Abstract
Neuroinflammation is implicated in central nervous system (CNS) diseases, but the molecular mechanisms involved are poorly understood. Progress may be accelerated by developing a comprehensive view of the pathogenesis of CNS disorders, including the immune and the chaperone systems (IS and CS). The latter consists of the molecular chaperones; cochaperones; and chaperone cofactors, interactors, and receptors of an organism and its main collaborators in maintaining protein homeostasis (canonical function) are the ubiquitin–proteasome system and chaperone-mediated autophagy. The CS has also noncanonical functions, for instance, modulation of the IS with induction of proinflammatory cytokines. This deserves investigation because it may be at the core of neuroinflammation, and elucidation of its mechanism will open roads toward developing efficacious treatments centered on molecular chaperones (i.e., chaperonotherapy). Here, we discuss information available on the role of three members of the CS—heat shock protein (Hsp)60, Hsp70, and Hsp90—in IS modulation and neuroinflammation. These three chaperones occur intra- and extracellularly, with the latter being the most likely involved in neuroinflammation because they can interact with the IS. We discuss some of the interactions, their consequences, and the molecules involved but many aspects are still incompletely elucidated, and we hope that this review will encourage research based on the data presented to pave the way for the development of chaperonotherapy. This may consist of blocking a chaperone that promotes destructive neuroinflammation or replacing or boosting a defective chaperone with cytoprotective activity against neurodegeneration.
1. Introduction
Neuroinflammation occurs in brain injury and chronic neurodegenerative diseases affecting the central nervous system (CNS) [1,2,3,4,5]. The CNS is characterized by two main types of cells: neurons and neuroglia. The former’s function is impulse transmission and signaling, while the latter play other roles [6]. For instance, microglia and astrocytes, resident antigen-presenting cells (APCs), rapidly respond to tissue damage that compromises the homeostasis of the local brain parenchyma [7]. Microglia activation is a highly regulated process involved in the generation of different and complex phenotypes, the reorganization of cell surface markers, and the release of soluble pro-and anti-inflammatory factors. Neuroinflammation is a complex cellular and biochemical response that increases inflammatory mediators (such as cytokines and chemokines) and activates glial cells and leukocyte invasion of brain tissue. These events have been correlated with an increased permeability of the blood–brain barrier (BBB). Microglial cells can remain activated for long periods, which causes the release of large amounts of cytokines and neurotoxic molecules that contribute to neurodegeneration [8]. It is important to bear in mind that inflammation is not necessarily deleterious because moderate inflammatory reactions are involved in diverse phenomena that protect cells and tissues from a variety of noxae [9]. Whether inflammation is good or bad for the organism depends mostly on the intensity and duration of the inflammatory reaction: the more intense and long lasting the reaction, the higher the probability of disease development or aggravation. In inflammatory and immune reactions, molecular chaperones interact with the immune system, especially when they are activated under stress conditions in different organs, including the brain. Molecular chaperones, many of which are heat shock proteins (Hsps), are the main components of the chaperone system [10,11]. They are ubiquitously expressed, and their canonical role is to assist in the folding of nascent polypeptides avoiding protein misfolding and aggregation, and to deliver damaged proteins to protein degradation machineries [10,12]. The levels of some chaperones change in response to stressors, for example, oxidative stress and DNA damage [13]. Typically, chaperones are cytoprotective, but they can also be pathogenic when they are structurally and/or functionally abnormal and can contribute to the mechanism of diseases termed chaperonopathies [10]. Chaperonopathies are involved in the development of some neurodegenerative diseases in which neuroinflammation is implicated. The role played by chaperones in neuroinflammation is under scrutiny and constitutes a promising area of research because it may lead to the discovery of novel treatment strategies centered on chaperonotherapy, namely, the use of chaperones as therapeutic targets or agents [14,15]. Here, we discuss molecular chaperones within the context of neurodegenerative diseases/neuroinflammation and the interactions between the immune system and the chaperoning system, focusing on extracellular Hsp60, Hsp70, and Hsp90 in Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple sclerosis (MS).
2. Immunomodulatory Function of Extracellular Hsp60, Hsp70, and Hsp90
Hsp60, Hsp70, and Hsp90 interact with the immune system in many ways and thereby have an impact on neurodegenerative diseases. Extracellular Hsp60, Hsp70, and Hsp90 influence both the innate and the adaptive immune responses. Generally, extracellular Hsp–receptor interaction involves specific receptors expressed on macrophages and dendritic and microglia cells, including toll-like receptors (TLRs), scavenger receptors (SR), and other molecules [16]. For example, Hsp70 and Hsp90 can interact with the SR LOX-1 [17], and Hsp70 interacts also with multiple members of the SR family [18]. The SR are expressed on different types of cells and they are involved in the binding and internalization of stress proteins [18]. Extracellular Hsp60, Hsp70, and Hsp90 can modulate the innate immune response, causing the secretion of proinflammatory cytokines by APCs [19]. This interaction elicits a proinflammatory response that involves mainly nuclear factor-kappa B (NF-kB). These chaperones are endogenous ligands for TLRs, and by interacting also with CD14 molecules, they can induce the production of cytokines (e.g., interleukin 1 beta (IL-1β), IL-6, inducible isoform of nitric oxide synthase (iNOS)) [20,21]. TLR4 is a receptor expressed on the microglia plasma cell membrane with a key role in the generation of immune responses in the nervous system, responses that are implicated in the development of neurodegenerative disorders [22]. For instance, Hsp60 can mediate neuroinflammation through a MyD88-dependent pathway by interacting with TLR4 on the microglia surface [21] and by inducing the production of proinflammatory factors via microglial LOX-1 [23]. Intrathecal injection of Hsp60 lead to neurodegeneration and demyelination by the activation of TLR4-MyD88 signaling in microglial cells [24]. Hsp70 can interact with microglia, dendritic cells, and macrophages through TLR2 and TLR4, leading to proinflammatory NF-kB activation and its associated pathways [25]. Hsp90 interacts with an extensive list of key mediators involved in pathways regulating inflammatory and immune responses. For example, among the protein clients of Hsp90, there is the receptor-interacting protein (RIP) kinase, which is involved in the innate immune response and in the cell-death signaling pathway. [26] RIP, following TLR4 activation, induces the expression of proinflammatory cytokines by NF-kB signaling [27] (Figure 1).
