Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential
Abstract
1. Introduction
2. Materials and Methods
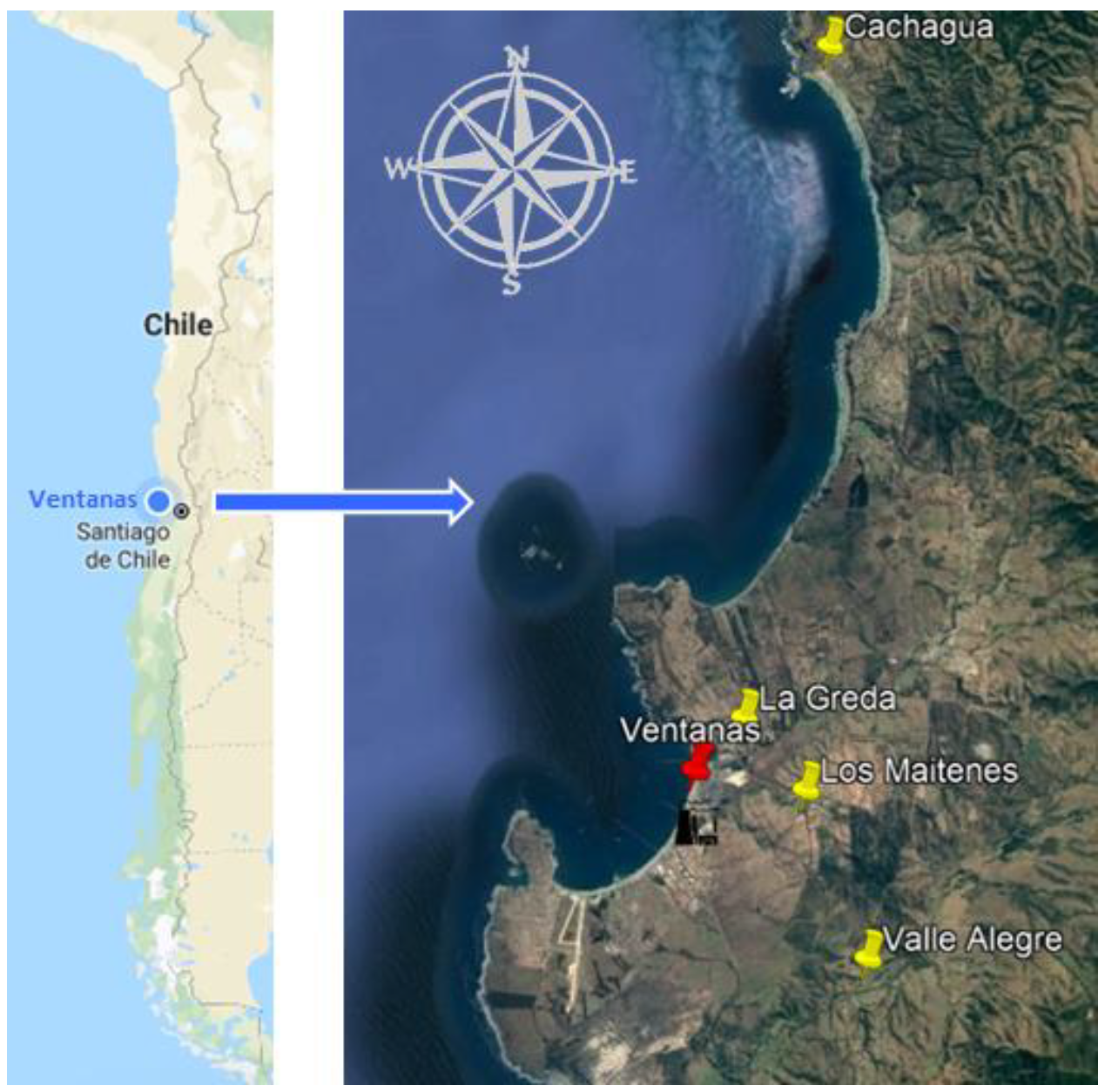
2.1. Site Description (Including Vegetation)
2.2. Plant Sampling and Identification
2.3. Reagents
2.4. Plant and Soil Analysis
2.5. Quality Assurance/Quality Control
2.6. Transfer Factor Calculations
3. Results and Discussion
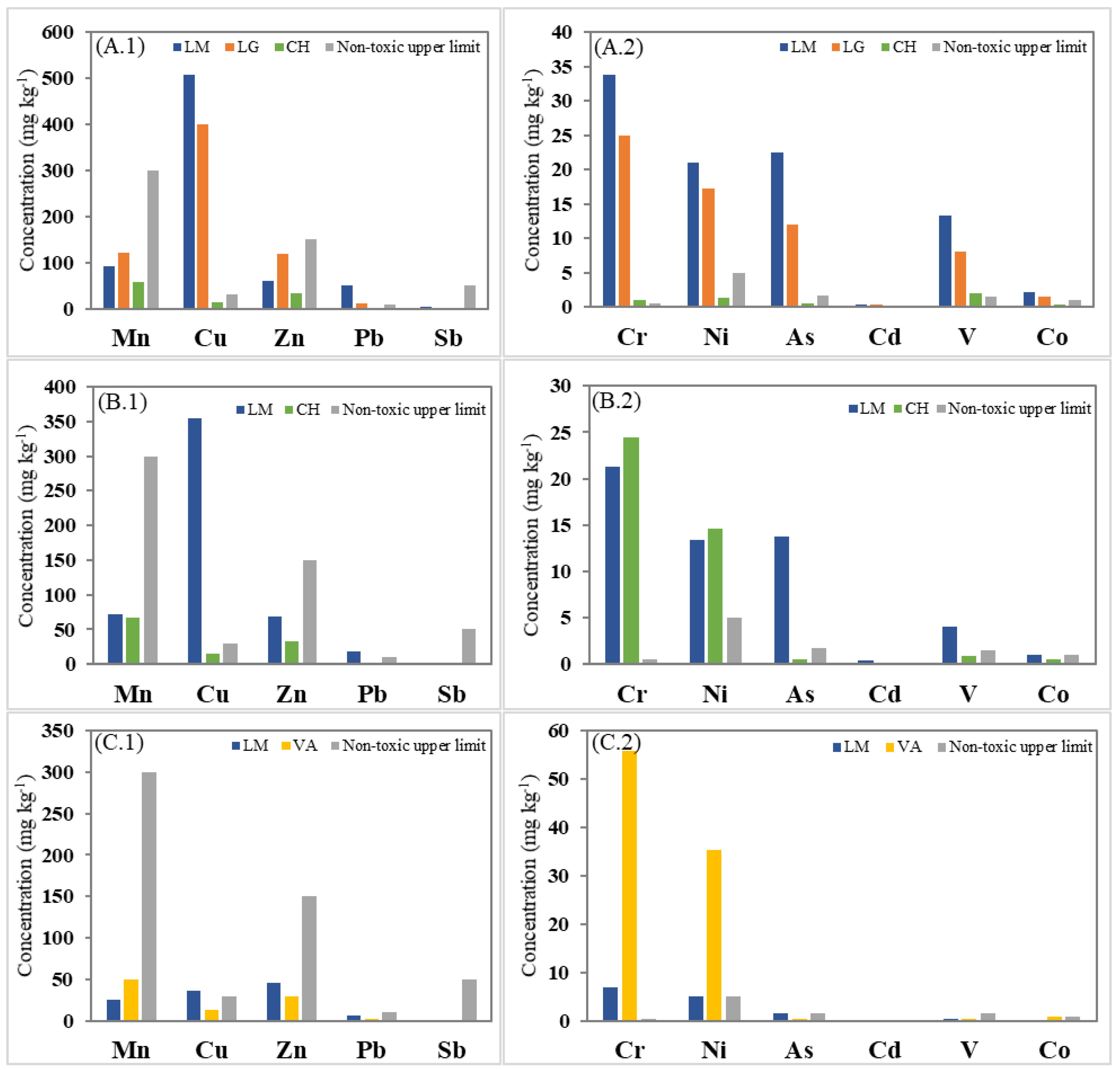
3.1. Trace Elements Concentrations in Plant Species
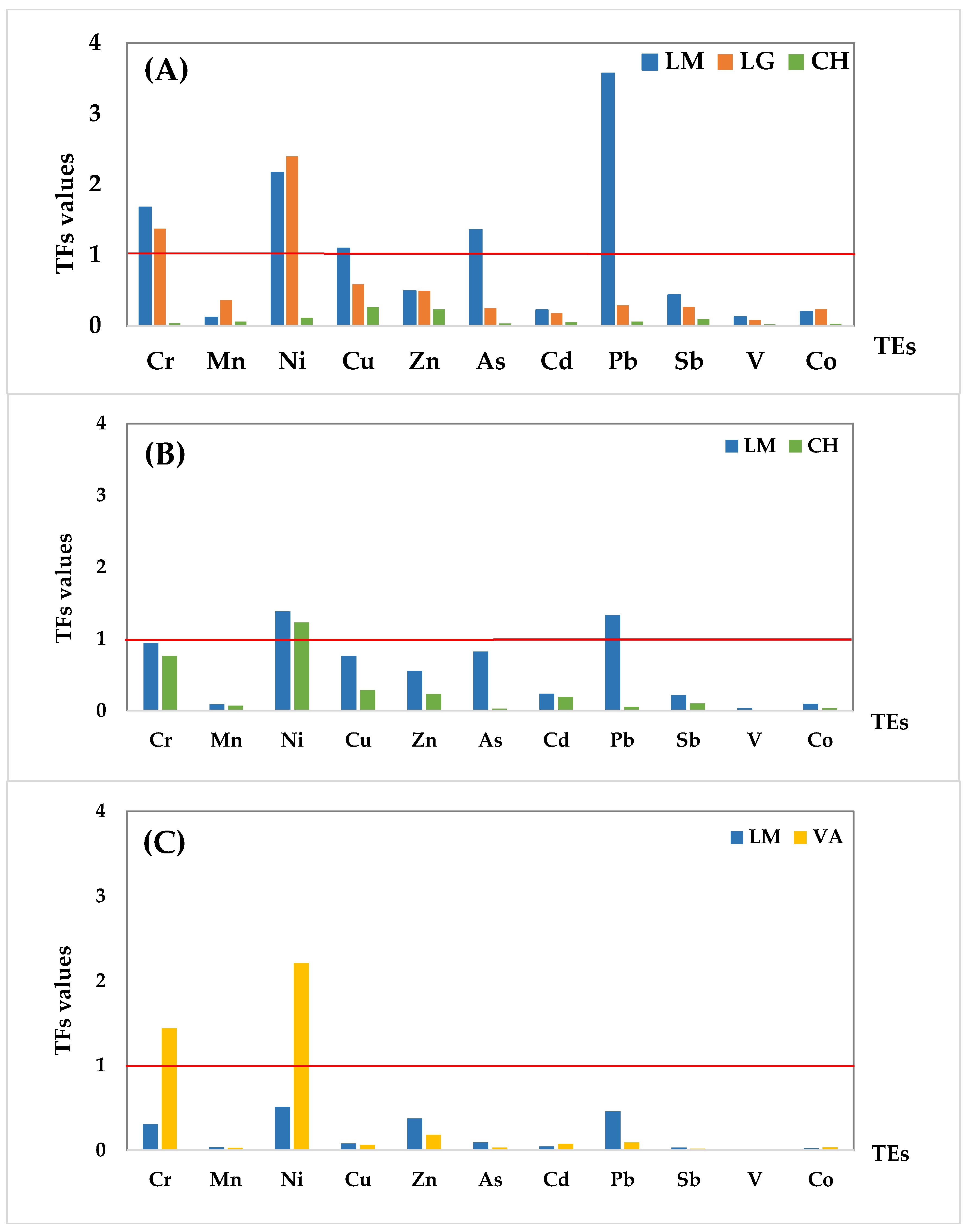
3.2. Soil-to-Plant Transfer Factors for Trace Elements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massa, N.; Andreucci, F.; Poli, M.; Aceto, M.; Barbato, R.; Berta, G. Screening for heavy metal accumulators amongst autochtonous plants in a polluted site in Italy. Ecotoxicol. Environ. Saf. 2010, 73, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Gorena, T.; Fadic, X.; Cereceda-Balic, F. Cupressus macrocarpa leaves for biomonitoring the environmental impact of an industrial complex: The case of Puchuncaví-Ventanas in Chile. Chemosphere 2020, 260, 127521. [Google Scholar] [CrossRef] [PubMed]
