Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Essential Oil Used
2.1.2. Clay Used
2.1.3. Polystyrene Used
2.2. Methods
2.2.1. Preparation of OO@NaMt and OO@OrgMt Nanostructures
2.2.2. Preparation of PS/OO@NaMt, PSOO@OrgMt, and PScoMA/OO@NaMt Films
2.3. XRD Analysis
2.4. FTIR Spectrometry
2.5. Tensile Properties
2.6. DMA
2.7. Water Vapor Transmission Rate (WVTR)
2.8. Oxygen Permeability (PeO2)
2.9. Antioxidant Activity
2.10. Antimicrobial Activity Assay
3. Results
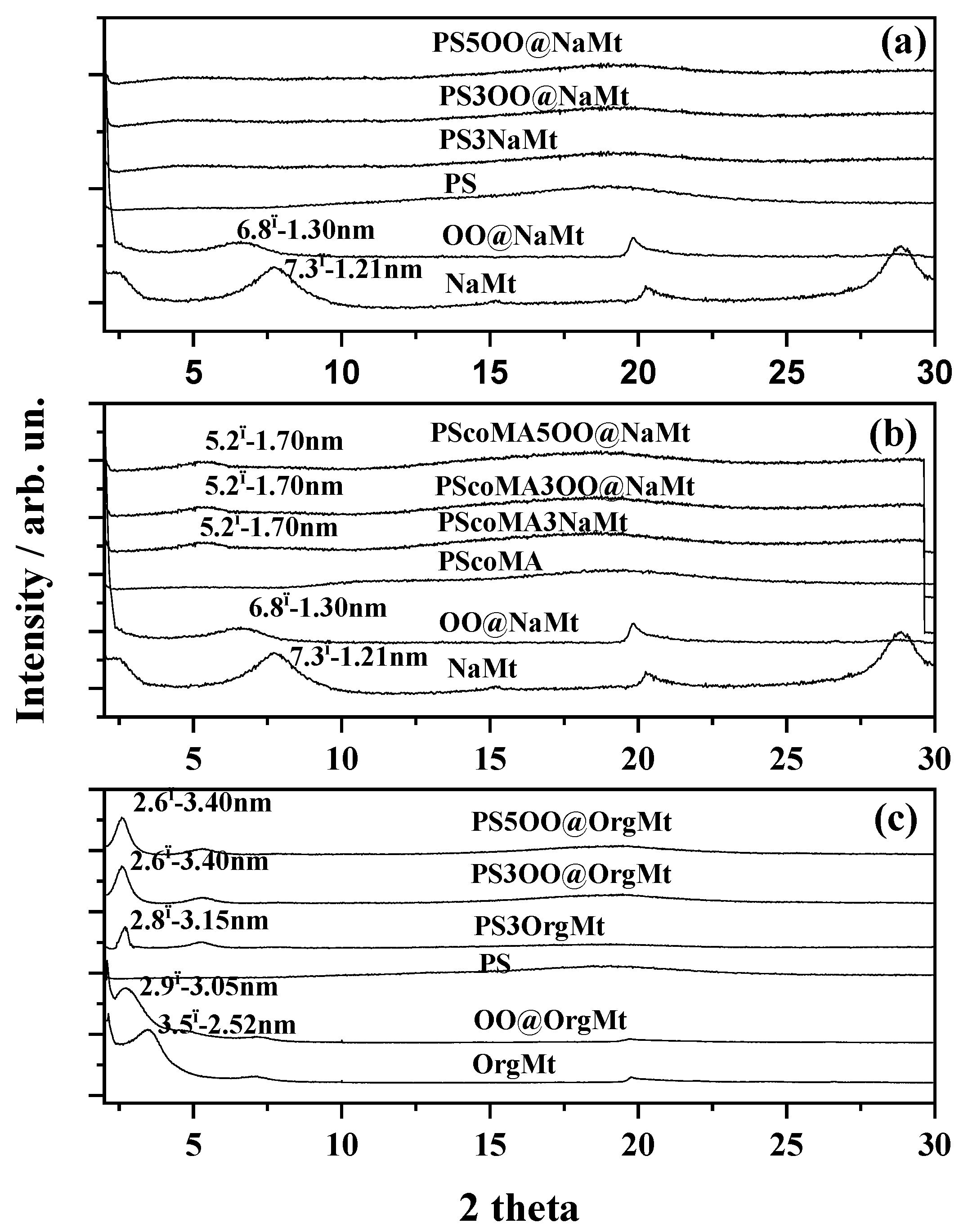
3.1. XRD Results
3.2. FTIR Results
3.3. Tensile Properties
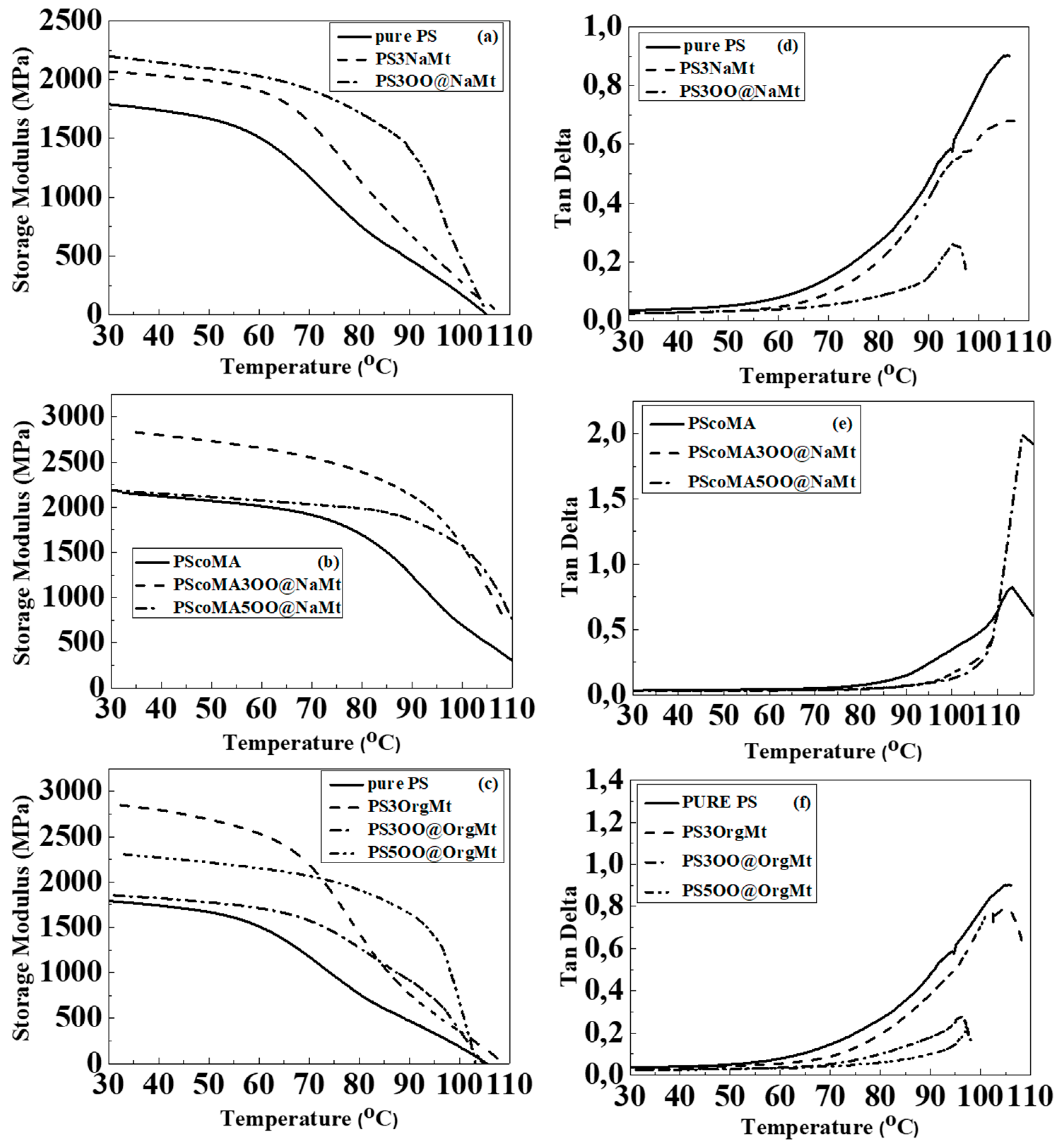
3.4. DMA Results
3.5. Barrier Properties
3.6. Antioxidant Activity
3.7. Antimicrobial Activity
3.8. Statistical Analysis of the Experimental Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Idumah, C.I.; Zurina, M.; Ogbu, J.; Ndem, J.U.; Igba, E.C. A Review on Innovations in Polymeric Nanocomposite Packaging Materials and Electrical Sensors for Food and Agriculture. Compos. Interfaces 2020, 27, 1–72. [Google Scholar] [CrossRef]
- Hári, J.; Pukánszky, B. Chapter 8—Nanocomposites: Preparation, Structure, and Properties. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2011; pp. 109–142. ISBN 978-1-4377-3514-7. [Google Scholar]
- Abolghasemi Fakhri, L.; Ghanbarzadeh, B.; Dehghannya, J.; Abbasi, F.; Ranjbar, H. Optimization of Mechanical and Color Properties of Polystyrene/Nanoclay/Nano ZnO Based Nanocomposite Packaging Sheet Using Response Surface Methodology. Food Packag. Shelf Life 2018, 17, 11–24. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Rico, B.P.M.; Vieira, M.C. Chapter 9—Food packaging and migration. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; pp. 281–301. ISBN 978-0-12-817190-5. [Google Scholar]
- Food Packaging Safety: A Critical Review of Materials. Available online: https://www.packagingdigest.com/food-packaging/food-packaging-safety-critical-review-materials (accessed on 19 September 2020).
- Tajeddin, B.; Ahmadi, B.; Sohrab, F.; Chenarbon, H.A. Chapter 14—Polymers for Modified Atmosphere Packaging Applications. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: London, UK, 2018; pp. 457–499. ISBN 978-0-12-811516-9. [Google Scholar]
