Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Activated-Carbon Preparation
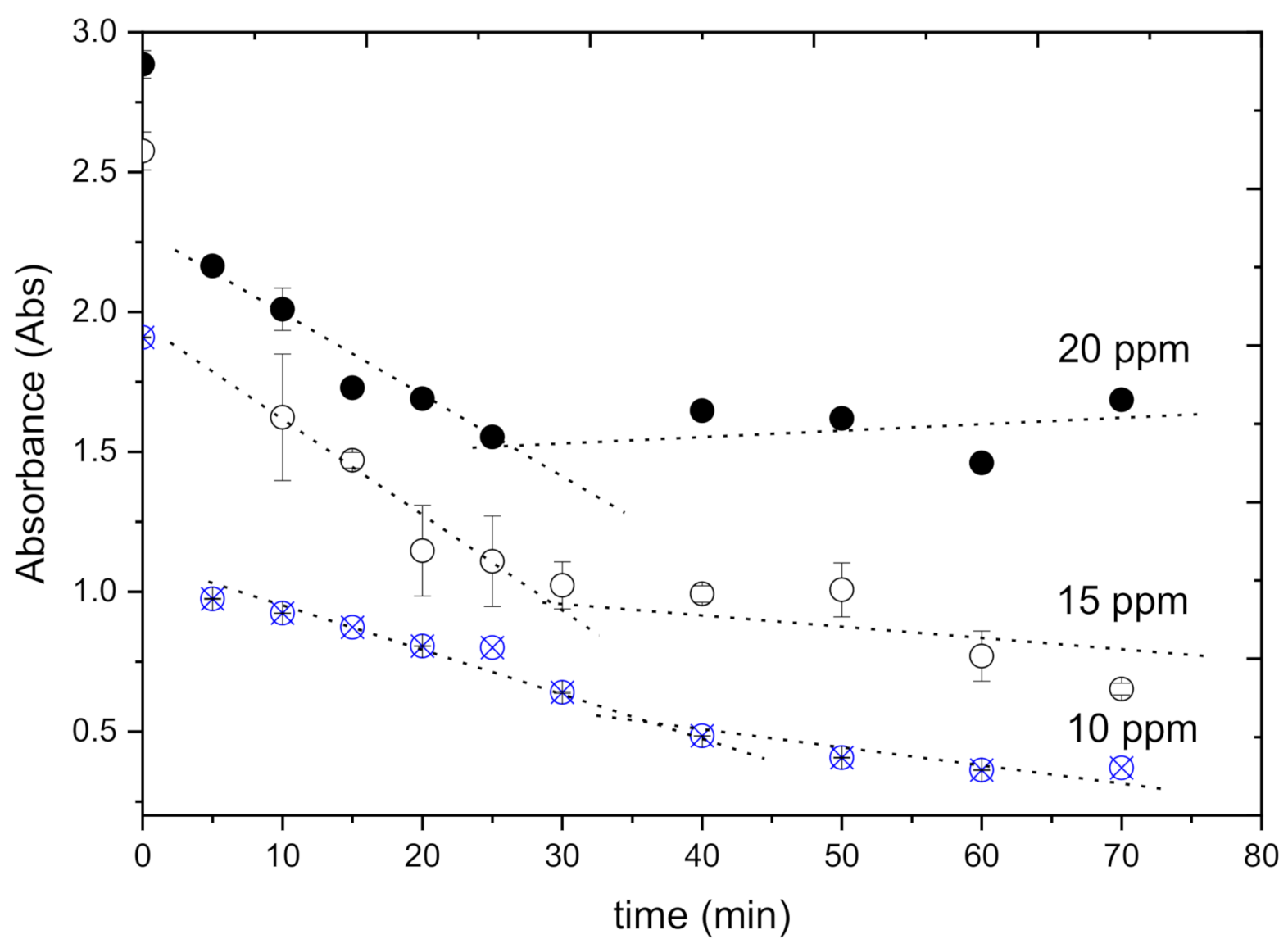
2.3. Adsorption Test
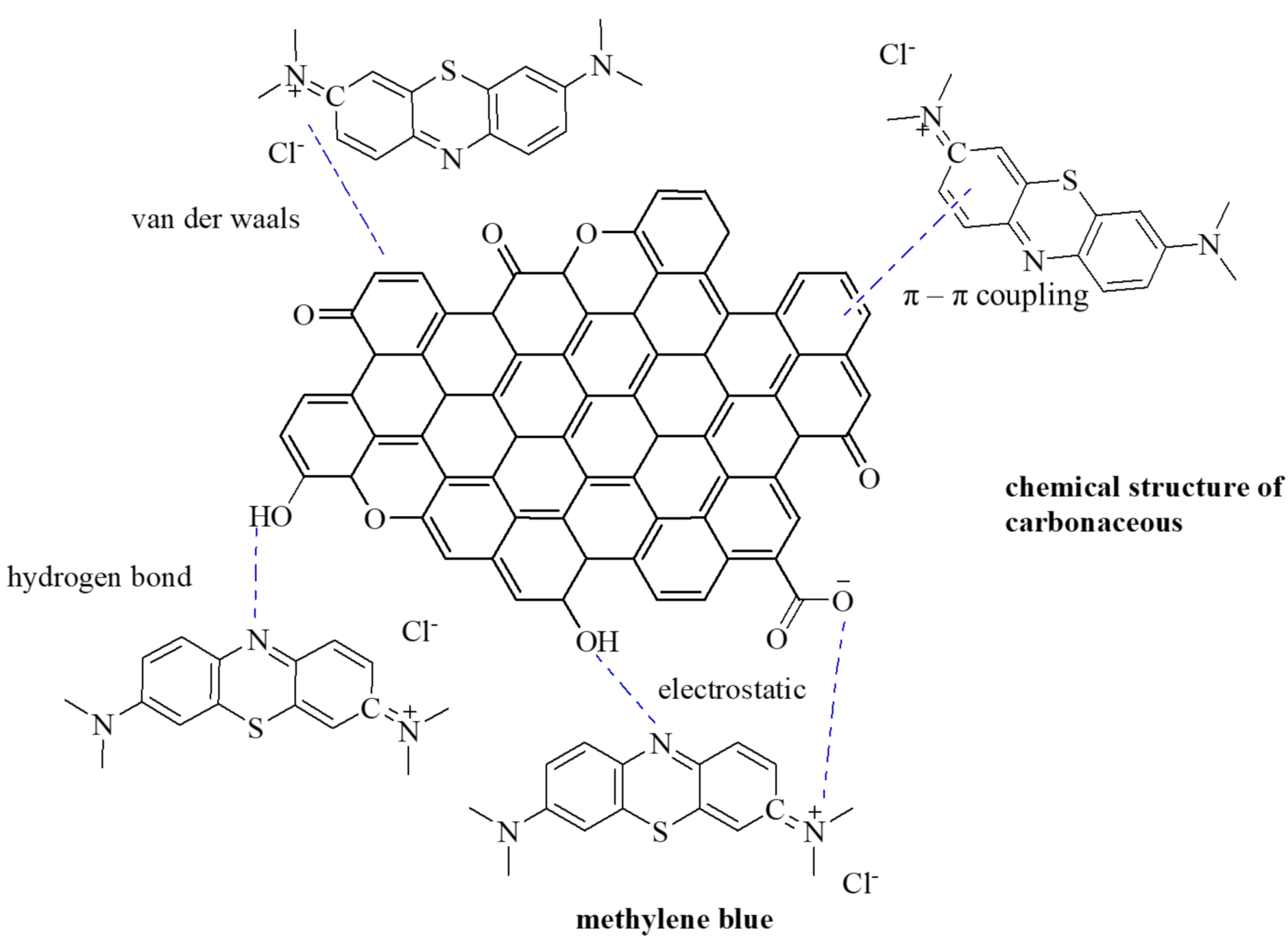
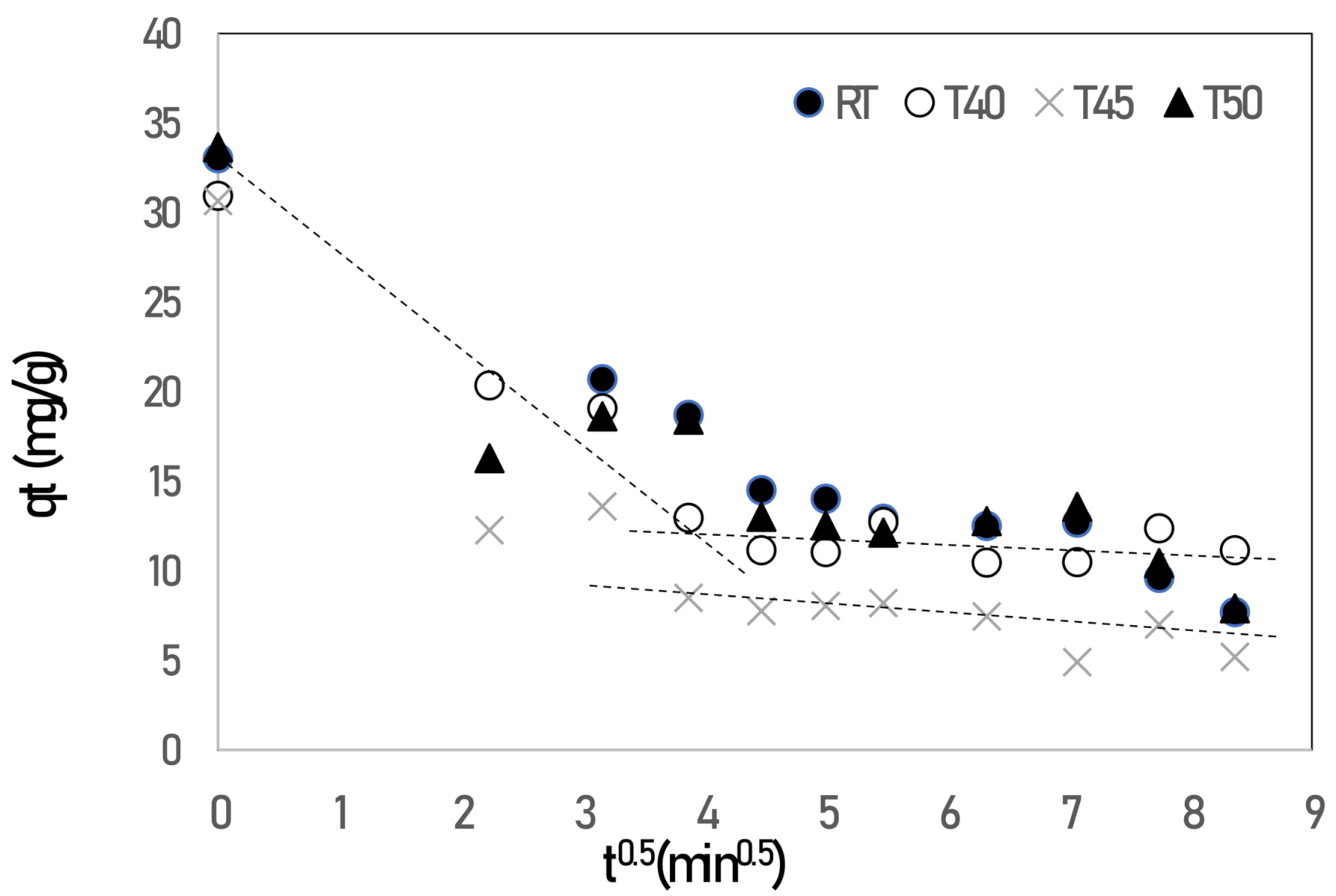
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sultan, M.; Miyazaki, T.; Koyama, S. Optimization of Adsorption Isotherm Types for Desiccant Air-Conditioning Applications. Renew. Energy 2018, 121, 441–450. [Google Scholar] [CrossRef]
- Yaningsih, I.; Wijayanta, A.T.; Thu, K.; Miyazaki, T. Influence of Phase Change Phenomena on the Performance of a Desiccant Dehumidification System. Appl. Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Oktariani, E.; Tahara, K.; Nakashima, K.; Noda, A.; Xue, B.; Wijayanta, A.T.; Nakaso, K.; Fukai, J. Experimental Investigation on the Adsorption Process for Steam Generation Using a Zeolite-Water System. J. Chem. Eng. Jpn. 2012, 45, 355–362. [Google Scholar] [CrossRef]
- Xue, B.; Tahara, K.; Nakashima, K.; Noda, A.; Oktariani, E.; Wijayanta, A.T.; Nakaso, K.; Fukai, J. Numerical Simulation for Steam Generation Process in a Novel Zeolite-Water Adsorption Heat Pump. J. Chem. Eng. Jpn. 2012, 45, 408–416. [Google Scholar] [CrossRef]
- Fukai, J.; Wijayanta, A.T. Potential Ability of Zeolite to Generate High-Temperature Vapor Using Waste Heat. AIP Conf. Proc. 2018, 1931, 020001. [Google Scholar] [CrossRef]
- Xu, Y.; Chai, X. Characterization of Coal Gasification Slag-Based Activated Carbon and Its Potential Application in Lead Removal. Environ. Technol. 2018, 39, 382–391. [Google Scholar] [CrossRef]
- Uraki, Y.; Tamai, Y.; Ogawa, M.; Gaman, S.; Tokurad, S. Preparation of Activated Carbon from Peat. BioResources 2009, 4, 205–213. [Google Scholar] [CrossRef]
