Abstract
Despite several studies having identified factors associated with successful treatment outcomes in locally advanced cervical cancer, there is the lack of accurate predictive modeling for progression-free survival (PFS) in patients who undergo radical hysterectomy after neoadjuvant chemotherapy (NACT). Here we investigated whether machine learning (ML) may have the potential to provide a tool to predict neoadjuvant treatment response as PFS. In this retrospective observational study, we analyzed patients with locally advanced cervical cancer (FIGO stages IB2, IB3, IIA1, IIA2, IIB, and IIIC1) who were followed in a tertiary center from 2010 to 2018. Demographic and clinical characteristics were collected at either treatment baseline or at 24-month follow-up. Furthermore, we recorded data about magnetic resonance imaging (MRI) examinations and post-surgery histopathology. Proper feature selection was used to determine an attribute core set. Three different machine learning algorithms, namely Logistic Regression (LR), Random Forest (RFF), and K-nearest neighbors (KNN), were then trained and validated with 10-fold cross-validation to predict 24-month PFS. Our analysis included n. 92 patients. The attribute core set used to train machine learning algorithms included the presence/absence of fornix infiltration at pre-treatment MRI as well as of either parametrium invasion and lymph nodes involvement at post-surgery histopathology. RFF showed the best performance (accuracy 82.4%, precision 83.4%, recall 96.2%, area under receiver operating characteristic curve (AUROC) 0.82). We developed an accurate ML model to predict 24-month PFS.
1. Introduction
Cervical cancer is the third most common cancer in women worldwide with 569,000 new cases each year [1].
Although early stage forms are often asymptomatic, symptoms that may occur in locally advance stages are abnormal vaginal bleeding, pelvic pain, hematuria, dysuria, or hematochezia [2].
The most common histopathologic type of cervical cancer is squamous cell carcinoma, accounting for more than 80% of the cervical malignancies. The others histotypes are adenocarcinoma (up to 15%) and adenosquamous carcinoma (less than 5%) [3]. Uncommon histopathologic types are small cell or neuroendocrine, serouspapillary and clear cell. Non squamous presentations are associated with the worst prognosis [4,5].
The most recent revision of the International Federation of Gynecology and Obstetrics (FIGO) staging system was announced in 2018 introducing the role of the imaging as a source of staging information [6,7].
For pretreatment local staging, pelvic magnetic resonance imaging (MRI) and/or transvaginal ultrasound are the gold standard examinations. This evaluations are useful to define pelvic tumor extent, allowing accurate assessment of either tumor size, stromal invasion depth, and parametrial invasion.
MRI examination is a valuable imaging method in the diagnostic work-up of macroscopically visible cervical cancers (stage ≥ IB) and represents a tool for monitoring the cervical tumor response to chemotherapy [8,9,10].
According to 2018 FIGO Staging System [7], in early stage forms (IA, IB1, IB2, IB3, and IIA) treatment typically consists of surgery as chemoradiation makes patients susceptible to more unpredictable long-term side effects and menopause, despite equally effective; patients may undergo surgery alone if no risk factors requiring adjuvant radiation treatment are identified [7] Conversely, in locally advanced cervical cancer (FIGO stage ≥ IIB), definitive management with concomitant chemoradiation is the preferred treatment [2,11].
In patients with stage IB2, IB3, IIA, or IIB, the choice of neoadjuvant chemotherapy followed by radical hysterectomy can improve disease control and reduce toxicity [12,13].
Additionally, several studies report that patients undergoing radical surgery after neoadjuvant chemotherapy may lead to improved survival outcomes compared with those on radiotherapy [14,15,16].
In several fields of science, machine learning (ML) is emerging as a promising tool for the implementation of complex multi-parametric decision algorithms [17]. In this regard, a ML approach is a potential gamechanger. In fact, in addition to detecting linear patterns in analyzed data, it can unravel complex non-linear relationships between patient attributes that cannot be solved by traditional statistical methods, merging them to output a forecast or a probability for a given outcome [18].
