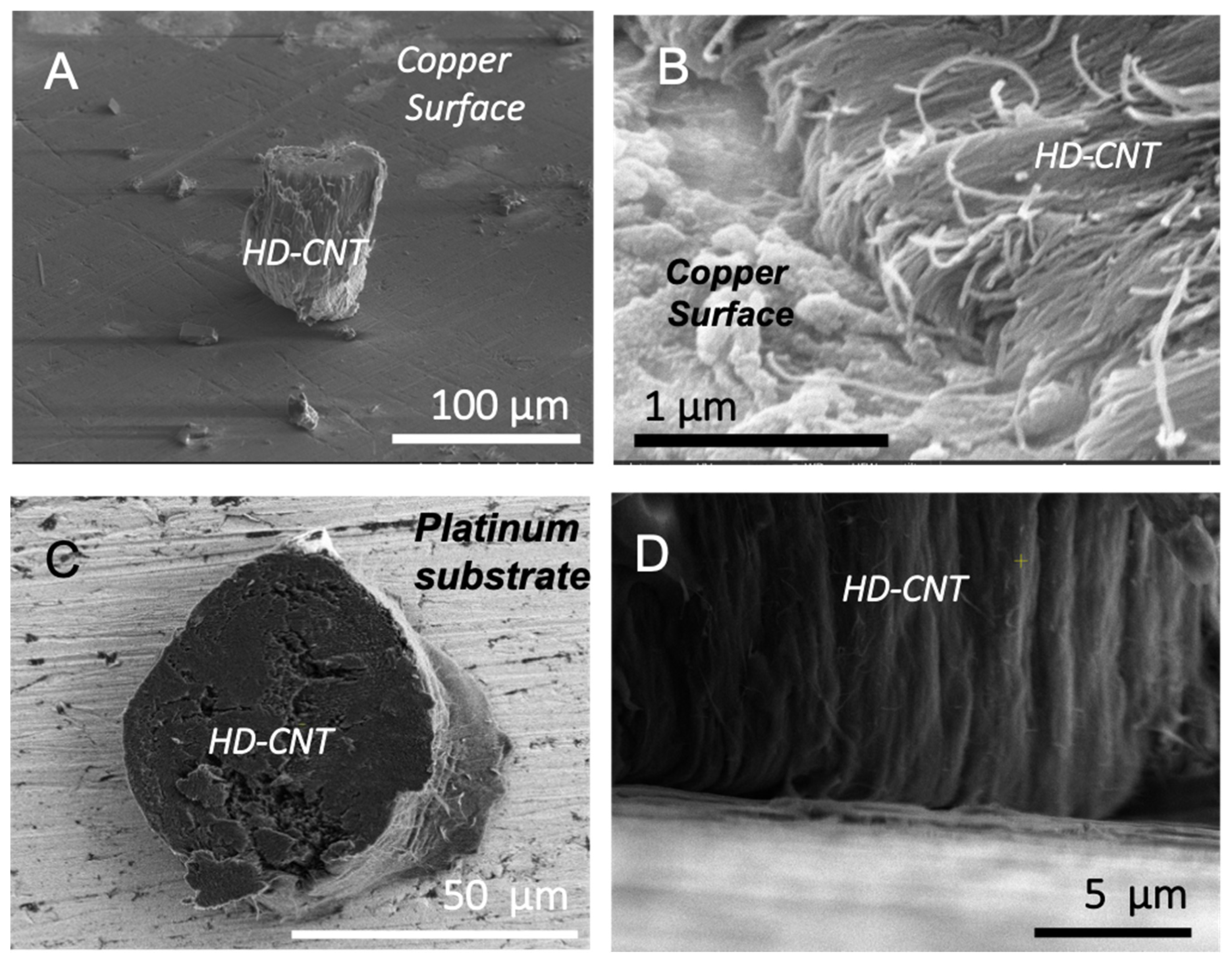
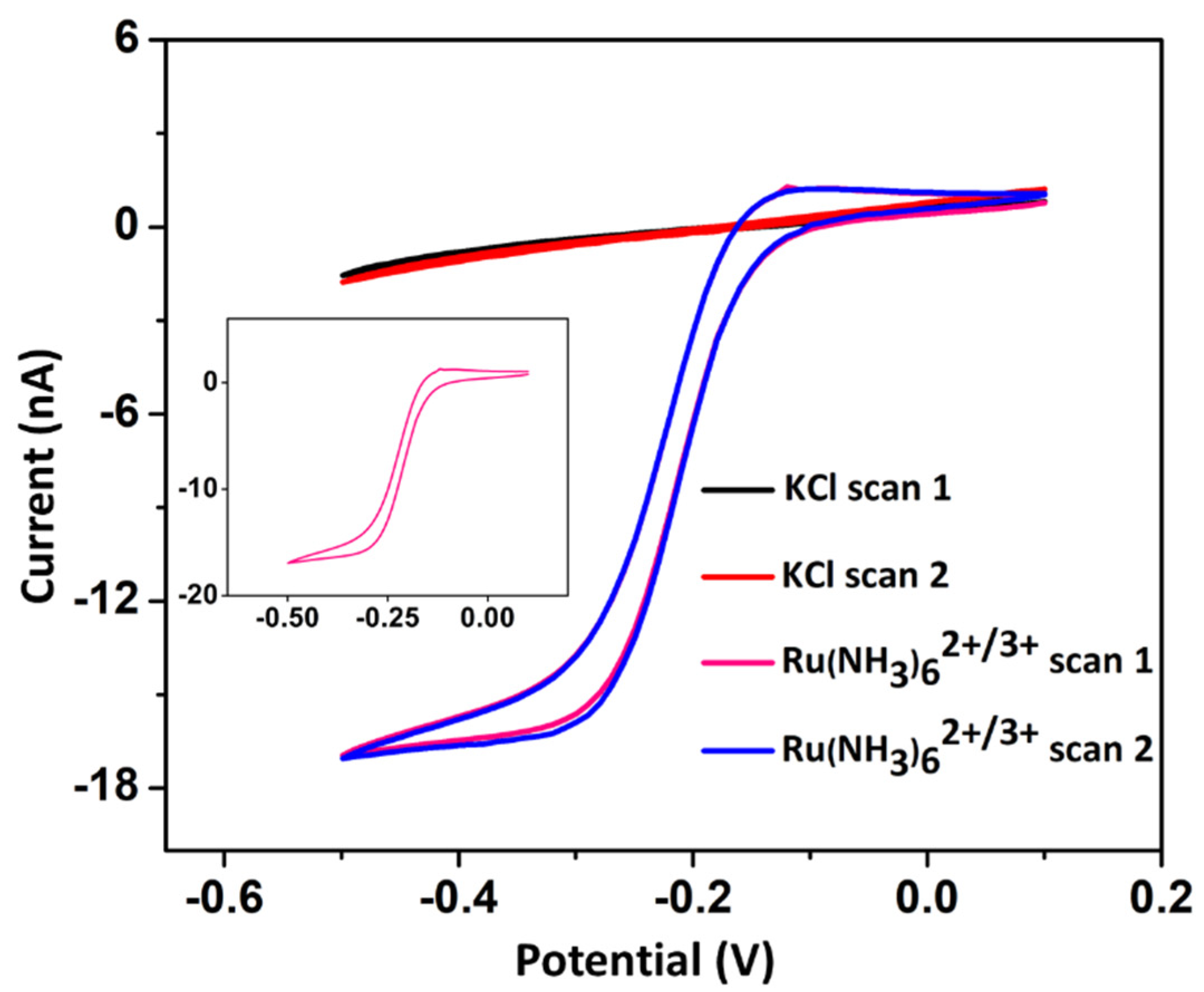
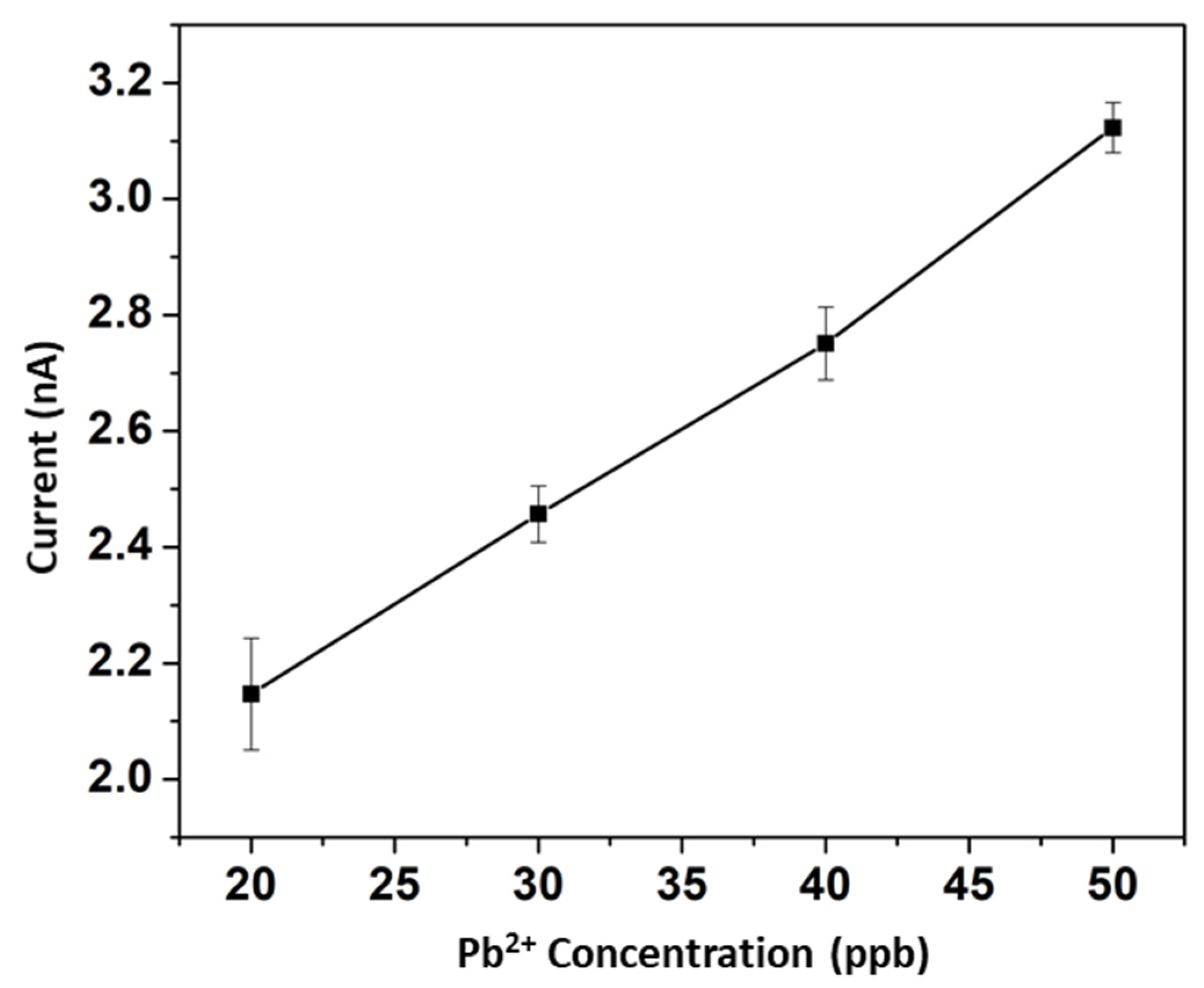
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who should be given the credit for the discovery of carbon nanotubes? Carbon N. Y. 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Kleinerman, O.; Wang, X.; Ma, A.W.K.; Bengio, E.A.; Ter Waarbeek, R.F.; de Jong, J.J.; Hoogerwerf, R.E.; et al. Strong, light, multifunctional fibers of carbon nanotubes with ultrahigh conductivity. Science 2013, 339, 182–186. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- White, C.T.; Todorov, T.N. Carbon nanotubes as long ballistic conductors. Nature 1998, 393, 240–241. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, R.; Ye, X.; Zhu, Z.; Xie, H.; Shen, B.; Cai, D.; Liu, B.; Zhang, C.; Jia, Z.; et al. Carbon nanotube bundles with tensile strength over 80 GPa. Nat. Nanotechnol. 2018, 13, 589–595. [Google Scholar] [CrossRef]
- Poncharal, P.; Berger, C.; Yi, Y.; Wang, Z.L.; de Heer, W.A. Room temperature ballistic conduction in carbon nanotubes. J. Phys. Chem. B 2002, 106, 12104–12118. [Google Scholar] [CrossRef] [Green Version]
- Bachtold, A.; Henny, M.; Terrier, C.; Strunk, C.; Schönenberger, C.; Salvetat, J.P.; Bonard, J.M.; Forró, L. Contacting carbon nanotubes selectively with low-ohmic contacts for four-probe electric measurements. Appl. Phys. Lett. 1998, 73, 274–276. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502-1–215502-4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One hundred fold increase in current carrying capacity in a carbon nanotube-copper composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Raravikar, N.; Helms, B.A.; Prasher, R.; Ogletree, D.F. Enhanced thermal transport at covalently functionalized carbon nanotube array interfaces. Nat. Commun. 2014, 5, 3082. [Google Scholar] [CrossRef] [Green Version]
- Milowska, K.Z.; Burda, M.; Wolanicka, L.; Bristowe, P.D.; Koziol, K.K.K. Carbon nanotube functionalization as a route to enhancing the electrical and mechanical properties of Cu-CNT composites. Nanoscale 2019, 11, 145–157. [Google Scholar] [CrossRef]