- Yorgun, S.; Yildiz, D. Preparation and Characterization of Activated Carbons from Paulownia Wood by Chemical Activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Laine, J.; Simoni, S.; Calles, R. Preparation of Activated Carbon from Coconut Shell in a Small Scale Cocurrent Flow Rotary Kiln. Chem. Eng. Commun. 1999, 99, 15–23. [Google Scholar] [CrossRef]
- Chairunnisa Mikšík, F.; Miyazaki, T.; Thu, K.; Miyawaki, J.; Nakabayashi, K.; Wijayanta, A.T.; Rahmawati, F. Enhancing Water Adsorption Capacity of Acorn Nutshell Based Activated Carbon for Adsorption Thermal Energy Storage Application. Energy Rep. 2020. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Wang, H.; Fan, M. Synthesis of Bamboo-Based Activated Carbons with Super-High Specific Surface Area for Hydrogen Storage. BioResources 2017, 12, 1246–1262. [Google Scholar] [CrossRef]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated Carbon from Spent Coffee Grounds: A Good Competitor of Commercial Carbons for Water Decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Hajiha, H.; Sain, M. The Use of Sugarcane Bagasse Fibres as Reinforcements in Composites. Biofiber Reinf. Compos. Mater. 2014, 525–549. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, N.; Zhang, Y.; Hu, H.; Luo, Y. Composites: Part A Effect of Mechanical Activation Pretreatment on the Properties of Sugarcane Bagasse/Poly (Vinyl Chloride) Composites. Compos. Part A 2012, 43, 114–120. [Google Scholar] [CrossRef]
- Azmi, N.B.; Bashir, M.J.K.; Sethupathi, S.; Aun, C.N.; Lam, G.C. Optimization of Preparation Conditions of Sugarcane Bagasse Activated Carbon via Microwave-Induced KOH Activation for Stabilized Landfill Leachate Remediation. Environ. Earth Sci. 2016, 75, 902. [Google Scholar] [CrossRef]