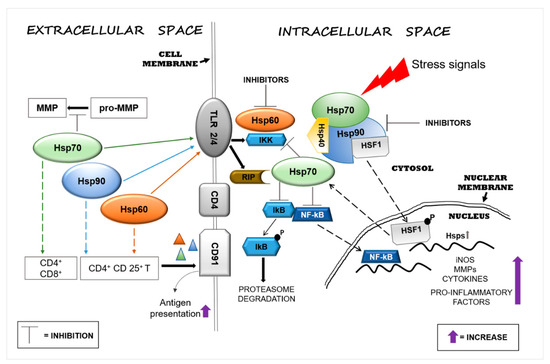
Figure 1.
Heat shock protein (Hsp)60, Hsp70, and Hsp90 modulate inflammatory reactions by interacting with factors involved in the regulation of innate and adaptive immune responses. Stressors can activate the immune system and, in turn, promote neurodegeneration by inducing Hsps in brain tissue as a mechanism of protection. Extracellular Hsp60, Hsp70, and Hsp90 interact with receptors present on the surface of cells of the neural tissue’s immune compartment (e.g., microglia) and elicit pro- or anti-inflammatory responses, depending on the local cellular status. The interaction of extracellular Hsp60, Hsp70, and Hsp90 with toll-like receptor (TLR)2/4 induces the activation of the nuclear factor-kappa B (NF-kB) inhibitor protein, which in turn triggers the activation of the NF-kB pathway, promoting an inflammatory response. Hsp60, Hsp70, and Hsp90 form complexes with antigens (represented by triangles) mediating their presentation via the CD91 cell surface receptor on antigen-presenting cells (APCs) [28]. Hsp90 plays a proinflammatory role through the interaction with its client proteins, such as members of the receptor-interacting protein (RIP) kinases and, thereby, activates the NF-kB pathway. Under physiological conditions, intracellular Hsp90, by blocking heat shock factor (HSF)1, prevents the transcription of Hsp genes, such as Hsp70, or other genes that code for anti-inflammatory molecules. The pharmacological inhibition of Hsp90 can lead to upregulation of the transcription of intracellular Hsp70 and of anti-inflammatory molecules by its release. Abbreviations: MMP, matrix metalloproteinase; TLR, toll-like receptor; IKK, inhibitor of κB kinase; RIP, receptor interaction protein; CD, cluster of differentiation; lkB, inhibitory subunit I kappa B-alpha; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; HSF1, heat shock factor 1; P, phosphate; Hsp, heat shock protein; iNOS, inducible isoform of nitric oxide synthase.
Extracellular Hsp60, Hsp70, and Hsp90 can also help antigen presentation in the adaptive immune responses by upregulating the expression of major histocompatibility complex (MHC) molecules and their load [29,30]. Extracellular Hsp70 and Hsp90 complexed with antigens elicit the responses of cluster of differentiation (CD)8+ or CD4+ T cells by adaptive receptors [16], while Hsp60 by itself can stimulate regulatory CD4+ and CD 25+ T cells (Tregs), leading to an immunosuppressive adaptive response without APC participation [16,31]. In addition, the chaperone–peptide complexes can also recognize the CD91 receptor of macrophages/dendritic cells and facilitate antigen presentation [28] (Figure 1). The activation of the adaptive response via Hsp70 might represent a negative reaction for the cell, but it could be considered advantageous for the development of immunological memory in preparation for rapid reaction against subsequent insults [32]. In contrast to the proinflammatory function of extracellular Hsp70, intracellular Hsp70 has an anti-inflammatory effect in the brain, especially when overexpressed following brain damage. Thus, Hsp70 can be anti-inflammatory because it can block the expression of proinflammatory molecules, such as matrix metalloproteinases [33], and it can also promote the reduction or the inhibition of NF-kB activity [34,35] (Figure 1). In addition, intracellular Hsp70 interferes also with genes involved in various neuronal pathways such as transmission of nerve impulses [36]. Therefore, extracellular Hsp70 could in principle have anti-inflammatory and neuroprotective effects similar to those of the intracellular counterpart [37]. Consequently, it is likely that an increase of intracellular Hsp70 will lead to an increase of functional extracellular Hsp70, contributing to the reduction of the inflammation associated with neurodegeneration. Pharmacological increase of the Hsp70 level in neurons and microglia by 17-N-allylamino-17-demethoxygeldanamycin (17-AAG) reduced the hemorrhagic volume in a mouse model of traumatic brain injury [38]. Likewise, 17-AAG inhibition of Hsp90 induced the expression of Hsp70 and Hsp60 [39]. Thus, it may be said that Hsp70, Hsp60, and Hsp90 promote inflammatory responses and, consequently, neuronal damage and are implicated in neuroinflammation and neurotoxicity. Cytosolic Hsp60 has been shown to directly interact with the inhibitor of κB kinase (IKK), promoting activation of NF-kB-dependent gene transcription by tumor necrosis factor-α (TNFα) [40] (Figure 1). Hsp90 can induce a proinflammatory response in different ways, for example, by sequestering the regulator transcriptional factor heat shock factor (HSF)1 and thereby inhibiting the expression of Hsps (e.g., Hsp70) or activating the NF-kB pathway through the activation of its protein clients RIP [41] (Figure 1). In view of these results, Hsp60 and Hsp90 modulators appear as potentially useful agents for controlling inflammation in the nervous system [42,43]. Currently, numerous compounds have been designed to inhibit Hsp90 activity, but few have been developed for Hsp60 [15]. Hsp90 inhibitors have been developed to directly act on the chaperone or on its client proteins. Some inhibitors block the Hsp90 folding activity linked to adenosine triphosphate (ATP)-dependent conformation changes [44], while others inactivate its client proteins via proteasomal degradation [45]. For example, geldanamycin induces the degradation of Hsp90 client proteins of the RIP family with the consequent inhibition of TNF-mediated IkB kinase and NF-kB activation [41]. Furthermore, Hsp90 forms a complex with HSF1, blocking its translocation to the nucleus and, thereby, impedes the upregulation of Hsp70 and other anti-inflammatory molecules [46] (Figure 1). Most of the compounds that inhibit Hsp70 function by targeting its ATP hydrolysis activity or specific cysteine residues [47].