- McIntyre, D.J.; Hirschman, R.K.; Puchades, I.; Landi, B.J. Enhanced copper–carbon nanotube hybrid conductors with titanium adhesion layer. J. Mater. Sci. 2020, 55, 6610–6622. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Sekiguchi, A.; Sekiya, M.; Yamada, T.; Hata, K. Copper/carbon nanotube composites: Research trends and outlook. R. Soc. Open Sci. 2018, 5, 180814. [Google Scholar] [CrossRef] [Green Version]
- Miura, S.; Yoshihara, Y.; Asaka, M.; Hasegawa, K.; Sugime, H.; Ota, A.; Oshima, H.; Noda, S. Millimeter-tall carbon nanotube arrays grown on aluminum substrates. Carbon N. Y. 2018, 130, 834–842. [Google Scholar] [CrossRef]
- Teblum, E.; Noked, M.; Grinblat, J.; Kremen, A.; Muallem, M.; Fleger, Y.; Tischler, Y.R.; Aurbach, D.; Nessim, G.D. Millimeter-tall carpets of vertically aligned crystalline carbon nanotubes synthesized on copper substrates for electrical applications. J. Phys. Chem. C 2014, 118, 19345–19355. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Xu, N.; Tan, Y.; Zhan, R.; Shen, Y.; Xu, Z.; Bai, X.; Chen, J.; She, J.; et al. Epitaxial growth of multiwall carbon nanotube from stainless steel substrate and effect on electrical conduction and field emission. Nanotechnology 2017, 28, 305704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baker-Fales, M.; Almkhelfe, H.; Gaede, N.R.; Harris, T.S.; Amama, P.B. Rational modification of a metallic substrate for CVD Growth of Carbon Nanotubes. Sci. Rep. 2018, 8, 4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [Green Version]
- Esconjauregui, S.; Xie, R.; Guo, Y.; Pfaendler, S.M.-L.; Fouquet, M.; Gillen, R.; Cepek, C.; Castellarin-Cudia, C.; Eslava, S.; Robertson, J. Electrical conduction of carbon nanotube forests through sub-nanometric films of alumina. Appl. Phys. Lett. 2013, 102, 113109. [Google Scholar] [CrossRef]
- Zhong, G.; Warner, J.H.; Fouquet, M.; Robertson, A.W.; Chen, B.; Robertson, J. Growth of ultrahigh density single-walled carbon nanotube forests by improved catalyst design. ACS Nano 2012, 6, 2893–2903. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, J.; Sugime, H.; Rao, R.; Zhao, J.; Liu, D.; Harutyunyan, A.; Robertson, J. Growth of high quality, high density single-walled carbon nanotube forests on copper foils. Carbon N. Y. 2016, 98, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Sugime, H.; Esconjauregui, S.; D’Arsié, L.; Yang, J.; Makaryan, T.; Robertson, J. Growth kinetics and growth mechanism of ultrahigh mass density carbon nanotube forests on conductive Ti/Cu supports. ACS Appl. Mater. Interfaces 2014, 6, 15440–15447. [Google Scholar] [CrossRef]
- Lettiere, B.R.; Chazot, C.A.C.; Cui, K.; John Hart, A. High-Density Carbon Nanotube Forest Growth on Copper Foil for Enhanced Thermal and Electrochemical Interfaces. ACS Appl. Nano Mater. 2020, 3, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Roumeli, E.; Diamantopoulou, M.; Serra-Garcia, M.; Johanns, P.; Parcianello, G.; Daraio, C. Characterization of vertically aligned carbon nanotube forests grown on stainless steel surfaces. Nanomaterials 2019, 9, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burda, M.; Lekawa-Raus, A.; Gruszczyk, A.; Koziol, K.K.K. Soldering of Carbon Materials Using Transition Metal Rich Alloys. ACS Nano 2015, 9, 8099–8107. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Kokabi, A.H. Friction stir soldering: A novel route to produce graphite-copper dissimilar joints. Mater. Des. 2017, 116, 599–608. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, S.; Zhou, C.; Xiao, Y.; Li, S.; Chan, M. Synthesis of vertical carbon nanotube interconnect structures using CMOS-compatible catalysts. Nanomaterials 2020, 10, 1918. [Google Scholar] [CrossRef]
- Vollebregt, S.; Tichelaar, F.D.; Schellevis, H.; Beenakker, C.I.M.; Ishihara, R. Carbon nanotube vertical interconnects fabricated at temperatures as low as 350 °C. Carbon N. Y. 2014, 71, 249–256. [Google Scholar] [CrossRef]
- Yokoyama, D.; Iwasaki, T.; Yoshida, T.; Kawarada, H.; Sato, S.; Hyakushima, T.; Nihei, M.; Awano, Y. Low temperature grown carbon nanotube interconnects using inner shells by chemical mechanical polishing. Appl. Phys. Lett. 2007, 91, 263101. [Google Scholar] [CrossRef]
- Nylander, A.N.; Fu, Y.; Huang, M.; Liu, J. Covalent anchoring of carbon nanotube-based thermal interface materials using epoxy-silane monolayers. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 9, 427–433. [Google Scholar] [CrossRef]