- Wirawan, R.; Sapuan, S.M. Sugarcane Bagasse-Filled Poly (Vinyl Chloride) Composites; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Foo, K.Y.; Lee, L.K.; Hameed, B.H. Bioresource Technology Preparation of Activated Carbon from Sugarcane Bagasse by Microwave Assisted Activation for the Remediation of Semi-Aerobic Landfill Leachate. Bioresour. Technol. 2013, 134, 166–172. [Google Scholar] [CrossRef]
- Rahmawati, F.; Wahyuningsih, S.; Irianti, D. The Photocatalytic Activity of SiO2-TiO2/Graphite and Its Composite with Silver and Silver Oxide. Bull. Chem. React. Eng. Catal. 2014, 9, 45–52. [Google Scholar] [CrossRef]
- Rahmawati, F.; Yuliati, L.; Alaih, I.S.; Putri, F.R.; Rahmawati, F. Carbon Rod of Zinc-Carbon Primary Battery Waste as a Substrate for CdS and TiO2 Photocatalyst Layer for Visible Light Driven Photocatalytic Hydrogen Production. Biochem. Pharmacol. 2017. [Google Scholar] [CrossRef]
- Carey, J.; Silva, S.R. Carbon Based Electronic Materials: Applications in Electron Field Emission. J. Mater. Sci. Mater. Electron. 2006, 17, 405–412. [Google Scholar] [CrossRef][Green Version]
- Luo, X.; Cai, Y.; Zeng, J. Cr (VI) Adsorption Performance and Mechanism of an Effective Activated Carbon Prepared from Bagasse with a One-Step Pyrolysis and ZnCl 2 Activation Method. Cellulose 2019, 26, 4921–4934. [Google Scholar] [CrossRef]
- Chaiwon, T.; Channei, D. Study of Adsorption Behavior of Methyl Orange Dye on Activated Carbon Prepared from Sugarcane Bagasse via Chemical Activation. Carbon Sci. Technol. 2017, 9, 32–38. [Google Scholar]