ML is a step towards precision medicine, leading to the improvement of patient profiling and treatment personalization. Supervised ML algorithms have proven effective in predicting treatment responses and disease progression in patients affected with heterogeneous diseases [19,20].
Despite several studies had identified factors correlated with successful treatment outcomes in locally advanced cervical cancer [21], there is the lack of accurate predictive modeling for long-term progression-free survival (PFS) after neoadjuvant therapy.
Here we investigated whether ML may have the potential to provide a tool to predict neoadjuvant treatment response in terms of PFS.
2. Materials and Methods
In this retrospective observational study, we analyzed patients with locally advanced cervical cancer who were followed in a tertiary center from 2010 to 2018. All patients of our cohort underwent a pre-treatment MRI and, consequently, a pretreatment radiologic stage, according to FIGO 2018 [7], was established. All patients had either IB2, IB3, IIA1, IIA2, IIB, or IIIC1 stage (ordinal variable). They also received neoadjuvant chemotherapy and a subsequent post-treatment MRI. The treatment response was assessed by variation in tumor size according to Response Evaluation Criteria In Solid Tumors (RECIST v. 1.1, ordinal variable) [22]. In case of complete response (CR), partial response (PR) or stable disease (SD), the patients underwent radical hysterectomy with pelvic and lombo-aortic lymphadenectomy. Radical hysterectomy type was C1. All surgery cases were performed by open surgery.
Demographic features (age), clinical characteristics (Body Mass Index (BMI), parity, menopause, regime of neoadjuvant therapy and number of cycles) and progression free survival (PFS) at 24-month were collected at either treatment baseline and 24-month follow-up. Furthermore, we recorded data about MRI examinations as well as information about post-surgery histopathology (histotypes, grading, lymph node involvement).
In pre and post-treatment MRI we recorded the largest diameter of lesion, the presence/absence of either lymph node involvement, fornix infiltration, parametrium infiltration, vescico-vaginal septum infiltration and recto-vaginal septum infiltration.
In total, the original database included n. 92 patients and n. 24 variables.
Proper feature selection was used to determine an attribute core set (see “Attributes Selection” paragraph for further details).
This study followed STARD guidelines [23] and the TRIPOD statement [24].
The ML algorithms were aimed at forecasting PFS at 24-month follow up.
Student’s t-test for paired samples or Wilcoxon matched-pair signed-rank test were used as appropriate to identify difference among continuous variables between different observation periods. McNemar’s test was used to identify the difference among dummy variables between different observation periods. The significance level at α = 0.05 was used.
The attribute core set used to train the algorithms was determined using a recursive feature elimination (RFE) wrapper based on a decision tree algorithm with extreme gradient boosting (XGBoost) [25]; in brief, this algorithm automatically selects among all the recorded attributes (n. 23) the best number of features upon their importance for predictions of the given outcome (PFS at 24 months). Feature selection may contrast overfitting problems and improves classification performance. RFE elimination method is one of the commonly used feature selection methods for small samples problems [26,27,28] (For further details about RFE see Supplementary Materials).
The whole analysis was implemented in a Python 3.6 environment using scikit-learn (ver.0.22.1) and XGBoost (ver. 1.1.0) libraries [25,29]. After z-score normalization, we ran a Bayesian ridge conditional imputation [30] for missing data. The latter method has proven to be the more accurate method of imputation for obstetrics and gynecology datasets [31] (see Supplementary Materials for further details).
Three different classifiers, either linear and non-linear, were trained and validated with 10-fold cross-validation using the attribute core set retrieved by the RFE for predicting 24-month PFS.