- Ataabadi, M.; Hoodaji, M.; Afi, A. Heavy Metals Biomonitoring by Plants Grown in an Industrial Area of Isfahan’ Mobarakeh Steel Company. J. Environ. Stud. 2010, 35, 83–92. [Google Scholar]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soils; Terry, N., Vangronsveld, J., Banuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 85–107. [Google Scholar]
- Mganga, N.; Manoko, M.L.K.; Rulangaranga, Z. Classification of plants according to their heavy metal content around North Mara Gold Mine, Tanzania: Implication for phytoremediation. Tanzan. J. Sci. 2011, 37, 109–119. [Google Scholar]
- Del Río, M.; Font, R.; Almela, C.; Vélez, D.; Montoro, R.; De Haro Bailón, A. Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcóllar mine. J. Biotechnol. 2002, 98, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, Q.; Zheng, D. Health risk of Hg, Pb, Cd, Zn, and Cu to the inhabitants around Huludao Zinc Plant in China via consumption of vegetables. Sci. Total Environ. 2007, 383, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mihali, C.; Michnea, A.; Oprea, G.; Gogoasa, I.; Pop, C.; Marin Senilă, L.G. Trace element transfer from soil to vegetables around the lead smelter in Baia Mare, NW Romania. J. Food Agric. Environ. 2012, 10, 828–834. [Google Scholar]