3. Extracellular Hsp60, Hsp70, and Hsp90 in Acute Nervous System Injury and Chronic Neurodegenerative Diseases
Neurodegenerative diseases are accompanied by inflammatory responses aimed at eliminating dead and damaged neuronal cells to restore the compromised area to its normal status [7]. It should be borne in mind that while short-lived inflammatory responses generally have a beneficial effect, excessive and persistent release of inflammatory mediators can be harmful to brain tissue [9]. Moreover, prolonged activation of microglia and astrocytes could also lead to the alteration of their beneficial functions, which they display under normal conditions [48]. Therefore, it is not surprising that neuroinflammation contributes to CNS diseases [49]. Although different in their origins, many neurodegenerative conditions are characterized by shared cellular responses that promote the upregulation of molecular chaperones as the first line of defense against misfolded, dysfunctional, and aggregation-prone proteins [50]. There is increasing evidence for the release of Hsp60, Hsp70, and Hsp90 into the extracellular environment, with functions that are complementary or independent of those of their intracellular counterparts. Since these chaperones lack a secretion signal in their sequences, the mechanisms by which they are released are poorly understood. In vitro and in vivo studies with Hsp60 have unveiled secretion pathways, involving lipid rafts and exosomes, which would explain the presence of Hsp60 in extramitochondrial sites such as interstitial space, cellular membrane, and biological fluids [51]. Similarly, nontraditional secretion mechanisms participate in the membrane delivery and release of Hsp70, involving lipid rafts [52] and lysosomes [53], in line with its role as a lysosomal stabilizer [54]. Secretion of Hsp90 via exosomes depends on its ATPase function and on the open or closed conformational state of the Hsp90 dimer: the open state promotes Hsp90 release via exosomes, whereas the closed state blocks this process [55]. Different types of CNS cells, including neurons and glial cells, can release exosomes with their cargo of specific molecules that could affect the function of acceptor cells [56]. At the extracellular level, Hsp60 is known to contribute to neuroinflammation with possible negative implications: this chaperone is highly expressed in activated microglia, and when released extracellularly, it induces neuroinflammation with neuronal cell death [57]. For this reason, inhibition of Hsp60 expression and its release represents a possible therapeutic mechanism applicable to neurodegenerative diseases. The pro- and anti-inflammatory effects of extracellular Hsp60, Hsp70, and Hsp90 in AD, PD, ALS, HD, and MS are summarized in Table 1 and discussed in the following paragraphs.

Table 1.
Anti- and proinflammatory effects of extracellular Hsp60, Hsp70, and Hsp90 in neurodegenerative diseases.
3.1. Alzheimer’s Disease
AD is a neurodegenerative disorder in which the amyloid-β peptide (Aβ) accumulates in extracellular deposits named plaque, whereas neurofibrillary tangles (NFTs) occur intracellularly with hyperphosphorylated tau [12,58]. Under pro-aggregating conditions (37 °C and stirring), extracellular Hsp60 inhibits the onset of Aβ cross-β-structure formation that typically accompanies the peptide assembly toward higher ordered structures [59]. The hypotheses formulated on the possible role of Hsp60 in the formation of protein deposits are mainly based on its holding activity. For instance, Hsp60 could act as a noncatalytic inhibitor of polypeptide aggregation by sequestering unfolded monomers via hydrophobic interactions. However, the stoichiometric ratio of the Aβpeptide/Hsp60 and the limitation of the methods applied for these measurements put a question mark on the validity of the results. In fact, the inhibition of amyloid formation appears discontinuous when passing from a 75:1 to 50:1 molar ratio. Furthermore, the method used, size-exclusion chromatography, cannot distinguish between Aβ monomers or peptide oligomers of very low molecular weight, such as dimers or trimers, nor can it discriminate between on-pathway and off-pathway species. These data suggest that Hsp60 exerts its inhibitory action only under stress conditions and, in particular, in the presence of other factors such as high temperature and stirring, which favor the formation of on-pathway seeding species. [59]. Higher levels of Hsp60 were found in lymphocytes isolated from AD subjects [60,61]. αβ immunization with peptides derived from Hsp60 induced a decrease of cerebral amyloid burden in a mouse model [62]. Like Hsp60, extracellular Hsp70 also interacts with Aβ oligomers, blocking their oligomerization into fibers and reducing their toxicity [63]. The engineered form of secreted Hsp70 (secHsp70) in Drosophila protects against the toxicity induced by Aβ42 deposits in the extracellular milieu [64]. Exogenous Hsp90 was found to induce microglial activation and to facilitate phagocytosis and clearance of Aβ directly via the TLR4 pathway, but when bound to the Aβ oligomers, it induced the production of IL-6 and TNF-α [65]. In another work, it was revealed that Hsp90 modulates the formation of the STIP1 (or Hsp70/Hsp90 organizing protein (HOP))/PrPC complex, which inhibits the neuroprotective role of STIP1 against amyloid-beta peptide [66]. However, it is still unclear whether extracellular Hsp70/Hsp90/STIP1 in AD brain exists separately or as a complex with the Aβ aggregate [66]. All these observations indicate that the understanding of Hsp90′s role in neurodegeneration deserves further investigation.
3.2. Parkinson’s Disease
PD is characterized by movement disorders and loss of dopaminergic neurons in the brain’s substantia nigra pars compacta [67,68]. The disease is also characterized by aggregated α-synuclein that forms nuclear inclusions called Lewy bodies [69]. A study in yeast cells has shown that null mutations in the Hsp60 gene are linked with defects in the folding of mitochondrial proteins, with accumulations of misfolded peptides analogous to the α-synuclein aggregates of PD [70]. Hsp60, Hsp70, and Hsp90 interact with α-synuclein in the Lewy bodies in PD patients. These inclusions consist not only of α-synuclein aggregates but also contain molecular chaperones which have been sequestered in the aggregates while attempting to impede or correct protein misfolding and aggregation [71,72]. This sequestration leads to a deficit of chaperones available for maintaining protein homeostasis, namely, a chaperonopathy by defect occurs, which contributes to the aggravation of the pathologic process leading to neurodegeneration. The interaction between Hsp70 and α-synuclein involves the central hydrophobic region of the pathological protein and the substrate-binding domain Hsp70 and is crucial for inhibiting assembly before the elongation stage [73]. The neuroprotective function of overexpressed Hsp70 has been confirmed in experimental models in vivo [74]. There is less information regarding the protective role of Hsp90 in the regulation of α-synuclein aggregation. Like in AD, Hsp60, Hsp70, and Hsp90 contribute to neuronal toxicity in PD. Hsp90 abolishes the binding of α-synuclein to vesicles and promotes the formation of fibrils [75]. In in vivo and in vitro models of PD, it was found that Hsp60 expression gradually decreased after 6-hydroxydopamine (6-OHDA) injection into dopaminergic neurons (DA). This result may be explained by the release of Hsp60 by the damaged neurons, as suggested by its presence in the cell culture medium [76]. In PD models and patients, activation of microglia plays a key role in the release of proinflammatory factors that aggravate the loss of DA neurons [77]. Astrocytes, which are the predominant glial cell type in the CNS, are also critically affected by stressors. The expression of Hsp60 on the surface of activated microglia suggests that Hsp60 is involved in the progression of PD. Extracellular release of Hsp60 from CNS cells undergoing necrotic or apoptotic death activates microglia in a TLR4- and MyD88-dependent manner [21]. Hsp60 was released from degenerated neurons to activate microglia in a rat PD model, providing a novel idea for developing a therapeutic strategy to slow or stop PD progression by preventing the release of Hsp60 or interfering with the interaction between Hsp60 and microglia [76].