While logistic regression (LR) had been almost always the algorithm of choice to find independent predictors in multivariate models, it must be noticed that the study hypotheses were usually based on the unreal assumption that the association between the prognostic factors and clinical outcomes is direct and isolated. On the contrary, LR is not suitable for the modeling of non-independent variables. For this reason, along with usual LR, for linear modeling we deployed the non-parametric K-nearest neighbors (KNN) and random forest (RFF) [30] algorithms. The latter models have recently proven able to accurately predict important outcomes for woman’s health, also in presence of non-linear patterns in data [32,33,34]. Additionally, we choose RFF as there is evidence of accurate performance in case of imbalanced data, which is often the case of clinical datasets [35]. We also ran RFF using cost-sensitive training (using the argument class weight = “balanced” in scikit-learn) to try to overcome imbalanced class issue.
A repeated grid-search with cross-validation was used for optimal hyperparameter tuning to maximize the classifiers’ performance [36] (See Supplementary Material for hyperparameter fine-tuning).
For each classifier, we plotted ROC curves, and then area under receiver operating characteristic curve (AUROC) was determined.
Then, based on the optimal probability cut-off (Youden’s Index) [37] classifiers’ performance was compared with the following metrics:
- Accuracy = ,
- Recall (True Positive Rate (TPR)) = ,
- Precision = .
In general, a classification model forecasts a binary outcome for a given observation and class. In the process of predicting, a model may output the probability of an observation belonging to each possible class. This case provides some flexibility both in the way predictions are interpreted and presented, allowing the choice of a threshold, as the above mentioned Youden’s index [38].
For a model to be reliable, the estimated class probabilities should be reflective of the true underlying probability of the sample. To check these assumptions, a diagnostic calibration curve for the candidate best classifier was also plotted [38].
3. Results
Our analysis included n. 92 patients with diagnosis of locally advanced cervical cancer.
Demographic and clinical data, MRI parameters and histological examination are shown in Table 1.

Table 1.
Demographic and clinical data, MRI parameters and histological examination.
Patients had a mean age (±SD) of 48.9 ± 11.5 years at diagnosis and n. 50 (54.3%) patients were premenopausal. All patients underwent neoadjuvant therapy; n. 46/92 (50%) paclitaxel-carboplatin, n. 39/92 (42.4%) cisplatin-vinorelbine, n. 6/92 (6.5%) topotecan-cisplatin, n. 1/92 (1.1%) paclitaxel-ifosfamide-cisplatin (TIP)). 24-month PFS was achieved by 70/92 patients (76.1%, imbalanced classes).
At pre-treatment MRI the largest diameter of lesion (mm), mean ± SD was 44.6 ± 10.5, lymph node involvement occurred in n. 19/92 (20.7%), fornix infiltration in n. 46/92 (50%), parametrium infiltration in 46/92 (50%), vescico-vaginal septum infiltration in n. 1 (1.1%), recto-vaginal septum infiltration in n. 1 (1.1%).
At post-treatment MRI the largest diameter of lesion (mm), mean ± SD was 19.6 ± 12.5, lymph node involvement occurred in n. 4/92 (4.3%), fornix infiltration in n. 5/92 (5.4%), parametrium infiltration in 4/92 (4.3%), vescico-vaginal septum infiltration in n. 0 (0%), recto-vaginal septum infiltration in n. 0 (0%).
RECIST criteria showed CR in n. 19/92 (21%), PR in n. 67/92 (73%), SD in n. 6/92 (6%).
At histopathological analysis histotypes were n. 70/92 (76.1%) squamous cell carcinoma, n. 18/92 (19.6%) adenocarcinoma, n. 4/92 (4.3%) adenosquamous carcinoma.
Grading was G1 in n. 13/92 (14%), G2 in n. 32/92 (35%), G3 in n. 47/92 (51). Lymph node involvement occurred in n. 16/92 (17%). Persistence of cervix disease after NACT was verified in 73/92 (79%); n. 25/92 patients (27%) needed further treatment.
RFE retrieved an attribute core set used to train machine learning algorithms including the presence/absence of fornix infiltration at pre-treatment MRI as well as the presence/absence of either parametrium invasion and lymph nodes involvement at post-radical surgery histopathology.
The final dataset had a dimensionality of 92 columns × 4 rows (n.3 selected attributes plus n. 1 target class (PFS at 24 months, as above mentioned).