- Pastor, J.; Aparicio, A.M.; Gutierrez-Maroto, A.; Hernández, A.J. Effects of two chelating agents (EDTA and DTPA) on the autochthonous vegetation of a soil polluted with Cu, Zn and Cd. Sci. Total Environ. 2007, 378, 114–118. [Google Scholar] [CrossRef]
- Salmanighabeshi, S.; Palomo-Marín, M.; Bernalte, E.; Rueda-Holgado, F.; Miró-Rodríguez, C.; Fadic-Ruiz, X.; Vidal-Cortez, V.; Cereceda-Balic, F.; Pinilla-Gil, E. Long-term assessment of ecological risk from deposition of elemental pollutants in the vicinity of the industrial area of Puchuncaví-Ventanas, central Chile. Sci. Total Environ. 2015, 527–528, 335–343. [Google Scholar] [CrossRef]
- Ginocchio, R. Effects of a copper smelter on a grassland community in the Puchuncaví Valley, Chile. Chemosphere 2000, 41, 15–23. [Google Scholar] [CrossRef]
- González, I.; Muena, V.; Cisternas, M.; Neaman, A. Copper accumulation in a plant community affected by mining contamination in Puchuncaví valley, central Chile. Rev. Chil. Hist. Nat. 2008, 81, 279–291. [Google Scholar]
- González, I.; Cortes, A.; Neaman, A.; Rubio, P. Biodegradable chelate enhances the phytoextraction of copper by Oenothera picensis grown in copper-contaminated acid soils. Chemosphere 2011, 84, 490–496. [Google Scholar] [CrossRef] [PubMed]
- De Gregori, I.; Lobos, G.; Lobos, S.; Pinochet, H.; Potin-Gautier, M.; Astruc, M. Copper and selenium in rainwater, soils and alfalfa from agricultural ecosystems of Valparaiso region, Chile. Boletín la Soc. Chil. Química 2000, 45. [Google Scholar] [CrossRef]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Cornejo, P. Different patterns of organic acid exudation in metallophyte and agricultural plants at increasing copper levels. In Proceedings of the the 19th World Congress of Soil Science; Soil Solutions for a Changing World; Gilkes, R., Prakongkep, N., Gilkes, R., Prakongkep, N., Eds.; International Union of Soil Sciences: Brisbane, Australia, 2010; pp. 17–20. [Google Scholar]
- Muena, V.; González, I.; Neaman, A. Efectos del encalado y la fertilización nitrogenada sobre el desarrollo de Oenothera affinis en un suelo afectado por la minería del cobre. Rev. la Cienc. del suelo y Nutr. Veg. 2010, 10. [Google Scholar] [CrossRef]
- Cochilco Anuario de Estadísticas del Cobre y Otros Minerales (1994-2013). Available online: https://biblioteca.cchc.cl/datafiles/33546-2.pdf (accessed on 13 December 2016).