- Gupta, P.; Tsai, K.; Ruhunage, C.K.; Gupta, V.K.; Rahm, C.E.; Jiang, D.; Alvarez, N.T. True Picomolar Neurotransmitter Sensor Based on Open-Ended Carbon Nanotubes. Anal. Chem. 2020, 92, 8536–8545. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Manohar, S.; Jagota, A.; Zheng, M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 2009, 460, 250–253. [Google Scholar] [CrossRef]
- Hároz, E.H.; Rice, W.D.; Lu, B.Y.; Ghosh, S.; Hauge, R.H.; Weisman, R.B.; Doorn, S.K.; Kono, J. Enrichment of armchair carbon nanotubes via density gradient ultracentrifugation: Raman spectroscopy evidence. ACS Nano 2010, 4, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Voggu, R.; Rao, K.V.; George, S.J.; Rao, C.N.R. A simple method of separating metallic and semiconducting single-walled carbon nanotubes based on molecular charge transfer. J. Am. Chem. Soc. 2010, 132, 5560–5561. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Li, F.; Pint, C.L.; Mayo, J.T.; Fisher, E.Z.; Tour, J.M.; Colvin, V.L.; Hauge, R.H. Uniform large diameter carbon nanotubes in vertical arrays from premade near-monodisperse nanoparticles. Chem. Mater. 2011, 23, 3466–3475. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Miller, P.; Haase, M.R.; Lobo, R.; Malik, R.; Shanov, V. Tailoring physical properties of carbon nanotube threads during assembly. Carbon N. Y. 2019, 144, 55–62. [Google Scholar] [CrossRef]
- Alvarez, N.T.; Miller, P.; Haase, M.; Kienzle, N.; Zhang, L.; Schulz, M.J.; Shanov, V. Carbon nanotube assembly at near-industrial natural-fiber spinning rates. Carbon N. Y. 2015, 86, 350–357. [Google Scholar] [CrossRef]
- Prasher, R. Nano and Micro Technology-Based Next-Generation Package-Level Cooling Solutions. Intel Technol. J. 2005, 9, 7539–7545. [Google Scholar] [CrossRef]
- Sun, S.; Samani, M.K.; Fu, Y.; Xu, T.; Ye, L.; Satwara, M.; Jeppson, K.; Nilsson, T.; Sun, L.; Liu, J. Improving Thermal Transport at Carbon Hybrid Interfaces by Covalent Bonds. Adv. Mater. Interfaces 2018, 5, 1800318. [Google Scholar] [CrossRef]