As reported in Table 2, at optimal cut-off (Youden’s index), RFF (n. estimators = 500, depth = 5) showed the best performance (accuracy 82.4%, precision 83.4%, TPR 96.2%, AUROC 0.82, Figure 1), outperforming LR (accuracy 77.9%, precision 80.1%, TPR 96.2%, AUROC 0.81), and KNN (n. of neighbors = 5) (accuracy 73.6%, precision 76.5%, TPR 96.2%, AUROC 0.67).

Table 2.
Algorithms Performance. In bold the algorithm with the best performance on 10-fold cross validation. Accuracy, Recall, Precision and AUROC for RFF, were significantly better than other algorithms’ ones.
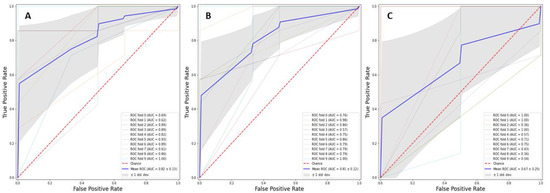
Figure 1.
Receiver operating characteristics curve for Random Forest (box (A)), Logistic Regression (box (B)) and K-nearest neighbors (box (C)) models.
In Figure 1, ROC curve for RFF (box A), LR (box B) and KNN (box C) models was reported.
In Figure 2 calibration diagnostic has been plotted for RFF; PFS roughly happened with an observed relative frequency consistent with the forecast value, showing an acceptable calibration curve. We would expect the match between predicted frequencies and observed frequencies to increase with a larger dataset.
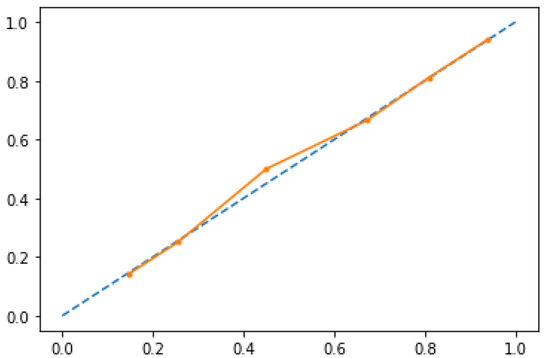
Figure 2.
Calibration diagnostics for RFF model. 24-month PFS roughly happened with an observed relative frequency consistent with the forecast value, showing good calibration.
4. Discussion
The pillar of survival analyses in oncologic research had historically been Cox proportional hazard regression model, being a surrogate for estimating treatment effectiveness and safety. This model is based on an assumption of linear association. However, many clinicopathologic features exhibit a nonlinear association in medicine [39].
Conversely in the area of cervical cancer research, ML can be used for supporting the study of human papillomavirus-related disease, evaluating either cervical cytology, colposcopy and genomic analysis [40,41,42,43,44,45,46,47,48,49,50,51]. However, there are only a few studies that have examined oncologic outcome [52].
This is the first study that wants to analyze the accuracy of a ML modeling to predict the response to neoadjuvant chemotherapy in patients with locally advanced cervical cancer.
Gadducci et al. [53] studied predictors of clinical outcome in patients with locally advanced cervical cancer treated with radical hysterectomy followed neoadjuvant chemotherapy using traditional statistics. This study stated that an optimal pathological response was the most relevant predictor for disease-free survival (DFS) and overall survival (OS). The involvement of the parameters and/or margins of surgical resection was the other independent predictor; vice versa, the lymph node status and the involvement of the lymphovascular spaces correlated with DFS and OS.
A study of Liang et al. established prognostic value of pathological response to neoadjuvant chemotherapy in 204 patients affected with stage IB2—IIA cervical squamous cell cancer. Clinical response and FIGO stage are variables statistically associated with DFS. Patient age, histological grade and chemotherapy regimen result not associated with DFS. An optimal pathological response to neoadjuvant chemotherapy has been shown to be associated with improved long-term outcome.