- AES Gener Principal. Available online: http://www.gener.cl/Paginas/Principal.aspx (accessed on 13 December 2016).
- Folchi, D.M. Historia Ambiental de las Labores de Beneficio en la Minería del Cobre en Chile, Siglos XIX y XX; Universidad de Barcelona: Barcelona, Spain, 2006. [Google Scholar]
- Ginocchio, R.; Carvallo, G.; Toro, I.; Bustamante, E.; Silva, Y.; Sepúlveda, N. Micro-spatial variation of soil metal pollution and plant recruitment near a copper smelter in Central Chile. Environ. Pollut. 2004, 127, 343–352. [Google Scholar] [CrossRef]
- González, I.; Neaman, A.; Rubio, P.; Cortés, A. Spatial distribution of copper and pH in soils affected by intensive industrial activities in Puchuncaví and Quintero, central Chile. J. Soil Sci. Plant Nutr. 2014. [Google Scholar] [CrossRef]
- Parra, S.; Bravo, M.A.; Quiroz, W.; Moreno, T.; Karanasiou, A.; Font, O.; Vidal, V.; Cereceda, F. Distribution of trace elements in particle size fractions for contaminated soils by a copper smelting from different zones of the Puchuncaví Valley (Chile). Chemosphere 2014, 111. [Google Scholar] [CrossRef]
- Tang, S.; Wilke, B.M.; Huang, C. The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant Soil 1999, 209, 225–232. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E.; Barceló, J. Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 2001, 230, 247–256. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Zereini, F.; Püttmann, W. Traffic-related trace element fate and uptake by plants cultivated in roadside soils in Toronto, Canada. Sci. Total Environ. 2013, 442, 86–95. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H.; Górecki, H. Bioavailability of heavy metals from polluted soils to plants. Sci. Total Environ. 2005, 337, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, Y.A.; Skipperud, L.; Meland, S.; Dadebo, E.; Einset, J.; Salbu, B. Trace element mobility and transfer to vegetation within the Ethiopian Rift Valley lake areas. J. Environ. Monit. 2012, 14, 2698. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ding, C.; Li, X.; Zhang, T.; Wang, X. Heavy metals in navel orange orchards of Xinfeng County and their transfer from soils to navel oranges. Ecotoxicol. Environ. Saf. 2015, 122, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bakhat, H.F.; Zia, Z.; Abbas, S.; Hammad, H.M.; Shah, G.M.; Khalid, S.; Shahid, N.; Sajjad, M.; Fahad, S. Factors controlling arsenic contamination and potential remediation measures in soil-plant systems. Groundw. Sustain. Dev. 2019, 9, 100263. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Rueda-Holgado, F.; Palomo-Marín, M.R.; Calvo-Blázquez, L.; Cereceda-Balic, F.; Pinilla-Gil, E. Fractionation of trace elements in total atmospheric deposition by filtrating-bulk passive sampling. Talanta 2014, 125. [Google Scholar] [CrossRef]
- Rueda-Holgado, F.; Calvo-Blázquez, L.; Cereceda-Balic, F.; Pinilla-Gil, E. A semiautomatic system for soluble lead and copper monitoring in atmospheric deposition by coupling of passive elemental fractionation sampling and voltammetric measurement on screen-printed gold electrodes. Microchem. J. 2016, 124. [Google Scholar] [CrossRef]
- Rueda-Holgado, F.; Calvo-Blázquez, L.; Cereceda-Balic, F.; Pinilla-Gil, E. Temporal and spatial variation of trace elements in atmospheric deposition around the industrial area of Puchuncaví-Ventanas (Chile) and its influence on exceedances of lead and cadmium critical loads in soils. Chemosphere 2016, 144. [Google Scholar] [CrossRef]
- CCME Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. Available online: https://www.esdat.net/environmental%20standards/canada/soil/rev_soil_summary_tbl_7.0_e.pdf (accessed on 25 November 2020).