- Ladavos, A.; Giannakas, A.E.; Xidas, P.; Giliopoulos, D.J.; Baikousi, M.; Gournis, D.; Karakassides, M.A.; Triantafyllidis, K.S. Preparation and Characterization of Polystyrene Hybrid Composites Reinforced with 2D and 3D Inorganic Fillers. Micro 2021, 1, 3–14. [Google Scholar] [CrossRef]
- Carastan, D.J.; Demarquette, N.R. Polystyrene/Clay Nanocomposites. Int. Mater. Rev. 2007, 52, 345–380. [Google Scholar] [CrossRef]
- Panwar, A.; Choudhary, V.; Sharma, D.k. Review: A Review: Polystyrene/Clay Nanocomposites. J. Reinf. Plast. Compos. 2011, 30, 446–459. [Google Scholar] [CrossRef]
- Giannakas, A.; Spanos, C.G.; Kourkoumelis, N.; Vaimakis, T.; Ladavos, A. Preparation, Characterization and Water Barrier Properties of PS/Organo-Montmorillonite Nanocomposites. Eur. Polym. J. 2008, 44, 3915–3921. [Google Scholar] [CrossRef]
- Giannakas, A.; Giannakas, A.; Ladavos, A. Preparation and Characterization of Polystyrene/Organolaponite Nanocomposites. Polym. Plast. Technol. Eng. 2012, 51, 1411–1415. [Google Scholar] [CrossRef]
- Pavía-Sanders, A.; Nissen, A.; O’Bryan, G. An Efficient Post-Polymerization Modification of Poly(Styrene-Co-Maleic Anhydride) for Thermally Reversible Nanocomposites. Macromol. Mater. Eng. 2018, 303, 1800278. [Google Scholar] [CrossRef]
- Salahuddin, N.; Akelah, A. Synthesis and Characterization of Poly(Styrene-Maleic Anhydride)-Montmorillonite Nanocomposite. Polym. Adv. Technol. 2002, 13, 339–345. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Giannakas, E.A.; Leontion, A. Montmorillonite Composite Materials and Food Packaging. Packag. Mater. 2018, 1–71. [Google Scholar]
- Gueu, S.; Tia, V.E.; Bartier, D.; Barres, O.; Soro, F.D. Adsorption of Lippia Multiflora Essential Oil on Two Surfactant Modified Clays: Qualitative Approach. Clay Miner. 2020, 55, 219–228. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Giannakas Na-Montmorillonite, V.s. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, R.; Krepker, M.; Natan, M.