- Prasher, R.; Tong, T.; Majumdar, A. An acoustic and dimensional mismatch model for thermal boundary conductance between a vertical mesoscopic nanowire/nanotube and a bulk substrate. J. Appl. Phys. 2007, 102, 104312. [Google Scholar] [CrossRef]
- Chen, J.; Walther, J.H.; Koumoutsakos, P. Covalently Bonded Graphene-Carbon Nanotube Hybrid for High-Performance Thermal Interfaces. Adv. Funct. Mater. 2015, 25, 7539–7545. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhao, L.; Wang, J.; Qiao, Y. Ultrafast Bonding of Wafer Scale Vertical Aligned Carbon Nanotubes onto Gold Surface by Induction Heating. Nanosci. Nanotechnol. Lett. 2018, 9, 2083–2087. [Google Scholar] [CrossRef]
- Daneshvar, F.; Zhang, T.; Aziz, A.; Sue, H.J.; Welland, M.E. Tuning the composition and morphology of carbon nanotube-copper interface. Carbon N. Y. 2020, 157, 583–593. [Google Scholar] [CrossRef]
- Svensson, J.; Campbell, E.E.B. Schottky barriers in carbon nanotube-metal contacts. J. Appl. Phys. 2011, 110, 111101. [Google Scholar] [CrossRef] [Green Version]
- Banhart, F. Interactions between metals and carbon nanotubes: At the interface between old and new materials. Nanoscale 2009, 1, 201–213. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Yao, M. Electrical resistance at carbon nanotube/copper interfaces: Capped versus open-end carbon nanotubes. Mater. Lett. 2012, 82, 184–187. [Google Scholar] [CrossRef]
- Banks, C.E.; Compton, R.G. New electrodes for old: From carbon nanotubes to edge plane pyrolytic graphite. Analyst 2006, 131, 15–21. [Google Scholar] [CrossRef]
- Moore, R.R.; Banks, C.E.; Compton, R.G. Basal plane pyrolytic graphite modified electrodes: Comparison of carbon nanotubes and graphite powder as electrocatalysts. Anal. Chem. 2004, 76, 2677–2682. [Google Scholar] [CrossRef]
- Banks, C.E.; Davies, T.J.; Wildgoose, G.G.; Compton, R.G. Electrocatalysis at graphite and carbon nanotube modified electrodes: Edge-plane sites and tube ends are the reactive sites. Chem. Commun. 2005, 7, 829–841. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Gong, K.; Dai, L. Structural evaluation along the nanotube length for super-long vertically aligned double-walled carbon nanotube arrays. J. Phys. Chem. C 2008, 112, 8136–8139. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Yao, M. Electronic structure and contact resistance at an open-end carbon nanotube and copper interface. Appl. Phys. Lett. 2010, 96, 102108. [Google Scholar] [CrossRef]