- Australian Department of Environment and Conservation Assessment Level for Soil, Sediment and Water. Available online: https://www.esdat.net/EnvironmentalStandards/Australia/WA/AssessmentLevels-2010.pdf (accessed on 18 September 2020).
- Ministry of Housing Spatial Planning and the Environment (Netherlands) Circular on Target Values and Intervention Values for Soil Remediation. Available online: https://www.esdat.net/EnvironmentalStandards/Dutch/annexS_I2000DutchEnvironmentalStandards.pdf (accessed on 12 September 2020).
- Wei, S.; Zhou, Q.; Wang, X. Identification of weed plants excluding the uptake of heavy metals. Environ. Int. 2005, 31. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Quadro, M.S.; Camargo, F.A.O.; Andreazza, R. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. J. Environ. Manag. 2020, 256. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Agrawal, M. Identification of metal tolerant plant species for sustainable phytomanagement of abandoned red mud dumps. Appl. Geochem. 2019, 104. [Google Scholar] [CrossRef]
- Al-Qahtani, K. Assessment of Heavy Metals Accumulation in Native Plant Species from Soils Contaminated in Riyadh City, Saudi Arabia. Life Sci. J. 2012, 9, 384–392. [Google Scholar]
- Badr, N.; Fawzy, M.; Al-Qahtani, K. Phytoremediation: An ecological solution to heavy-metal-polluted soil and evaluation of plant removal ability. World Appl. Sci. J. 2012, 16, 1292–1301. [Google Scholar]
- Chaffee, M.A.; Berry, K.H. Abundance and distribution of selected elements in soils, stream sediments, and selected forage plants from desert tortoise habitats in the Mojave and Colorado deserts, USA. J. Arid Environ. 2006, 67. [Google Scholar] [CrossRef]
- Wetl, R.; Bensko-Tarsitano, B.; Johnson, K.; Sweat, K.G.; Cahill, T. Uptake of uranium into desert plants in an abandoned uranium mine and its implications for phytostabilization strategies. J. Environ. Radioact. 2020, 220‒221, 106293. [Google Scholar] [CrossRef]
| Elements | SRM | Certified Values (mg kg−1) ± SD | Measured Concentration (mg kg−1) ± SD |
|---|---|---|---|
| Cr | NIST 1573a | 1.99 ± 0.06 | 1.44 ± 0.13 |
| Mn | NIST 1573a | 246 ± 8 | 199 ± 12 |
| BCR 281 | 81.6 ± 2.6 | 62.6 ± 0.1 | |
| Ni | NIST 1573a | 1.59 ± 0.07 | 1.74 ± 0.12 |
| BCR 281 | 3.00 ± 0.17 | 2.25 ± 0.003 | |
| Cu | NIST 1573a | 4.70 ± 0.14 | 4.03 ± 0.25 |
| BCR 281 | 9.65 ± 0.68 | 9.17 ± 0.02 | |
| Zn | NIST 1573a | 30.9 ± 0.7 | 35.5 ± 3.08 |
| As | NIST 1573a | 0.112 ± 0.004 | 0.24 ± 0.03 |
| BCR 281 | 0.057 ± 0.004 | 0.095 ± 0.00 | |
| Cd | NIST 1573a | 1.52 ± 0.04 | 1.77 ± 0.20 |
| BCR 281 | 0.120 ± 0.003 | 0.165 ± 0.000 | |
| Pb | BCR 281 | 2.38 ± 0.11 | 2.71 ± 0.00 |
| Sb | NIST 1573a | 0.063 ± 0.006 | 0.088 ± 0.008 |
| BCR 281 | 0.047 ± 0.005 | 0.062 ± 0.000 | |