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. RSC Advances Novel LDPE/Halloysite Nanotube Fi Lms with Sustained Carvacrol Release for Broad-Spectrum Antimicrobial Activity †. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Angulo, I.; Lagarón, J.M.; Paseiro-Losada, P.; Cruz, J.M. Development of New Active Packaging Films Containing Bioactive Nanocomposites. Innov. Food Sci. Emerg. Technol. 2014, 26, 310–318. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic Starch/Clay Nanocomposites Loaded with Essential Oil Constituents as Packaging for Strawberries—In Vivo Antimicrobial Synergy over Botrytis Cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Sanfuentes, E.A. The Synergistic Antimicrobial Effect of Carvacrol and Thymol in Clay/Polymer Nanocomposite Films over Strawberry Gray Mold. LWT—Food Sci. Technol. 2015, 64, 390–396. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. J. Food Process. Preserv. 2019, 44. [Google Scholar] [CrossRef]
- Dias, M.V.; de Medeiros, H.S.; Soares, N.d.F.F.; de Melo, N.R.; Borges, S.V.; Carneiro, J.d.D.S.; Pereira, J.M.T.d.A.K. Development of Low-Density Polyethylene Films with Lemon Aroma. LWT—Food Sci. Technol. 2013, 50, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Leontiou, A.; Siasou, Z.; Karakassides, M.A. Development of Poly(L-Lactic Acid)/Chitosan/Basil Oil Active Packaging Films via a Melt-Extrusion Process Using Novel Chitosan/Basil Oil Blends. Processes 2021, 9, 88. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Loukidi, A.; Chatzimichailidou, S.; Salmas, C.E.; Giannakas, A.E.; Achilias, D.S. Effect of Na- and Organo-Modified Montmorillonite/Essential Oil Nanohybrids on the Kinetics of the In Situ Radical Polymerization of Styrene. Nanomaterials 2021, 11, 474. [Google Scholar] [CrossRef]
- Krishna, S.V.; Pugazhenthi, G. Properties and Thermal Degradation Kinetics of Polystyrene/Organoclay Nanocomposites Synthesized by Solvent Blending Method: Effect of Processing Conditions and Organoclay Loading. J. Appl. Polym. Sci. 2011, 120, 1322–1336. [Google Scholar] [CrossRef]
- Vermeesch, I.; Groeninckx, G. Chemical Modification of Poly(Styrene-Co-Maleic Anhydride) with Primary N-Alkylamines by Reactive Extrusion. J. Appl. Polym. Sci. 1994, 53, 1365–1373. [Google Scholar] [CrossRef]