- Andriotis, A.N.; Menon, M. Structural and conducting properties of metal carbon-nanotube contacts: Extended molecule approximation. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 045412. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Qu, J.; Yao, M. Effects of local structural defects on the electron transport in a carbon nanotube between Cu electrodes. Appl. Phys. Lett. 2010, 97, 242112. [Google Scholar] [CrossRef]
- Gupta, P.; Lazenby, R.A.; Rahm, C.E.; Heineman, W.R.; Buschbeck, E.; White, R.J.; Alvarez, N.T. Electrochemistry of Controlled Diameter Carbon Nanotube Fibers at the Cross Section and Sidewall. ACS Appl. Energy Mater. 2019, 2, 8757–8766. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Marandi, M.; Matisen, L.; Sammelselg, V.; Podvorica, F.I.; Tammeveski, K. Surface and electrochemical characterization of aryl films grafted on polycrystalline copper from the diazonium compounds using the rotating disk electrode method. J. Electroanal. Chem. 2018, 817, 89–100. [Google Scholar] [CrossRef]
- Chamoulaud, G.; Bélanger, D. Spontaneous derivatization of a copper electrode with in situ generated diazonium cations in aprotic and aqueous media. J. Phys. Chem. C 2007, 111, 7501–7507. [Google Scholar] [CrossRef]
- Hurley, B.L.; McCreery, R.L. Covalent Bonding of Organic Molecules to Cu and Al Alloy 2024 T3 Surfaces via Diazonium Ion Reduction. J. Electrochem. Soc. 2004, 151, B252. [Google Scholar] [CrossRef] [Green Version]
- Segut, O.; Herlem, G.; Lakard, B.; Blondeau-Patissier, V.; Nardin, M.; Gree, S.; Rauch, J.Y. Electrochemically deposited polyethyleneimine films and their characterization. Synth. Met. 2010, 160, 1359–1364. [Google Scholar] [CrossRef]
- Herlem, G.; Goux, C.; Fahys, B.; Dominati, F.; Gonçalves, A.M.; Mathieu, C.; Sutter, E.; Trokourey, A.; Penneau, J.F. Surface modification of platinum and gold electrodes by anodic oxidation of pure ethylenediamine. J. Electroanal. Chem. 1997, 435, 259–265. [Google Scholar] [CrossRef]