| V | NIST 1573a | 0.835 ± 0.01 | 0.879 ± 0.05 |
| Co | NIST 1573a | 0.57 ± 0.02 | 0.38 ± 0.10 |
| Elements | Cr | Mn | Ni | Cu | Zn | As | Cd | Pb | Sb | V | Co | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration(mg kg−1) | Non–toxic | 0.1–0.5 | 30–300 | 0.1–5 | 5–30 | 27–150 | 1–1.7 | 0.05–0.2 | 5–10 | 7–50 | 0.2–1.5 | 0.02–1 |
| Toxic | 5–30 | 400–1000 | 10–100 | 20–100 | 100–400 | 5–20 | 5–30 | 30–300 | 150.00 | 5–10 | 15–50 | |
| Hyperaccumulation Limit | 1000 | 10,000 | 1000 | 1000 | 10,000 | - | 100 | 1000 | - | - | 1000 | |
| Species | Location | Concentrations (mg kg−1) | ||||||||||
| O. picensis (OP) | LM | 30.64–35.46 (33.77) | 83.67–99.24 (92.34) | 19.70–21.68 (21.00) | 467.92–548.44 (507.77) | 55.24–66.66 (60.35) | 20.52–25.17 (22.47) | 0.36–0.45 (0.40) | 34.03–78.84 (49.64) | 3.65–5.07 (4.17) | 11.78–14.44 (13.29) | 2.03–2.22 (2.11) |
| LG | 23.01–26.16 (24.95) | 114.99–126.46 (120.70) | 16.26–17.90 (17.24) | 390.53–407.94 (399.41) | 115.46–119.26 (117.87) | 11.42–12.93 (12.01) | 0.30–0.33 (0.31) | 11.36–12.73 (11.99) | 1.60–2.19 (1.84) | 7.51–8.92 (8.15) | 1.38–1.67 (1.49) | |
| LG (leaves) | 8.99–9.63 (9.20) | 117.09–127.71 (123.66) | 9.06–9.83 (9.40) | 190.34–204.99 (196.53) | 135.01–142.57 (138.84) | 3.36–4.03 (3.80) | 0.27–0.29 (0.28) | 5.55–6.07 (5.80) | 0.56–0.64 (0.60) | 1.23–1.27 (1.25) | 0.48–0.59 (0.52) | |
| CH | 0.82–1.27 (1.02) | 52.71–60.55 (56.80) | 1.20–1.47 (1.30) | 12.52–14.99 (13.51) | 31.46–35.08 (33.34) | 0.49–0.64 (0.58) | 0.03–0.03 (0.03) | 1.06–1.14 (1.11) | 0.08–0.09 (0.09) | 1.83–2.30 (2.02) | 0.28–0.42 (0.35) | |
| S. velutina (SV) | LM | 20.73–21.80 (21.23) | 68.90–73.29 (71.56) | 12.85–13.97 (13.40) | 347.95–364.70 (355.22) | 66.54–69.88 (68.63) | 12.75–14.24 (13.70) | 0.41–0.45 (0.43) | 17.96–19.10 (18.47) | 2.05–2.11 (2.07) | 4.00–4.11 (4.06) | 1.02–1.11 (1.05) |
| CH | 23.48–25.52 (24.42) | 63.95–70.14 (67.05) | 14.07–15.07 (14.58) | 14.40–15.43 (14.84) | 30.92–36.47 (33.38) | 0.46–0.52 (0.49) | 0.12–0.13 (0.12) | 0.82–1.57 (1.09) | 0.09–0.10 (0.10) | 0.89–0.92 (0.91) | 0.41–0.52 (0.47) | |
| A. subfusiformis (AS) | LM | 6.72–7.10 (6.91) | 24.33–26.88 (25.90) | 4.81–5.05 (4.97) | 33.67–40.02 (36.52) | 43.40–47.75 (45.90) | 1.48–1.61 (1.52) | 0.06–0.09 (0.07) | 1.99–8.55 (6.34) | 0.25–0.35 (0.28) | 0.47–0.51 (0.49) | 0.13–0.38 (0.23) |
| VA | 49.02–63.31 (55.72) | 47.22–52.03 (49.56) | 30.16–41.19 (35.35) | 13.20–14.03 (13.75) | 27.51–33.16 (30.16) | 0.26–0.39 (0.33) | 0.06–0.10 (0.08) | 0.74–4.20 (1.97) | 0.06–0.09 (0.07) | 0.40–0.42 (0.41) | 0.82–0.98 (0.88) | |
| Location | pH | Organic Matter (%) | C.E.C * (meq 100 g−1) | Sand/Silt/Clay (%) | Mean Concentrations of Element in Soil (mg kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Ni | Cu | Zn | As | Cd | Pb | Sb | V | Co | |||||