- Pugazhenthi, G.; Suresh, K.; Vinoth Kumar, R.; Kumar, M.; Rajkumar Surin, R. A Simple Sonication Assisted Solvent Blending Route for Fabrication of Exfoliated Polystyrene (PS)/Clay Nanocomposites: Role of Various Clay Modifiers. Mater. Today Proc. 2018, 5, 13191–13210. [Google Scholar] [CrossRef]
- Ding, C.; Guo, B.; He, H.; Jia, D.; Hong, H. Preparation and Structure of Highly Confined Intercalated Polystyrene/Montmorillonite Nanocomposite via a Two-Step Method. Eur. Polym. J. 2005, 41, 1781–1786. [Google Scholar] [CrossRef]
- Uthirakumar, P.; Song, M.-K.; Nah, C.; Lee, Y.-S. Preparation and Characterization of Exfoliated Polystyrene/Clay Nanocomposites Using a Cationic Radical Initiator-MMT Hybrid. Eur. Polym. J. 2005, 41, 211–217. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-Chemical Properties of Whey Protein Isolate Films Containing Oregano Oil and Their Antimicrobial Action against Spoilage Flora of Fresh Beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–Clay Nanocomposites: Exfoliation of Organophilic Montmorillonite Nanolayers in Polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Mascia, L. The Influence of Deformation Mode on the Dynamic Mechanical Spectra of Lightly Plasticised PVC Compositions. Polym. Test. 1987, 7, 109–120. [Google Scholar] [CrossRef]
- Mascia, L.; Wooldridge, P.G.; Stokell, M.J. Antiplasticization of Polyvinyl Chloride in Relation to Crazing and Fracture Behaviour. J. Mater. Sci. 1989, 24, 2775–2780. [Google Scholar] [CrossRef]
- Mascia, L.; Margetts, G. Viscoelasticity and Plasticity Aspects of Antiplasticization Phenomena: Strain Rate and Temperature Effects. J. Macromol. Sci. Part B 1987, 26, 237–256. [Google Scholar] [CrossRef]
- Mascia, L.; Kouparitsas, Y.; Nocita, D.; Bao, X. Antiplasticization of Polymer Materials: Structural Aspects and Effects on Mechanical and Diffusion-Controlled Properties. Polymers 2020, 12, 769. [Google Scholar] [CrossRef] [Green Version]