3.3. Amyotrophic Lateral Sclerosis
ALS is a chronic inflammatory demyelinating disease that affects motor neurons and is characterized by atrophy and paralysis of muscles, with progressive aggravation over the years [78]. This disease occurs sporadically, but a small percentage is familial with mutations in specific genes, such as the gene encoding the free-radical-scavenging enzyme superoxide dismutase-1 (SOD1) [79]. An important aspect of SOD1-associated ALS is the deposition of SOD1 in large insoluble aggregates in motor neurons. The SOD1 mutated protein mediates the induction of the disease through the dysregulation of the heat shock response (HSR)–apoptosis axis [80]. The development of ALS is linked to the formation of intracellular aggregates of misfolded proteins [78]. Few data are available regarding the involvement of molecular chaperones in ALS onset. Motor neurons of ALS patients have an intrinsic deficit in the ability to activate the HSR and, consequently, do not readily regulate Hsp expression, as shown, for example, for Hsp70 [81]. It has been observed that the Hsp70/Hsp40 pair is complexed with the mutant form of the SOD1 protein in cultured neuronal cells [82]. However, data indicate that the increase of Hsp70 level alone is not sufficient to ameliorate mutant SOD1-protein-mediated toxicity in mouse models [83]. Histamine is neuroprotective through the HSR in motor neurons and microglia cell cultures, and in vivo in spinal cord and cortex from symptomatic SOD1-G93A mice [84]. These results emphasize the relevance of histidine-induced Hsp70 stimulation for preserving motor function [84]. Further, the intraperitoneal administration of human recombinant exogenous Hsp70 increased lifespan, delayed the onset of symptoms, preserved locomotor function, and prolonged motoneuron survival in a mouse model of ALS [85]. Extracellular Hsp70 stimulates the survival of neurons following injury [86] and overexpressed Hsp70 induces the survival of astrocytes [87]. Under stress, astrocytes increase the release of exosomes enriched in Hsp70, with positive implications on the survival of nearby neurons [88]. Interestingly, exosomes derived from cancer cells express Hsp70 on their surface, which allows their interaction with target cells carrying surface Hsp receptors [15]. Hsp70 (DnaJC5/Hsc70 complex) is also believed to be involved in the extracellular release of proteins associated with neurodegenerative disease as part of its chaperoning functions [89]. Exogenous Hsp70 protects from oxidative damage death in motor neurons through binding and sequestration of toxic proteins [90].
3.4. Huntington’s Disease
HD is a progressive neurodegenerative disease caused by excess repeats of glutamine residues, called polyQ repeats, in the huntingtin (Htt) protein, causing protein misfolding [91]. The accumulation of misfolded Htt is associated with cognitive decline and motor defects [92]. Few studies have investigated the involvement of Hsp60 in HD. For instance, Hsp60 plays a protective role in HD by inhibiting polyglutamine aggregate formation and toxicity in vitro [93]. In HD, using confocal microscopy, it was observed that exogenous Hsp70 helps to reduce the number and size of polyQ inclusions [94]. Under normal conditions, the Htt proteins are under the quality control of the chaperone system, particularly Hsp90 and Hsp70. Hsp90 co-immunoprecipitates with both mutant and wild-type forms of Htt, and its inhibition blocks the interaction [95]. Hsp90 preferentially binds the mutant huntingtin (mHtt) rather than normal Htt proteins and also binds other proteins, such as the transcriptional repressor RE1-silencing transcription factor (REST) [96]. REST is normally quiescent in differentiated neurons, but its levels and activity increase as a consequence of neuronal damage [97]. Under physiological conditions, Htt indirectly regulates REST nuclear traffic through the formation of a complex that causes REST retention in the cytoplasm, whereas under pathological conditions, the binding of mHtt to this complex induces a conformational change that leads to the release of REST and its subsequent translocation to the nucleus [96]. A direct effect of this pathological transport of REST is the repression of neuronal genes containing RE1 sequences, including the brain-derived neurotrophic factor (BDNF), a survival factor for striatal neurons. Hsp90-specific inhibitors dramatically reduce Htt stability and REST levels, providing neuroprotective activity [96]. Given the complexity of the mechanism regulating REST expression and the way by which REST modulates the expression of its target genes, further studies are needed to understand the Hsp90–mHtt and Hsp90–REST interactions. Other studies report the involvement of Hsp70 in the pathogenesis of HD. Hsp70 has not only a neuroprotective role as an intracellular chaperone but it has also important extracellular functions. Extracellular Hsp70 can reach, for example, the hippocampus, leading to the initiation and propagation of generalized tonic–clonic seizures [98,99].
3.5. Multiple Sclerosis
MS is a disease of the CNS with autoimmune components that provoke the damage of myelin around nerves and axons, impairing the transmission of information between brain and the rest of the body [100]. There is little information on the role that chaperones might play in MS. For example, it is not yet established if Hsp60 plays a role in the immunopathogenesis of MS. Serum and cerebrospinal fluid (CSF) samples with untreated, relapsing–remitting MS showed antibody signatures targeting epitopes of various proteins, including Hsp60 [101]. Although extracellular Hsp70 is associated with neuroprotective functions in AD and PD as it helps in lowering the levels of misfolded proteins, in MS, it may intensify the immune response. Hsp70 has been found in MS lesions, often in association with the two major myelin proteins of the myelin sheath, namely, myelin basic protein (MBP) and proteolipid protein (PLP) [102]. In the experimental autoimmune encephalomyelitis (EAE) model, Hsp70 promotes an immunological response mediated by its myelin peptide adjuvant capacity [103]. Moreover, Hsp70 overexpression in vitro leads to enhanced presentation of MBP in an MHC class-II-dependent manner [104]. It may be hypothesized that the association of Hsp70 with myelin proteins would be required also for remyelination during the repair process, and that its deficiency could compromise this process. This view is supported by findings with autopsy tissue of MS lesions which show a quantitative reduction of Hsp70 compared with normal brain tissues, a reduction that parallels the impairment of the remyelination process [105]. Oligodendrocyte precursor cells (OPCs) are the targets of autoimmune attack in MS, which prevents remyelination. CSF from MS patients contains antibodies that can specifically recognize Hsp90 molecules located on OPCs with consequent activation of the complement and significant reduction of the OPCs [106]. These features indicate that the use of Hsp90 inhibitors could be beneficial in EAE and probably also in MS.