- Herlem, G.; Reybier, K.; Trokourey, A.; Fahys, B. Electrochemical Oxidation of Ethylenediamine: New Way to Make Polyethyleneimine-Like Coatings on Metallic or Semiconducting Materials. J. Electrochem. Soc. 2000, 147, 597. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon N. Y. 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Samuelson, J.J.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano Lett. 2013, 13, 4387–4392. [Google Scholar] [CrossRef]
- Chełmecka, E.; Pasterny, K.; Kupka, T.; Stobiński, L. DFT studies of COOH tip-functionalized zigzag and armchair single wall carbon nanotubes. J. Mol. Model. 2012, 18, 2241–2246. [Google Scholar] [CrossRef] [Green Version]
- Kanakaraj, S.N.; Alvarez, N.T.; Gbordzoe, S.; Lucas, M.S.; Maruyama, B.; Noga, R.; Hsieh, Y.Y.; Shanov, V. Improved dry spinning process at elevated temperatures for making uniform and high strength CNT fibers. Mater. Res. Express 2018, 5, 065036. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Covalent functionalization of single-walled carbon nanotubes for materials applications. J. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano Lett. 2012, 12, 4742–4747. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.G.; Gao, F.; BenDhiab, I.; Teplyakov, A. Carbon Nanotubes Covalently Attached to Functionalized Surfaces Directly through the Carbon Cage. Langmuir 2017, 33, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Adenier, A.; Chehimi, M.M.; Gallardo, I.; Pinson, J.; Vilà, N. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces. Langmuir 2004, 20, 8243–8253. [Google Scholar] [CrossRef] [PubMed]
- Barbier, B.; Pinson, J.; Desarmot, G.; Sanchez, M. Electrochemical Bonding of Amines to Carbon Fiber Surfaces Toward Improved Carbon-Epoxy Composites. J. Electrochem. Soc. 1990, 137, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Lyskawa, J.; Bélanger, D. Direct modification of a gold electrode with aminophenyl groups by electrochemical reduction of in situ generated aminophenyl monodiazonium cations. Chem. Mater. 2006, 18, 4755–4763. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef] [PubMed]