| LM | 5.3 | 0.34 | 2.88 | 82/3/15 | 22.6 | 817.3 | 9.7 | 465.5 | 123.6 | 16.6 | 1.81 | 13.9 | 9.6 | 109.8 | 10.8 |
| LG | 5.0 | 1.40 | 4.96 | 83/2/15 | 18.2 | 335.6 | 7.2 | 685.9 | 239.7 | 49.0 | 1.78 | 42.0 | 7.0 | 104.1 | 6.4 |
| VA | 6.0 | 7.73 | 14.70 | 72/5/23 | 38.7 | 1888.2 | 16.0 | 224.1 | 166.6 | 10.9 | 1.06 | 21.8 | 4.3 | 121.0 | 26.6 |
| CH | 7.3 | 5.65 | 6.88 | 67/8/25 | 32.0 | 977.5 | 11.9 | 52.2 | 144.7 | 18.9 | 0.64 | 19.9 | 1.0 | 107.8 | 12.9 |
| Reference Soil Quality Standards | |||||||||||||||
| Canada [35] | 87 | - | 50 | 91 | 360 | - | 22 | 600 | - | 130 | - | ||||
| Australia [36] | - | 60 | 100 | 200 | 20 | 3 | 1500 | - | 50 | - | |||||
| Netherlands [37] | 380 | - | 210 | 190 | 720 | 55 | 12 | 530 | 15 | 0 | 240 | ||||
| Species | Location | Cr | Mn | Ni | Cu | Zn | As | Cd | Pb | Sb | V | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O. picensis (OP) | LM | 1.671 | 0.113 | 2.165 | 1.091 | 0.488 | 1.354 | 0.219 | 3.571 | 0.433 | 0.121 | 0.195 |
| LG | 1.371 | 0.360 | 2.394 | 0.582 | 0.492 | 0.245 | 0.177 | 0.285 | 0.264 | 0.078 | 0.233 | |
| CH | 0.032 | 0.058 | 0.109 | 0.259 | 0.230 | 0.031 | 0.047 | 0.056 | 0.092 | 0.019 | 0.027 | |
| S. velutina (SV) | LM | 0.939 | 0.088 | 1.381 | 0.763 | 0.555 | 0.825 | 0.237 | 1.329 | 0.216 | 0.037 | 0.097 |
| CH | 0.763 | 0.069 | 1.225 | 0.284 | 0.231 | 0.026 | 0.191 | 0.055 | 0.101 | 0.008 | 0.036 | |
| A. subfusiformis (AS) | LM | 0.306 | 0.032 | 0.512 | 0.078 | 0.371 | 0.092 | 0.041 | 0.456 | 0.029 | 0.005 | 0.021 |
| VA | 1.440 | 0.026 | 2.209 | 0.061 | 0.181 | 0.030 | 0.073 | 0.090 | 0.018 | 0.003 | 0.033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmani-Ghabeshi, S.; Fadic-Ruiz, X.; Miró-Rodríguez, C.; Pinilla-Gil, E.; Cereceda-Balic, F. Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential. Appl. Sci. 2021, 11, 713. https://doi.org/10.3390/app11020713
Salmani-Ghabeshi S, Fadic-Ruiz X, Miró-Rodríguez C, Pinilla-Gil E, Cereceda-Balic F. Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential. Applied Sciences. 2021; 11(2):713. https://doi.org/10.3390/app11020713
Chicago/Turabian StyleSalmani-Ghabeshi, Soroush, Ximena Fadic-Ruiz, Conrado Miró-Rodríguez, Eduardo Pinilla-Gil, and Francisco Cereceda-Balic. 2021. "Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential" Applied Sciences 11, no. 2: 713. https://doi.org/10.3390/app11020713
APA StyleSalmani-Ghabeshi, S., Fadic-Ruiz, X., Miró-Rodríguez, C., Pinilla-Gil, E., & Cereceda-Balic, F. (2021). Trace Element Levels in Native Plant Species around the Industrial Site of Puchuncaví-Ventanas (Central Chile): Evaluation of the Phytoremediation Potential. Applied Sciences, 11(2), 713. https://doi.org/10.3390/app11020713