4. Conclusions
Neuroinflammation and protein misfolding and aggregation are currently recognized as important players in neurodegenerative diseases. The chaperone system, the main component of which are molecular chaperones, is critical for maintaining protein homeostasis. While protein quality control encompasses the canonical functions of chaperones, these also have noncanonical functions, and both have an impact on the nervous system in health and disease. A malfunction of a chaperone may cause disease, a chaperonopathy. Thus, while normal chaperones are typically cytoprotective, abnormal ones can be pathogenic and contribute to the initiation–progression of neuropathies. This knowledge opens the road for considering chaperonotherapy as a therapeutic resource in the field of neurodegenerative diseases. If the chaperones are working in a cytoprotective mode, namely, promoting protein folding, preventing misfolding and aggregation, and dissolving reversible aggregates, their levels ought to be enhanced if necessary, for example, when they become quantitatively insufficient because of excessive demand and/or depletion because of sequestration in the aggregates. In these chaperonopathies by defect, positive chaperonotherapy would be appropriate and would involve administration of chaperone stimulators or the chaperones themselves as proteins or via gene therapy. The same would apply in other instances of chaperonopathies by defect, for example, when a chaperone is structurally damaged by mutation or by an aberrant post-translation modification. If, on the contrary, a chaperone plays an etiologic–pathogenic role and favors development of neuroinflammation and neurodegeneration, negative chaperonotherapy would be required. The pathogenic chaperone protein or its gene should be blocked or eliminated. These are options now open for investigating novel therapeutic approaches targeting neuroinflammation and neurodegeneration. For example, arimoclomol, which induces expression of chaperone genes, is a potential agent to potentiate neuroprotection in ALS [107,108,109]. Progress in these kinds of therapeutic strategies centering on chaperonotherapy is desperately needed, considering the severity of most neurodegenerative diseases and the current scarcity of efficacious treatments.
Author Contributions
Conceptualization A.J.L.M. and A.M.G.; original draft preparation G.A., L.P., and A.M.V.; supervision E.C.d.M., C.C., and C.C.B., writing and editing, A.J.L.M. and A.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
A.J.L.M. and E.C.d.M. were partially supported by IMET and IEMEST. This is IMET contribution number IMET 21-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Aβ, Amyloid-β peptide; ALS, Amyotrophic Lateral Sclerosis; AD, Alzeheimer’s Disease; APC, Antigen Presenting Cell; ATP, Adenosine Triphosphate; BBB, Blood-Brain Barrier; BDNF, Brain-derived Neurotrophic Factor; CD14, Cluster of Differentiation 14; CNS, Central Nervous System; CSF, Cerebrospinal Fluid; EAE, Experimental Autoimmune Encephalomyelitis; HD, Huntington’s Disease; HOP, Hsp70/Hsp90 organizing protein; HSF1, Heat Shock Factor 1; Hsp, Heat Shock Protein; HSR, Heat Shock Response; Htt, Huntingtin; IL-1β, Interleukin 1 beta; IL-6, Interleukin 6; lkB, Inhibitory Subunit I kappa B-alpha; IKK, inhibitor of κB kinasen; iNOS, Inducible Isoform of Nitric Oxide Synthase; MBP, Myelin Basic Protein; MHC, Major Histocompatibility Complex; mHtt, Mutant Huntingtin; MMP, Matrix Metalloproteinase; MS, Multiple Sclerosis; NF-kB, Nuclear Factor-kappa B; 6-OHDA, 6-hydroxydopamine; OPC, Oligodendrocyte Precursor Cell; PLP, Proteolipid Protein; P, Phosphate; PD, Parkinson’s Disease; REST, RE1-Silencing Transcription factor; RIP, Receptor Interacting Protein Kinase; SOD1, Superoxide Dismutase-1; SR, Scavenger Receptor; TLR, Toll-like Receptor; TNFα, Tumor Necrosis Factor-α; NTF, Neurofibrillary Tangle.
References
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Bader, V.; Winklhofer, K.F. Mitochondria at the interface between neurodegeneration and neuroinflammation. Semin. Cell Dev. Biol. 2020, 99, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.; Privat, A.L.; Bressac, L.; Toulorge, D. CD38 in Neurodegeneration and Neuroinflammation. Cells 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Liyanagamage, D.S.N.K.; Martinus, R.D. Role of Mitochondrial Stress Protein HSP60 in Diabetes-Induced Neuroinflammation. Mediators Inflamm. 2020, 2020, 8073516. [Google Scholar] [CrossRef] [PubMed]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D. The fine structure of the nervous system: The neurons and supporting cells. J. Neurol. Neurosurg. Psychiatry 1978, 41, 191. [Google Scholar] [CrossRef][Green Version]
- Banjara, M.; Ghosh, C. Sterile Neuroinflammation and Strategies for Therapeutic Intervention. Int. J. Inflamm. 2017, 8385961. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef]
- Chaperone proteins and chaperonopathies. In Stress Physiology, Biochemistry, and Pathology; Handbook of Stress; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 135–152.