- Kamel, N.; Saied, M.; Ramadan, R.; Abd-El-Messieh, S. A Study of the Biophysical Properties of Polystyrene Films Incorporated with Clove Oil as Bio-Based Plasticizer. Egypt. J. Chem. 2021, 64, 3111–3120. [Google Scholar] [CrossRef]


| PS g | PScoMA g | NaMt g-%wt. | OrgMt g-%wt. | OO@NaMt g-%wt. | OO@OrgMt g-%wt. | |
|---|---|---|---|---|---|---|
| PS | 5 | - | - | - | - | - |
| PS3NaMt | 4.85 | - | 0.15–3 | - | - | - |
| PS3OO@NaMt | 4.85 | - | - | - | 0.15–3 | - |
| PS5OO@NaMt | 4.75 | - | - | - | 0.25–5 | - |
| PScoMa | - | 5 | - | - | - | - |
| PScoMA3OO@NaMt | - | 4.85 | - | - | 0.15–3 | |
| PScoMA5OO@NaMt | - | 4.75 | - | - | 0.25–5 | |
| PS3OrgMt | 4.85 | - | - | 0.15–3 | - | - |
| PS3OO@OrgMt | 4.85 | - | - | - | - | 0.15–3 |
| PS 5OO@OrgMt | 4.75 | - | - | - | - | 0.25–5 |
| Young’s Modulus-E (Mean.Dev) (N/mm2) | σ (Mean.Dev) (N/mm2) | %ε (Mean.Dev) | |
|---|---|---|---|
| PS | 1832.7 (44.1) | 31.2 (2.0) | 1.9 (0.1) |
| PS3NaMt | 1740.2 (75.1) | 25.9 (2.5) | 0.8 (0.2) |
| PS3OO@NaMt | 1620.5 (69.4) | 23.9 (2.3) | 1.1 (0.1) |
| PS5OO@NaMt | 1590.5 (71.0) | 22.7 (3.3) | 1.0 (0.2) |
| PScoMa | 1815.7 (36.7) | 25.6 (4.4) | 1.5 (0.1) |
| PScoMA3OO@NaMt | 2270.5 (66.1) | 34.5 (4.1) | 1.3 (0.2) |
| PScoMA5OO@NaMt | 2380.8 (43.4) | 35.9 (4.0) | 1.3 (0.2) |
| PS3OrgMt | 1960.4 (61.2) | 32.8 (2.9) | 1.3 (0.2) |
| PS3OO@OrgMt | 2056.3 (58.8) | 33.3 (3.6) | 1.4 (0.2) |
| PS 5OO@OrgMt | 2120.5 (53.1) | 33.6 (3.9) | 1.5 (0.2) |
| Sample | E′ (40 °C) | E′ (100 °C) | Glass Transition Temperature (Tg) |
|---|---|---|---|
| PS | 1739 MPa | 172 MPa | 105 °C |
| PS3NaMt | 2034 MPa | 280 MPa | 107 °C |
| PS3OO@NaMt | 2146 MPa | 510 MPa | 96 °C |
| PScoMA | 2121 MPa | 689 MPa | 112 °C |
| PScoMA3OO@NaMt | 2799 MPa | 1542 MPa | 110 °C |
| PScoMA5OO@NaMt | 2151 MPa | 1556 MPa | 116 °C |
| PS3OrgMt | 2795 MPa | 356 MPa | 104 °C |
| PS3OO@OrgMt | 1823 MPa | 356 MPa | 96 °C |
| PS5OO@OrgMt | 2271MPa | 683 MPa | 97 °C |
| Code Name | Aver. Film Thick. (mm) | W.V.T.R ×106 (g/s) (Mean.Dev) | DW × 1010 (cm2/s) | Aver. Film Thick. (mm) | O.T.R. (cc/m2/Day) (Mean.Dev) | PeO2 × 103 (cc/m/Day) (Mean.Dev) | Antiox. Activ. after 24 h |
|---|---|---|---|---|---|---|---|