In this study tumor regression results to be an independent prognostic factor for survival performing the multivariate analysis. Moreover, in patients without extra-cervical deposits, an association between survival rate and chemotherapy response grade was shown, while this was not true in patients with extra-cervical deposits. This implies that when the tumor is confined to the cervix, residual viable tumor cells has an impact on prognosis. Although this is not true when tumor cells have spread outside the cervix [54].
The role of extra-cervical deposits (vaginal disease, nodal metastasis, parametrial involvement) in determining the prognosis of cervical cancer patients after NACT has been reported in previous studies. Uegaki et al. demonstrated that pelvic lymph node metastasis was the only histopathologically independent prognostic factor (p = 0.0029) [55].
Benedetti-Panici et al. showed that lymph node metastases and involved parametria were the only two independent factors for survival [56].
From all the recorded variables in our cohort, the automated attribute selection algorithm selected the presence/absence of fornix infiltration at pre-treatment MRI as well as presence/absence of either parametrium invasion and lymph nodes involvement at post-radical surgery histopathology as the attribute core set to be used in ML models training and validation.
The presence/absence of either parametrium invasion and lymph nodes involvement at post-radical surgery histopathology had been already evaluated as predictors in previous studies [53,55,56]. On the other hand, the presence/absence of fornix infiltration at pre-treatment MRI was not considered as a classical predictor of response to neoadjuvant chemotherapy.
On the contrary, for predictive modeling RFF, a non-linear algorithm, showed a slightly higher performance than LR in terms of accuracy and precision.
The lack of balance in target class (PFS at 24 months) may be responsible of the better performance of RFF, especially in terms of precision, when compared to KNN and LR.
The main strength of our model is its capability of predicting sustained remission basing on easy-to-gather attributes that are widely available at treatment baseline visit and come with no added cost.
Despite good performance, the main limitation of this study remains the sample size. Although our sample size for training and validation is similar or larger than those recently published [54], it must be noticed that ML algorithms score dramatically better when huge cohorts (i.e., thousands of patients) are used for training.
5. Conclusions
In gynecologic oncology, ML is a step towards precision medicine, leading to the improvement of patient profiling and treatment personalization.
We developed an accurate model to predict 24-month PFS in patients with locally advanced cervical cancer on neoadjuvant therapy, based on an ML algorithm requiring few easy-to-collect attributes. Our results are promising but need to be tested prospectively.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/2/823/s1.
Author Contributions
Conceptualization, F.A. and G.C.; methodology, V.V.; software, V.V.; validation, V.V.; formal analysis, V.V.; investigation, F.A. and C.L.; resources, F.A. and V.L.; data curation, F.A. and C.L.; writing—original draft preparation, F.A. and G.C.; writing—review and editing, F.A. and E.C.; visualization, V.V.; supervision, V.L., D.L.F., M.M., E.C., and A.S.T.; project administration, F.A., D.L.F., M.M., A.S.T., and G.C.; funding acquisition, F.A. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Azienda Ospedaliera Policlinico Consorziale—University of Bari, IT (protocol code 6398, date of approval 10 June 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study at baseline consultation.
Data Availability Statement
Data are not freely available due to local Ethics Committee privacy issues. Authors will consider data sharing upon specific request to local Ethics Committee.