- Marino Gammazza, A.; Caruso Bavisotto, C.; Barone, R.; de Macario, E.C.; Macario, A.J.L. Alzheimer’s disease and molecular chaperones: Current knowledge and the future of chaperonotherapy. Curr. Pharm. Des. 2016, 22, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Didelot, C.; Schmitt, E.; Brunet, M.; Maingret, L.; Parcellier, A.; Garrido, C. Heat shock proteins: Endogenous modulators of apoptotic cell death. In Molecular Chaperones in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2006; Volume 172, pp. 171–198. [Google Scholar]
- Macario, A.J.L.; Conway de Macario, E.; Cappello, F. The Chaperonopathies. In Diseases with Defective Molecular Chaperones; Springer: New York, NY, USA; London, UK, 2013; pp. 1–116. [Google Scholar]
- Cappello, F.; Marino Gammazza, A.; Palumbo Piccionello, A.; Campanella, C.; Pace, A.; Conway de Macario, E.; Macario, A.J.L. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert. Opin. Ther. Targets 2014, 18, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Theriault, J.; Gong, J.; Calderwood, S.K. Investigating receptors for extracellular heat shock proteins. Methods Mol. Biol. 2011, 787, 289–302. [Google Scholar] [PubMed]
- Thériault, J.R.; Adachi, H.; Calderwood, S.K. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J. Immunol. 2006, 177, 8604–8611. [Google Scholar] [CrossRef]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Lehnardt, S.; Schott, E.; Trimbuch, T.; Laubisch, D.; Krueger, C.; Wulczyn, G.; Nitsch, R.; Weber, J.R. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 2008, 28, 2320–2331. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, L.; Zhu, H.; Wang, L.; Wu, W.; Xie, J.; Gu, J. Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity. Neurochem. Int. 2012, 61, 1021–1035. [Google Scholar] [CrossRef]
- Rosenberger, K.; Dembny, P.; Derkow, K.; Engel, O.; Krüger, C.; Wolf, S.A.; Kettenmann, H.; Schott, E.; Meisel, A.; Lehnardt, S. Intrathecal heat shock protein 60 mediates neurodegeneration and demyelination in the CNS through a TLR4- and MyD88-dependent pathway. Mol. Neurodegener. 2015, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Giffard, R.G.; Han, R.; Emery, J.F.; Duan, M.; Pittet, J.F. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: The complex roles of heat shock protein 70. Anesthesiology 2008, 109, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.K.; He, S.D. Heat shock protein 90 regulates necroptosis by modulating multiple signaling effectors. Cell Death Dis. 2016, 7, e2126. [Google Scholar] [CrossRef]
- Mifflin, L.; Ofengeim, D.; Yuan, J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 2020, 19, 553–571. [Google Scholar] [PubMed]
- Binder, R.J.; Zhou, Y.J.; Messmer, M.N.; Pawaria, S. CD91-Dependent Modulation of Immune Responses by Heat Shock Proteins: A Role in Autoimmunity. Autoimmune Dis. 2012, 2012, 863041. [Google Scholar]
- Cohen-Sfady, M.; Nussbaum, G.; Pevsner-Fischer, M.; Mor, F.; Carmi, P.; Zanin-Zhorov, A.; Lider, O.; Cohen, I.R. Heat shock protein 60 activates B cells via the TLR4-MyD88 pathway. J. Immunol. 2005, 175, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y. Toll-like receptors and immune regulation: Their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology 2007, 122, 149–156. [Google Scholar]
- Chen, J.; Graham, S.H.; Zhu, R.L.; Simon, R.P. Stress proteins and tolerance to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1996, 16, 566–577. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, Y.J.; Kim, J.Y.; Lee, W.T.; Yenari, M.A.; Giffard, R.G. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport 2004, 15, 499–502. [Google Scholar] [CrossRef]
- Zheng, Z.; Kim, J.Y.; Ma, H.; Lee, J.E.; Yenari, M.A. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J. Cereb. Blood Flow Metab. 2008, 28, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.W.; Sun, X.; Khammash, M.; Giffard, R.G. Overexpression of heat shock protein 72 attenuates NF-κB activation using a combination of regulatory mechanisms in microglia. PLoS Comput. Biol. 2014, 10, e1003471. [Google Scholar] [CrossRef] [PubMed]
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Ferat-Osorio, E.; Sánchez-Anaya, A.; Gutiérrez-Mendoza, M.; Boscó-Gárate, I.; Wong-Baeza, I.; Pastelin-Palacios, R.; Pedraza-Alva, G.; Bonifaz, L.C.; Cortés-Reynosa, P.; Pérez-Salazar, E.; et al. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. 2014, 11, 19. [Google Scholar] [CrossRef]
- Kim, N.; Kim, J.Y.; Yenari, M.A. Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience 2015, 284, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.Q.; Heldt, S.A.; Xu, H.; Liao, F.F. Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 2014, 34, 2464–2470. [Google Scholar] [CrossRef]
- Chun, J.N.; Choi, B.; Lee, K.W.; Lee, D.J.; Kang, D.H.; Lee, J.Y.; Song, I.S.; Kim, H.I.; Lee, S.H.; Kim, H.S.; et al. Cytosolic Hsp60 is involved in the NF-kappaB-dependent survival of cancer cells via IKK regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef]
- Lewis, J.; Devin, A.; Miller, A.; Lin, Y.; Rodriguez, Y.; Neckers, L.; Liu, Z.G. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J. Biol. Chem. 2000, 275, 10519–10526. [Google Scholar] [CrossRef]
- Nakamura, H.; Minegishi, H. HSP60 as a drug target. Curr. Pharm. Des. 2013, 19, 441–451. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, J.; Liao, F.; Yan, X.; Li, J.; Huang, L.; Liu, F. Inhibition of Heat Shock Protein 90 by 17-AAG Reduces Inflammation via P2X7 Receptor/NLRP3 Inflammasome Pathway and Increases Neurogenesis after Subarachnoid Hemorrhage in Mice. Front. Mol. Neurosci. 2018, 11, 401. [Google Scholar] [CrossRef]
- Peterson, L.B.; Blagg, B.S. To fold or not to fold: Modulation and consequences of Hsp90 inhibition. Future Med. Chem. 2009, 1, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.A.; Caplan, A.J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta 2012, 1823, 683–688. [Google Scholar] [CrossRef]