| PS | 0.1 | 1.3704 (0.02) | 1.221 | 0.375 | 1715.0 (13.2) | 643.1 (4.4) | n.d. |
| PS3NaMt | 0.1 | 1.5000 (0.02) | 1.336 | 0.375 | 1934.0 (15.8) | 725.2 (5.3) | n.d. |
| PS3OO@NaMt | 0.1 | 1.4593 (0.03) | 1.300 | 0.375 | 1885.0 (15.6) | 706.9 (5.2) | 28.1 (2.2) |
| PS5OO@NaMt | 0.1 | 1.4482 (0.03) | 1.290 | 0.375 | 1932.0 (15.4) | 724.5 (5.1) | 31.2 (1.9) |
| PScoMa | 0.1 | 1.3778 (0.02) | 1.227 | 0.375 | 1731.0 (13.2) | 649.1 (4.4) | n.d. |
| PScoMA3OO@NaMt | 0.1 | 1.3482 (0.02) | 1.201 | 0.375 | 1684.0 (13.0) | 631.5 (4.4) | 27.3 (2.0) |
| PScoMA5OO@NaMt | 0.1 | 1.2407 (0.01) | 1.105 | 0.375 | 1602.0 (12.8) | 600.8 (4.3) | 32.6 (1.7) |
| PS3OrgMt | 0.1 | 1.1630 (0.02) | 1.036 | 0.375 | 1325.0 (12.7) | 496.9 (4.2) | n.d. |
| PS3OO@OrgMt | 0.1 | 1.1222 (0.02) | 1.000 | 0.375 | 1245.0 (12.7) | 466.9 (4.2) | 37.2 (2.1) |
| PS 5OO@OrgMt | 0.1 | 1.0963 (0.01) | 0.977 | 0.375 | 1142.0 (12.6) | 428.3 (4.2) | 50.4 (1.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakas, A.E.; Salmas, C.E.; Karydis-Messinis, A.; Moschovas, D.; Kollia, E.; Tsigkou, V.; Proestos, C.; Avgeropoulos, A.; Zafeiropoulos, N.E. Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films. Appl. Sci. 2021, 11, 9364. https://doi.org/10.3390/app11209364
Giannakas AE, Salmas CE, Karydis-Messinis A, Moschovas D, Kollia E, Tsigkou V, Proestos C, Avgeropoulos A, Zafeiropoulos NE. Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films. Applied Sciences. 2021; 11(20):9364. https://doi.org/10.3390/app11209364
Chicago/Turabian StyleGiannakas, Aris E., Constantinos E. Salmas, Andreas Karydis-Messinis, Dimitrios Moschovas, Eleni Kollia, Vasiliki Tsigkou, Charalampos Proestos, Apostolos Avgeropoulos, and Nikolaos E. Zafeiropoulos. 2021. "Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films" Applied Sciences 11, no. 20: 9364. https://doi.org/10.3390/app11209364
APA StyleGiannakas, A. E., Salmas, C. E., Karydis-Messinis, A., Moschovas, D., Kollia, E., Tsigkou, V., Proestos, C., Avgeropoulos, A., & Zafeiropoulos, N. E. (2021). Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films. Applied Sciences, 11(20), 9364. https://doi.org/10.3390/app11209364