- Amer, I.; Brandt, S. Synthesis and characterization of Poly(p-phenylenediamine) and its derivatives using aluminium triflate as a co-catalyst. Cogent Eng. 2018, 5, 1499701. [Google Scholar] [CrossRef]
- Akalin, E.; Akyüz, S. Theoretical study of IR spectra of paraphenylenediamine. Vib. Spectrosc. 2000, 22, 3–10. [Google Scholar] [CrossRef]
- Guo, X.; Kulkarni, A.; Doepke, A.; Halsall, H.B.; Iyer, S.; Heineman, W.R. Carbohydrate-based label-free detection of escherichia coli ORN 178 using electrochemical impedance spectroscopy. Anal. Chem. 2012, 84, 241–246. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, J.; Song, X. Microstructural investigation of CNT-metal bonding behavior through computational simulations. J. Nano Res. 2015, 33, 118–125. [Google Scholar] [CrossRef]
- Baranton, S.; Bélanger, D. Electrochemical derivatization of carbon surface by reduction of in situ generated diazonium cations. J. Phys. Chem. B 2005, 109, 24401–24410. [Google Scholar] [CrossRef] [PubMed]
- Allongue, P.; Delamar, M.; Desbat, B.; Fagebaume, O.; Hitmi, R.; Pinson, J.; Savéant, J.M. Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 1997, 119, 201–207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawarathne, C.P.; Hoque, A.; Ruhunage, C.K.; Rahm, C.E.; Alvarez, N.T. Chemical Bond Formation between Vertically Aligned Carbon Nanotubes and Metal Substrates at Low Temperatures. Appl. Sci. 2021, 11, 9529. https://doi.org/10.3390/app11209529
Nawarathne CP, Hoque A, Ruhunage CK, Rahm CE, Alvarez NT. Chemical Bond Formation between Vertically Aligned Carbon Nanotubes and Metal Substrates at Low Temperatures. Applied Sciences. 2021; 11(20):9529. https://doi.org/10.3390/app11209529
Chicago/Turabian StyleNawarathne, Chaminda P., Abdul Hoque, Chethani K. Ruhunage, Connor E. Rahm, and Noe T. Alvarez. 2021. "Chemical Bond Formation between Vertically Aligned Carbon Nanotubes and Metal Substrates at Low Temperatures" Applied Sciences 11, no. 20: 9529. https://doi.org/10.3390/app11209529
APA StyleNawarathne, C. P., Hoque, A., Ruhunage, C. K., Rahm, C. E., & Alvarez, N. T. (2021). Chemical Bond Formation between Vertically Aligned Carbon Nanotubes and Metal Substrates at Low Temperatures. Applied Sciences, 11(20), 9529. https://doi.org/10.3390/app11209529