- Tao, H.; Zhang, H.; Li, J.; Ding, W. Biomass Based Activated Carbon Obtained from Sludge and Sugarcane Bagasse for Removing Lead Ion from Wastewater. Bioresour. Technol. 2015, 192, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Solís-fuentes, J.A.; Galán-méndez, F.; Hernández-medel, M.R.; García-gómez, R.S.; Bernal-gonzález, M.; Mendoza-pérez, S.; Durán-domínguez-de-bazúa, C. Food Bioscience Effectiveness of Bagasse Activated Carbon in Raw Cane Juice Clarification. Food Biosci. 2019, 32, 100437. [Google Scholar] [CrossRef]
- Xia, J.; Noda, K.; Kagawa, S.; Wakao, N. Production of Activated Carbon from Bagasse (Waste) of Sugarcane Grown in Brazil. J. Chem. Eng. Jpn. 1998, 31, 987–990. [Google Scholar] [CrossRef]
- Jaguaribe, E.F.; Medeiros, L.L.; Barreto, M.C.S.; Araujo, L.P. The Performance of Activated Carbons from Sugarcane Bagasse, Babassu, and Coconut Shells in Removing Residual Chlorine. Braz. J. Chem. Eng. 2005, 22, 41–47. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Tümsek, F. Chemical Engineering Research and Design Production of Activated Carbon from Olive Bagasse by ˙ Lknur Demiral, Belgin Karabacako Glu. Chem. Eng. Res. Des. 2010, 9, 206–213. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Effect of the Activation Method on the Properties and Adsorption Behavior of Bagasse Fly Ash-Based Activated Carbon. Fuel Process. Technol. 2012, 102, 132–139. [Google Scholar] [CrossRef]
- Muthmann, J.; Bläker, C.; Pasel, C.; Luckas, M.; Schledorn, C.; Bathen, D. Characterization of Structural and Chemical Modifications during the Steam Activation of Activated Carbons. Microporous Mesoporous Mater. 2020, 309. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Ayad, H.M. Comparison of the Experimental Results with the Langmuir and Freundlich Models for Copper Removal on Limestone Adsorbent. Appl. Water Sci. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Kipling, J.J.; Wilson, R.B. Adsorption of Methylene Blue in the Determination of Surface Areas. J. Appl. Chem. 1960, 10, 109–113. [Google Scholar]
- Pathania, D.; Sharma, S.; Singh, P. Removal of Methylene Blue by Adsorption onto Activated Carbon Developed from Ficus Carica Bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef]
- Wei, W.; Yang, L.; Zhong, W.H.; Li, S.Y.; Cui, J.; Wei, Z.G. Fast Removal of Methylene Blue from Aqueous Solution by Adsorption onto Poorly Crystalline Hydroxyapatite Nanoparticles. Dig. J. Nanomater. Biostruct. 2015, 10, 1343–1363. [Google Scholar]
- De Oliveira Brito, S.M.; Adrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil Nut Shells as a New Biosorbent to Remove Methylene Blue and Indigo Carmine from Aqueous Solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.K.; Mondal, P. Physicochemical Characterization and Pyrolysis Kinetic Study of Sugarcane Bagasse Using Thermogravimetric Analysis. J. Energy Resour. Technol. Trans. ASME 2016, 138, 1–11. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C J. Carbon Res. 2018, 4, 41. [Google Scholar] [CrossRef]
- Antal, M.; Gronly, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- De Oliveira, G.F.; De Andrade, R.C.; Trindade, M.A.G.; Andrade, H.M.C.; De Carvalho, C.T. Thermogravimetric and Spectroscopic Study (Tg-DTA/FT-IR) of Activated Carbon from the Renewable Biomass Source Babassu. Quim. Nova 2017, 40, 284–292. [Google Scholar] [CrossRef]
- De Andrade, R.C.; Menezes, R.S.G.; Fiuza, R.A., Jr.; Andrade, H.M.C. Activated Carbon Microspheres Derived from Hydrothermally Treated Mango Seed Shells for Acetone Vapor Removal. Carbon Lett. 2020, 1–15. [Google Scholar] [CrossRef]