Acknowledgments
We thank the association “ACTO—alleanza contro il tumore ovarico” for supporting the research activity of Francesca Arezzo with the “Adele Leone” grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv262. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Sun, C.C.; Schover, L.R.; Munsell, M.F.; Jhingran, A.; Wharton, J.T.; Eifel, P.; Bevers, T.B.; Levenback, C.F.; Gershenson, D.M.; et al. Quality of life and sexual functioning in cervical cancer survivors. J. Clin. Oncol. 2005, 23, 7428–7436. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Bourgioti, C.; Chatoupis, K.; Moulopoulos, L.A. Current imaging strategies for the evaluation of uterine cervical cancer. World J. Radiol. 2016, 8, 342–354. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 22–36. [Google Scholar] [CrossRef]
- Lee, S.I.; Atri, M. 2018 FIGO Staging System for Uterine Cervical Cancer: Enter Cross-sectional Imaging. Radiology 2019, 292, 15–24. [Google Scholar] [CrossRef]
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2011, 21, 1102–1110. [Google Scholar] [CrossRef]
- Hameeduddin, A.; Sahdev, A. Diffusion-weighted imaging and dynamic contrast-enhanced MRI in assessing response and recurrent disease in gynaecological malignancies. Cancer Imaging 2015, 15, 3. [Google Scholar] [CrossRef]
- Bipat, S.; Glas, A.S.; van der Velden, J.; Zwinderman, A.H.; Bossuyt, P.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol. Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, T.; Wang, J.; Yang, Y.; Gao, Y.; Gao, J.; Gao, S.; Wang, Y.; Zhou, X.; Liu, Z. Radical hysterectomy with adjuvant radiotherapy versus radical radiotherapy for FIGO stage IIB cervical cancer. BMC Cancer 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Cormio, G.; Vicino, M.; Selvaggi, L. Neoadjuvant chemotherapy: An alternative option of treatment for locally advanced cervical cancer. Gynecol. Obstet. Investig. 2008, 65, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Del Vecchio, V.; Crupano, F.M.; Minicucci, V.; Fumarulo, V.V.; Resta, L.; Vimercati, A.; Bettocchi, S.; Cicinelli, E.; Cormio, G. A phase II study: Dose-dense carboplatin and paclitaxel as neoadjuvant chemotherapy in locally advanced cervical cancer. J. Chemother. 2018, 30, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Benedetti-Panici, P.; Greggi, S.; Colombo, A.; Amoroso, M.; Smaniotto, D.; Giannarelli, D.; Amunni, G.; Raspagliesi, F.; Zola, P.; Mangioni, C.; et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: Results from the Italian multicenter randomized study. J. Clin. Oncol. 2002, 20, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus class III radical hysterectomy in stage IB-IIA cervical cancer: A prospective randomized study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-Analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur. J. Cancer 2003, 39, 2470–2486. [Google Scholar] [CrossRef]
- Venerito, V.; Angelini, O.; Cazzato, G.; Lopalco, G.; Maiorano, E.; Cimmino, A.; Iannone, F. A convolutional neural network with transfer learning for automatic discrimination between low and high-grade synovitis: A pilot study. Intern. Emerg. Med. 2021. ePub ahead of print. [Google Scholar] [CrossRef]
- Johnson, K.W.; Soto, J.T.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Pandit, A.; Radstake, T. Machine learning in rheumatology approaches the clinic. Nat. Rev. Rheumatol. 2020, 16, 69–70. [Google Scholar] [CrossRef]
- Baldini, C.; Ferro, F.; Luciano, N.; Bombardieri, S.; Grossi, E. Artificial neural networks help to identify disease subsets and to predict lymphoma in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 137–144. [Google Scholar]
- Rose, P.G.; Java, J.; Whitney, C.W.; Stehman, F.B.; Lanciano, R.; Thomas, G.M.; DiSilvestro, P.A. Nomograms Predicting Progression-Free Survival, Overall Survival, and Pelvic Recurrence in Locally Advanced Cervical Cancer Developed From an Analysis of Identifiable Prognostic Factors in Patients From NRG Oncology/Gynecologic Oncology Group Randomized Trials of Chemoradiotherapy. J. Clin. Oncol. 2015, 33, 2136–2142. [Google Scholar] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; De Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Casalino, G.; Vessio, G.; Consiglio, A. (Eds.) Evaluation of Cognitive Impairment in Pediatric Multiple Sclerosis with Machine Learning: An Exploratory Study of miRNA Expressions. In Proceedings of the 2020 IEEE Conference on Evolving and Adaptive Intelligent Systems (EAIS), Bari, Italy, 27–29 May 2020. [Google Scholar]
- Kamel, E.; Sheikh, S.; Huang, X. Data-driven predictive models for residential building energy use based on the segregation of heating and cooling days. Energy 2020, 206, 118045. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, Y.; Tao, C.; Alphen, D. (Eds.) Feature Selection Using Recursive Feature Elimination for Handwritten Digit Recognition. In Proceedings of the 2009 Fifth International Conference on Intelligent Information Hiding and Multimedia Signal Processing, Kyoto, Japan, 12–14 September 2009. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Buitinck, L.; Louppe, G.; Blondel, M.; Pedregosa, F.; Mueller, A.; Grisel, O.; Prettenhofer, P.; Gramfort, A.; Grobler, J.; Layton, R.; et al. API Design for Machine Learning Software: Experiences from the Scikit-Learn Project 1 September 2013. arXiv 2013, arXiv:1309.0238. Available online: https://ui.adsabs.harvard.edu/abs/2013arXiv1309.0238B (accessed on 11 November 2020).