- Luo, W.; Sun, W.; Taldone, T.; Rodina, A.; Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.J.; Thrash, J.C.; Walter, B. The cellular response in neuroinflammation: The role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 2006, 6, 237–245. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Bogár, F.; Crul, T.; Sántha, M.; Tóth, M.E.; Vígh, L. Heat Shock Proteins and Autophagy Pathways in Neuroprotection: From Molecular Bases to Pharmacological Interventions. Int. J. Mol. Sci. 2018, 19, 325. [Google Scholar] [CrossRef]
- Campanella, C.; Bucchieri, F.; Merendino, A.M.; Fucarino, A.; Burgio, G.; Corona, D.F.; Barbieri, G.; David, S.; Farina, F.; Zummo, G.; et al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS ONE 2012, 7, e42008. [Google Scholar] [CrossRef] [PubMed]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef]
- Mambula, S.S.; Stevenson, M.A.; Ogawa, K.; Calderwood, S.K. Mechanisms for Hsp70 secretion: Crossing membranes without a leader. Methods 2007, 43, 168–175. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef]
- Lauwers, E.; Wang, Y.C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, J.; Xu, Y.; Zhang, X. Paraquat-induced inflammatory response of microglia through HSP60/TLR4 signaling. Hum. Exp. Toxicol. 2018, 37, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mangione, M.R.; Vilasi, S.; Marino, C.; Librizzi, F.; Canale, C.; Spigolon, D.; Bucchieri, F.; Fucarino, A.; Passantino, R.; Cappello, F.; et al. Hsp60, amateur chaperone in amyloid-beta fibrillogenesis. Biochim. Biophys. Acta 2016, 1860, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Angileri, F.; Conway de Macario, E.; Macario, A.J.L. Chaperonopathies and chaperonotherapy. Hsp60 as therapeutic target in cancer: Potential benefits and risks. Curr. Pharm. Des. 2013, 19, 452–457. [Google Scholar] [CrossRef]
- Cappello, F.; Conway de Macario, E.; Marino Gammazza, A.; Bonaventura, G.; Carini, F.; Czarnecka, A.M.; Farina, F.; Zummo, G.; Macario, A.J.L. Hsp60 and human aging: Les liaisons dangereuses. Front. Biosci. 2013, 18, 626–637. [Google Scholar] [CrossRef]
- Nemirovsky, A.; Fisher, Y.; Baron, R.; Cohen, I.R.; Monsonego, A. Amyloid beta-HSP60 peptide conjugate vaccine treats a mouse model of Alzheimer’s disease. Vaccine 2011, 29, 4043–4050. [Google Scholar] [CrossRef]
- Arispe, N.; De Maio, A. Memory Loss and the Onset of Alzheimer’s Disease Could Be under the Control of Extracellular Heat Shock Proteins. J. Alzheimers Dis. 2018, 63, 927–934. [Google Scholar] [CrossRef]
- De Mena, L.; Chhangani, D.; Fernandez-Funez, P.; Rincon-Limas, D.E. secHsp70 as a tool to approach amyloid-β42 and other extracellular amyloids. Fly 2017, 11, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kakimura, J.; Kitamura, Y.; Takata, K.; Umeki, M.; Suzuki, S.; Shibagaki, K.; Taniguchi, T.; Nomura, Y.; Gebicke-Haerter, P.J.; Smith, M.A.; et al. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002, 16, 601–603. [Google Scholar] [CrossRef]
- Maciejewski, A.; Ostapchenko, V.G.; Beraldo, F.H.; Prado, V.F.; Prado, M.A.; Choy, W.Y. Domains of STIP1 responsible for regulating PrPC-dependent amyloid-β oligomer toxicity. Biochem. J. 2016, 473, 2119–2130. [Google Scholar] [CrossRef]
- Wirdefeldt, K.; Adami, H.O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and etiology of Parkinson’s disease: A review of the evidence. Eur. J. Epidemiol. 2011, 26, S1–S58. [Google Scholar] [CrossRef]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Parkinson disease. Nat. Rev. Dis. Primers 2018, 159, 173–193. [Google Scholar]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Hartl, F.U.; Martin, J.; Pollock, R.A.; Kalousek, F.; Neupert, W.; Hallberg, E.M.; Hallberg, R.L.; Horwich, A.L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature 1989, 337, 620–625. [Google Scholar] [CrossRef]
- Pemberton, S.; Melki, R. The interaction of Hsc70 protein with fibrillar α-Synuclein and its therapeutic potential in Parkinson’s disease. Commun. Integr. Biol. 2012, 5, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Bohush, A.; Bieganowski, P.; Filipek, A. Hsp90 and Its Co-Chaperones in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 4976. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M. Interactions between Hsp70 and the hydrophobic core of alpha-synuclein inhibit fibril assembly. Biochemistry 2008, 47, 12614–12625. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Shin, Y.; Masliah, E.; Hyman, B.T.; McLean, P.J. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J. Biol. Chem. 2004, 279, 25497–25502. [Google Scholar] [PubMed]
- Falsone, S.F.; Kungl, A.J.; Rek, A.; Cappai, R.; Zangger, K. The molecular chaperone Hsp90 modulates intermediate steps of amyloid assembly of the Parkinson-related protein alpha-synuclein. J. Biol. Chem. 2009, 284, 31190–31199. [Google Scholar] [PubMed]
- Feng, M.; Zhang, L.; Liu, Z.; Zhou, P.; Lu, X. The expression and release of Hsp60 in 6-OHDA induced in vivo and in vitro models of Parkinson’s disease. Neurochem. Res. 2013, 38, 2180–2189. [Google Scholar] [PubMed]
- Lecours, C.; Bordeleau, M.; Cantin, L.; Parent, M.; Paolo, T.D.; Tremblay, M.È. Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front. Cell Neurosci. 2018, 12, 282. [Google Scholar]
- Robberecht, W.; Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013, 14, 248–264. [Google Scholar]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar]
- McAlary, L.; Aquilina, J.A.; Yerbury, J.J. Susceptibility of mutant SOD1 to form a destabilized monomer predicts cellular aggregation and toxicity but not in vitro aggregation propensity. Front. Neurosci. 2016, 10, 499. [Google Scholar]
- San Gil, R.; Ooi, L.; Yerbury, J.J.; Ecroyd, H. The heat shock response in neurons and astroglia and its role in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 65. [Google Scholar]
- Takeuchi, H.; Kobayashi, Y.; Yoshihara, T.; Niwa, J.; Doyu, M.; Ohtsuka, K.; Sobue, G. Hsp70 and Hsp40 improve neurite outgrowth and suppress intracytoplasmic aggregate formation in cultured neuronal cells expressing mutant SOD1. Brain Res. 2002, 949, 11–22. [Google Scholar]
- Liu, J.; Shinobu, L.A.; Ward, C.M.; Young, D.; Cleveland, D.W. Elevation of the Hsp70 chaperone does not effect toxicity in mouse models of familial amyotrophic lateral sclerosis. J. Neurochem. 2005, 93, 875–882. [Google Scholar]