- Sun, S.; Yu, Q.; Li, M.; Zhao, H.; Wu, C. Preparation of Coffee-Shell Activated Carbon and Its Application for Water Vapor Adsorption. Renew. Energy 2019, 142, 11–19. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, X.; Jia, X.; Liang, S.; Qian, L.; Rao, Z. Synthesis of Biomass Carbon Electrode Materials by Bimetallic Activation for the Application in Supercapacitors. J. Electroanal. Chem. 2019, 844, 105–115. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Suárez-García, F.; Tascón, J.M.D. Synthetic Carbons Activated with Phosphoric-Acid I. Surface Chemistry and Ion Binding Properties. Carbon N. Y. 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.Y.; Liang, Y.; Chen, Q.; Li, Z.S.; Li, C.H.; Wang, Z.H.; Li, W. Coconut-Based Activated Carbon Fibers for Efficient Adsorption of Various Organic Dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Ahmed Baloch, H.; Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Dumbre, D.K.; Srinivasan, M.P.; Griffin, G.J. Sub-Supercritical Liquefaction of Sugarcane Bagasse for Production of Bio-Oil and Char: Effect of Two Solvents. J. Environ. Chem. Eng. 2018, 6, 6589–6601. [Google Scholar] [CrossRef]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and Characterization of Activated Carbon Produced from Kenaf Core Fiber Using H3PO4 Activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Wu, J.; Xia, H.; Zhang, L.; Xia, Y.; Peng, J.; Wang, S.; Zheng, Z.; Zhang, S. Effect of Microwave Heating Conditions on the Preparation of High Surface Area Activated Carbon from Waste Bamboo. High Temp. Mater. Process. 2015, 34, 667–674. [Google Scholar] [CrossRef]