- Altukhova, O. Choice of method imputation missing values for obstetrics clinical data. Procedia Comput. Sci. 2020, 176, 976–984. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, C.; Fu, B.; Yang, S.; Zhu, S.; Yang, D.; Lei, B.; Huang, R.; Lei, J. Risk prediction for postpartum depression based on random forest. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 1215–1222. [Google Scholar]
- Rawashdeh, H.; Awawdeh, S.; Shannag, F.; Henawi, E.; Faris, H.; Obeid, N.; Hyett, J. Intelligent system based on data mining techniques for prediction of preterm birth for women with cervical cerclage. Comput. Biol. Chem. 2020, 85, 107233. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Ding, R.; Shen, L.; Gao, P.; Xu, H.; Xiu, C.; Zhang, H.; Song, D.; Han, B. Characterization and imaging of surgical specimens of invasive breast cancer and normal breast tissues with the application of Raman spectral mapping: A feasibility study and comparison with randomized single-point detection method. Oncol. Lett. 2020, 20, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Khalilia, M.; Chakraborty, S.; Popescu, M. Predicting disease risks from highly imbalanced data using random forest. BMC Med. Inform. Decis. Mak. 2011, 11, 51. [Google Scholar] [CrossRef]
- Krstajic, D.; Buturovic, L.J.; Leahy, D.E.; Thomas, S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminform. 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Berrar, D. Performance Measures for Binary Classification. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Oxford, UK, 2019; pp. 546–560. [Google Scholar]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling; Springer: New York, NY, US, 2013. [Google Scholar]
- Matsuo, K.; Purushotham, S.; Jiang, B.; Mandelbaum, R.S.; Takiuchi, T.; Liu, Y.; Roman, L.D. Survival outcome prediction in cervical cancer: Cox models vs. deep-learning model. Am. J. Obstet. Gynecol. 2019, 220, 381.e1–381.e14. [Google Scholar] [CrossRef] [PubMed]
- Komagata, H.; Ichimura, T.; Matsuta, Y.; Ishikawa, M.; Shinoda, K.; Kobayashi, N.; Sasaki, A. Feature analysis of cell nuclear chromatin distribution in support of cervical cytology. J. Med. Imaging 2017, 4, 047501. [Google Scholar] [CrossRef] [PubMed]
- Mariarputham, E.J.; Stephen, A. Nominated texture based cervical cancer classification. Comput. Math. Methods Med. 2015, 2015, 586928. [Google Scholar] [CrossRef] [PubMed]
- Kahng, J.; Kim, E.H.; Kim, H.G.; Lee, W. Development of a cervical cancer progress prediction tool for human papillomavirus-positive Koreans: A support vector machine-based approach. J. Int. Med. Res. 2015, 43, 518–525. [Google Scholar] [CrossRef]
- Sato, M.; Horie, K.; Hara, A.; Miyamoto, Y.; Kurihara, K.; Tomio, K.; Yokota, H. Application of deep learning to the classification of images from colposcopy. Oncol. Lett. 2018, 15, 3518–3523. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Yang, P.; Chen, Y.; Zhu, Y.; Tong, M.; Hao, Z.; Li, X. Identification of cervical cancer using laser-induced breakdown spectroscopy coupled with principal component analysis and support vector machine. Lasers Med. Sci. 2018, 33, 1381–1386. [Google Scholar] [CrossRef]