- Apolloni, S.; Caputi, F.; Pignataro, A.; Amadio, S.; Fabbrizio, P.; Ammassari-Teule, M.; Volonté, C. Histamine Is an Inducer of the Heat Shock Response in SOD1-G93A Models of ALS. Int. J. Mol. Sci. 2019, 20, 3793. [Google Scholar] [CrossRef] [PubMed]
- Gifondorwa, D.J.; Robinson, M.B.; Hayes, C.D.; Taylor, A.R.; Prevette, D.M.; Oppenheim, R.W.; Caress, J.; Milligan, C.E. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2007, 27, 13173–13180. [Google Scholar] [CrossRef] [PubMed]
- Tytell, M. Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int. J. Hyperth. 2005, 21, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Giffard, R.G.; Yenari, M.A. Many mechanisms for hsp70 protection from cerebral ischemia. J. Neurosurg. Anesth. 2004, 16, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Robinson, M.B.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Regulation of heat shock protein 70 release in astrocytes: Role of signaling kinases. Dev. Neurobiol. 2007, 67, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.N.; Zheng, D.; Sabbagh, J.J.; Martin, M.D.; Chaput, D.; Darling, A.; Trotter, J.H.; Stothert, A.R.; Nordhues, B.A.; Lussier, A.; et al. DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 2016, 35, 1537–1549. [Google Scholar] [CrossRef]
- Robinson, M.B.; Taylor, A.R.; Gifondorwa, D.J.; Tytell, M.; Milligan, C.E. Exogenous Hsc70, but not thermal preconditioning, confers protection to motoneurons subjected to oxidative stress. Dev. Neurobiol. 2008, 68, 1–17. [Google Scholar] [CrossRef]
- Kim, S.D.; Fung, V.S. An update on Huntington’s disease: From the gene to the clinic. Curr Opin Neurol 2014, 27, 477–483. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Wang, H.Q.; Xu, Y.X.; Zhao, X.Y.; Zhao, H.; Yan, J.; Sun, X.B.; Guo, J.C.; Zhu, C.Q. Overexpression of F(0)F(1)-ATP synthase alpha suppresses mutant huntingtin aggregation and toxicity in vitro. Biochem. Biophys. Res. Commun. 2009, 390, 1294–1298. [Google Scholar] [CrossRef]
- Novoselova, T.V.; Margulis, B.A.; Novoselov, S.S.; Sapozhnikov, A.M.; van der Spuy, J.; Cheetham, M.E.; Guzhova, I.V. Treatment with extracellular HSP70/HSC70 protein can reduce polyglutamine toxicity and aggregation. J. Neurochem. 2005, 94, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.; Weiss, A.; Parker, C.N.; Bibel, M.; Paganetti, P.; Kaupmann, K. A screen for enhancers of clearance identifies huntingtin as a heat shock protein 90 (Hsp90) client protein. J. Biol. Chem. 2012, 287, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Díaz, R.; Sánchez-Álvarez, A.; Hernández-Hernández, J.M.; Tapia-Ramírez, J. The interaction between RE1-silencing transcription factor (REST) and heat shock protein 90 as new therapeutic target against Huntington’s disease. PLoS ONE 2019, 14, e0220393. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Hwang, J.Y.; Follenzi, A.; Athanasiadou, R.; Miyawaki, T.; Greally, J.M.; Bennett, M.V.; Zukin, R.S. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, E962–E971. [Google Scholar] [CrossRef] [PubMed]
- Ekimova, I.V.; Nitsinskaya, L.E.; Romanova, I.V.; Pastukhov, Y.F.; Margulis, B.A.; Guzhova, I.V. Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J. Neurochem. 2010, 115, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Behrends, C.; Langer, C.A.; Boteva, R.; Böttcher, U.M.; Stemp, M.J.; Schaffar, G.; Rao, B.V.; Giese, A.; Kretzschmar, H.; Siegers, K.; et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol. Cell 2006, 23, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.C.; Douglas, J.N.; Meyers, L.; Lee, S.; Shin, Y.; Gardner, L.A. Neurodegeneration in multiple sclerosis involves multiple pathogenic mechanisms. Degener. Neurol. Neuromuscul. Dis. 2014, 4, 49–63. [Google Scholar] [CrossRef]
- Quintana, F.J.; Farez, M.F.; Izquierdo, G.; Lucas, M.; Cohen, I.R.; Weiner, H.L. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology 2012, 78, 532–539. [Google Scholar] [CrossRef]
- Cwiklinska, H.; Mycko, M.P.; Luvsannorov, O.; Walkowiak, B.; Brosnan, C.F.; Raine, C.S.; Selmaj, K.W. Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int. Immunol. 2003, 15, 241–249. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Costa, C.; Eixarch, H.; Tepavcevic, V.; Castillo, M.; Martin, R.; Lubetzki, C.; Aigrot, M.S.; Montalban, X.; Espejo, C. Hsp70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e105737. [Google Scholar] [CrossRef]
- Mycko, M.P.; Cwiklinska, H.; Walczak, A.; Libert, C.; Raine, C.S.; Selmaj, K.W. A heat shock protein gene (Hsp70.1) is critically involved in the generation of the immune response to myelin antigen. Eur. J. Immunol. 2008, 38, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Aquino, D.A.; Capello, E.; Weisstein, J.; Sanders, V.; Lopez, C.; Tourtellotte, W.W.; Brosnan, C.F.; Raine, C.S.; Norton, W.T. Multiple sclerosis: Altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J. Neuropathol. Exp. Neurol. 1997, 56, 664–672. [Google Scholar] [CrossRef]
- Cid, C.; Alvarez-Cermeño, J.C.; Camafeita, E.; Salinas, M.; Alcázar, A. Antibodies reactive to heat shock protein 90 induce oligodendrocyte precursor cell death in culture. Implications for demyelination in multiple sclerosis. FASEB J. 2004, 18, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Lu, C.H.; Greensmith, L. The role of heat shock proteins in Amyotrophic Lateral Sclerosis: The therapeutic potential of Arimoclomol. Pharmacol. Ther. 2014, 141, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Atassi, N.; David, W.; Cudkowicz, M.; Schoenfeld, D. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology 2018, 90, e565–e574. [Google Scholar] [CrossRef] [PubMed]
- Kuta, R.; Larochelle, N.; Fernandez, M.; Pal, A.; Minotti, S.; Tibshirani, M.; St Louis, K.; Gentil, B.J.; Nalbantoglu, J.N.; Hermann, A.; et al. Depending on the stress, histone deacetylase inhibitors act as heat shock protein co-inducers in motor neurons and potentiate arimoclomol, exerting neuroprotection through multiple mechanisms in ALS models. Cell Stress Chaperones 2020, 25, 173–191. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).