- Ridassepri, A.F.; Rahmawati, F.; Heliani, K.R.; Miyawaki, J.; Wijayanta, A.T. Activated Carbon from Bagasse and Its Application for Water Vapor Adsorption. Evergr. Jt. J. Nov. Carbon Resour. Green Asia Strateg. 2020, 7, 409–416. [Google Scholar] [CrossRef]
- Plermjai, K.; Boonyarattanakalin, K.; Mekprasart, W.; Pavasupree, S.; Phoohinkong, W.; Pecharapa, W. Extraction and Characterization of Nanocellulose from Sugarcane Bagasse by Ball-Milling-Assisted Acid Hydrolysis. AIP Conf. Proc. 2018, 2010. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Nasir, M.; Ibrahim, M. The Use of Date Palm as a Potential Adsorbent for Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2012, 19, 1464–1484. [Google Scholar] [CrossRef]
- Ghaffar, A.; Younis, M.N. Interaction and Thermodynamics of Methylene Blue Adsorption on Oxidized Multi-Walled Carbon Nanotubes. Green Process. Synth. 2015, 4, 209–217. [Google Scholar] [CrossRef]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W. Adsorption Characteristics of Acrylonitrile, p-Toluenesulfonic Acid, 1-Naphthalenesulfonic Acid and Methyl Blue on Graphene in Aqueous Solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of Organic Compounds by Carbon Nanomaterials in Aqueous Phase: Polanyi Theory and Its Application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, B.J. Ammonia Removal of Activated Carbon Fibers Produced by Oxyfluorination. J. Colloid Interface Sci. 2005, 291, 597–599. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Adsorption of Methylene Blue by an Efficient Activated Carbon Prepared from Citrullus Lanatus Rind: Kinetic, Isotherm, Thermodynamic, and Mechanism Analysis. Water Air Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J. Silkworm Exuviae—A New Non-Conventional and Low-Cost Adsorbent for Removal of Methylene Blue from Aqueous Solutions. J. Hazard. Mater. 