- Gu, J.; Fu, C.Y.; Ng, B.K.; Liu, L.B.; Lim-Tan, S.K.; Lee, C.G. Enhancement of early cervical cancer diagnosis with epithelial layer analysis of fluorescence lifetime images. PLoS ONE 2015, 10, e0125706. [Google Scholar] [CrossRef]
- Baltzer, N.; Sundstrom, K.; Nygard, J.F.; Dillner, J.; Komorowski, J. Risk stratification in cervical cancer screening by complete screening history: Applying bioinformatics to a general screening population. Int. J. Cancer 2017, 141, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Torheim, T.; Malinen, E.; Hole, K.H.; Lund, K.V.; Indahl, U.G.; Lyng, H.; Kvaal, K.; Futsæther, C. Autodelineation of cervical cancers using multiparametric magnetic resonance imaging and machine learning. Acta Oncol. 2017, 56, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chen, Z.; Liang, Y.; Shen, W.; Yang, F.; Dai, R.; Wu, N.; Tian, J. Staging of cervical cancer based on tumor heterogeneity characterized by texture features on (18)F-FDG PET images. Phys. Med. Biol. 2015, 60, 5123–5139. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Chang, S.W.; Cheah, P.L.; Yap, H.J. Integrative machine learning analysis of multiple gene expression profiles in cervical cancer. PeerJ 2018, 6, e5285. [Google Scholar] [CrossRef]
- Wilhelm, T. Phenotype prediction based on genome-wide DNA methylation data. BMC Bioinform. 2014, 15, 193. [Google Scholar] [CrossRef]
- Weegar, R.; Kvist, M.; Sundstrom, K.; Brunak, S.; Dalianis, H. Finding Cervical Cancer Symptoms in Swedish Clinical Text using a Machine Learning Approach and NegEx. AMIA Annu. Symp. Proc. 2015, 2015, 1296–1305. [Google Scholar]
- Obrzut, B.; Kusy, M.; Semczuk, A.; Obrzut, M.; Kluska, J. Prediction of 5-year overall survival in cervical cancer patients treated with radical hysterectomy using computational intelligence methods. BMC Cancer 2017, 17, 840. [Google Scholar] [CrossRef]
- Gadducci, A.; Teti, G.; Barsotti, C.; Tana, R.; Fanucchi, A.; Orlandini, C.; Fabrini, M.G.; Genazzani, A.R. Clinicopathological variables predictive of clinical outcome in patients with FIGO stage Ib2-IIb cervical cancer treated with cisplatin-based neoadjuvant chemotherapy followed by radical hysterectomy. Anticancer Res. 2010, 30, 201–208. [Google Scholar]
- Liang, Y.; Lu, B.; Chen, X.; Qin, J.; Cheng, X.; Xie, X.; Lü, W. Prognostic value of pathological response to neoadjuvant chemotherapy in bulky stage Ib2 and IIa cervical squamous cell cancer patients. Virchows Arch. 2016, 468, 329–336. [Google Scholar] [CrossRef]
- Uegaki, K.; Shimada, M.; Sato, S.; Deura, I.; Naniwa, J.; Sato, S.; Oishi, T.; Itamochi, H.; Harada, T.; Kigawa, J. Outcome of stage IB2-IIB patients with bulky uterine cervical cancer who underwent neoadjuvant chemotherapy followed by radical hysterectomy. Int. J. Clin. Oncol. 2014, 19, 348–353. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Greggi, S.; Scambia, G.; Amoroso, M.; Salerno, M.G.; Maneschi, F.; Cutillo, G.; Paratore, M.; Scorpiglione, N.; Mancuso, S. Long-term survival following neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Eur. J. Cancer 1998, 34, 341–346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).