2011, 186, 1320–1327. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle Diffusion Processes during Acid Dye Adsorption onto Chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Mohan, D.; Gupta, V.K.; Srivastava, S.K.; Chander, S. Kinetics of Mercury Adsorption from Wastewater Using Activated Carbon Derived from Fertilizer Waste. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 169–181. [Google Scholar] [CrossRef]
- Liu, Q.X.; Zhou, Y.R.; Wang, M.; Zhang, Q.; Ji, T.; Chen, T.Y.; Yu, D.C. Adsorption of Methylene Blue from Aqueous Solution onto Viscose-Based Activated Carbon Fiber Felts: Kinetics and Equilibrium Studies. Adsorpt. Sci. Technol. 2019, 37, 312–332. [Google Scholar] [CrossRef]
- Gao, J.J.; Qin, Y.B.; Zhou, T.; Cao, D.D.; Xu, P.; Hochstetter, D.; Wang, Y.F. Adsorption of Methylene Blue onto Activated Carbon Produced from Tea (Camellia Sinensis L.) Seed Shells: Kinetics, Equilibrium, and Thermodynamics Studies. J. Zhejiang Univ. Sci. B 2013, 14, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Pal, U. Isotherm and Kinetic Studies of Adsorption of Methylene Blue Using Activated Carbon from Ackee Apple Pods. Chem. Data Collect. 2020, 31, 100607. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic Analysis of Sorption of Methylene Blue Dye on Granular and Powdered Activated Carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
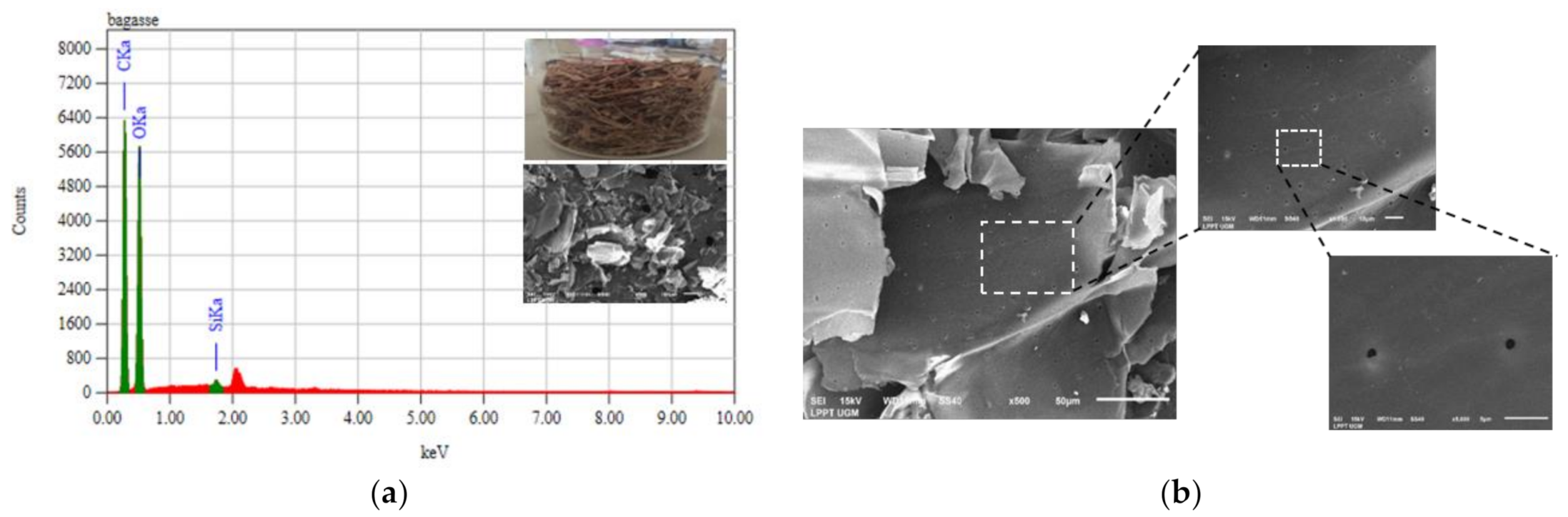

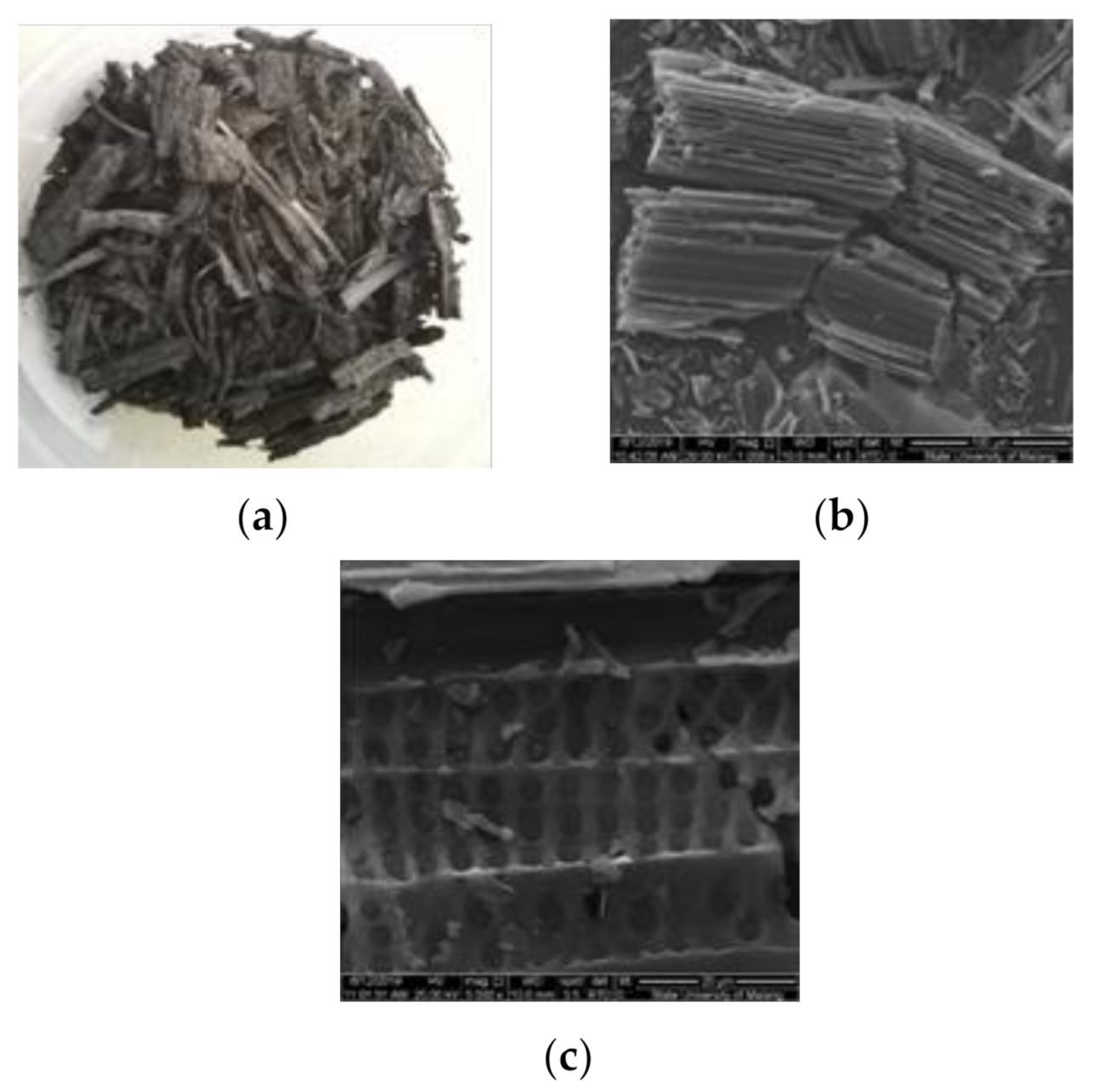

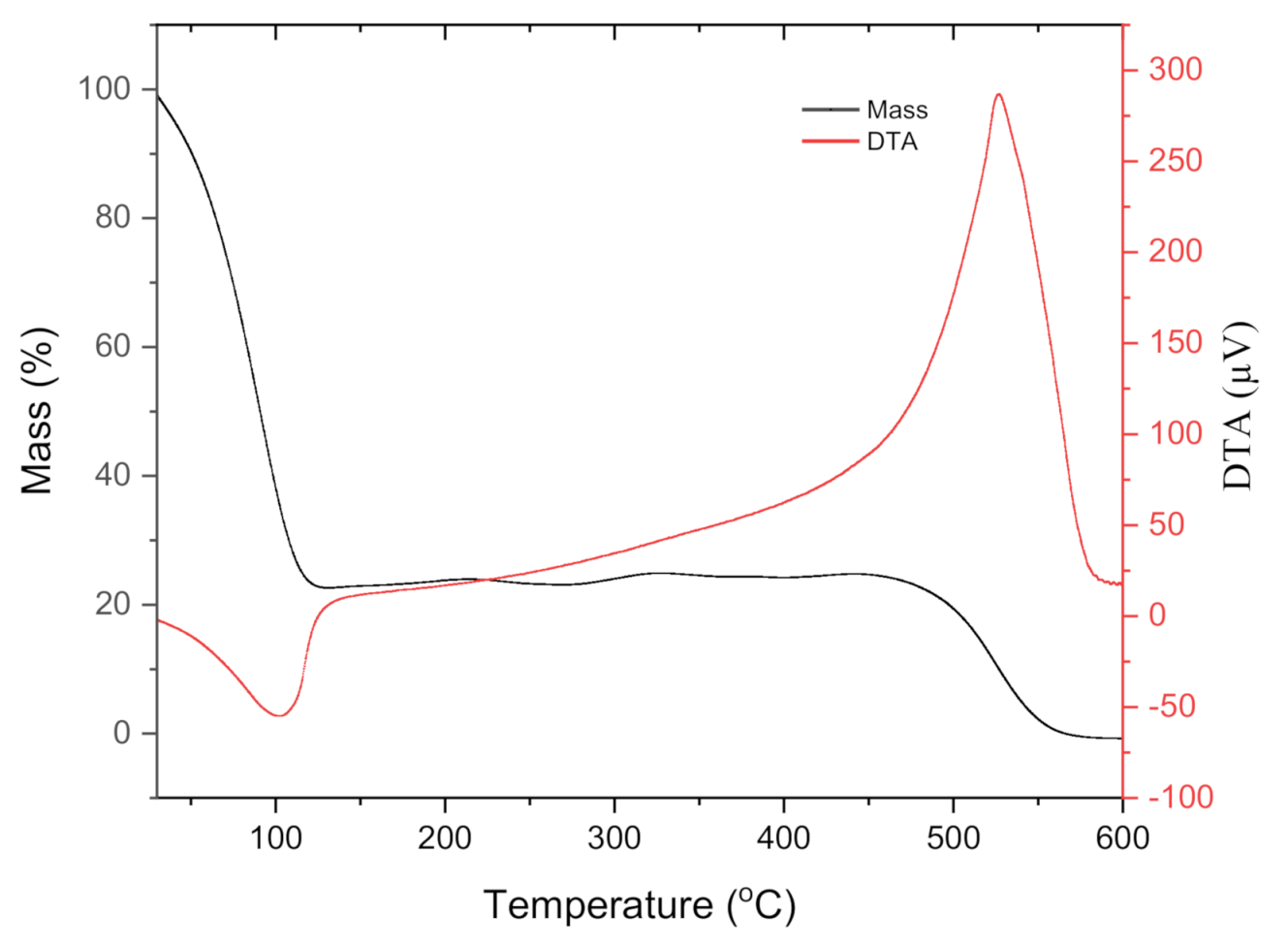
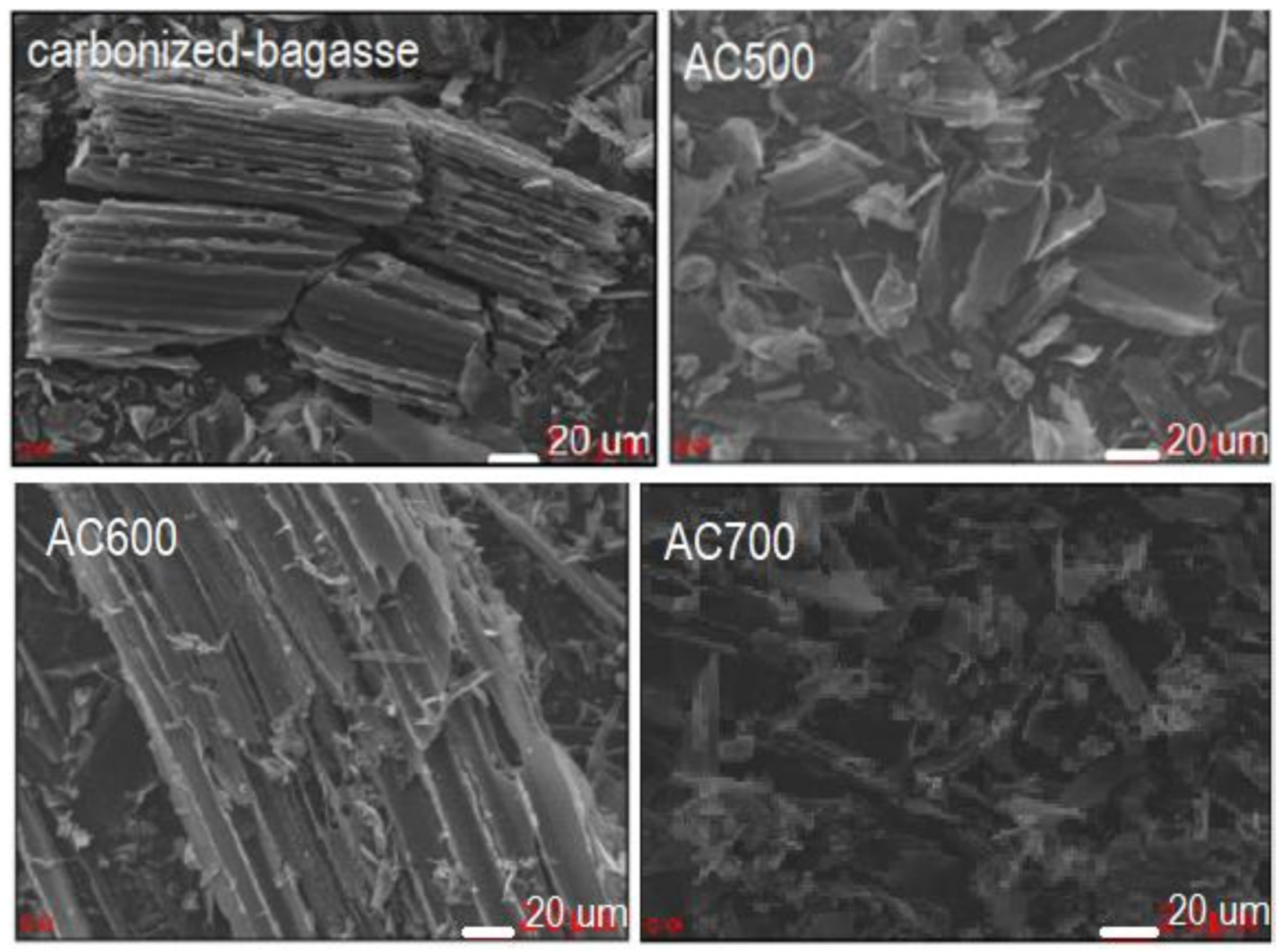
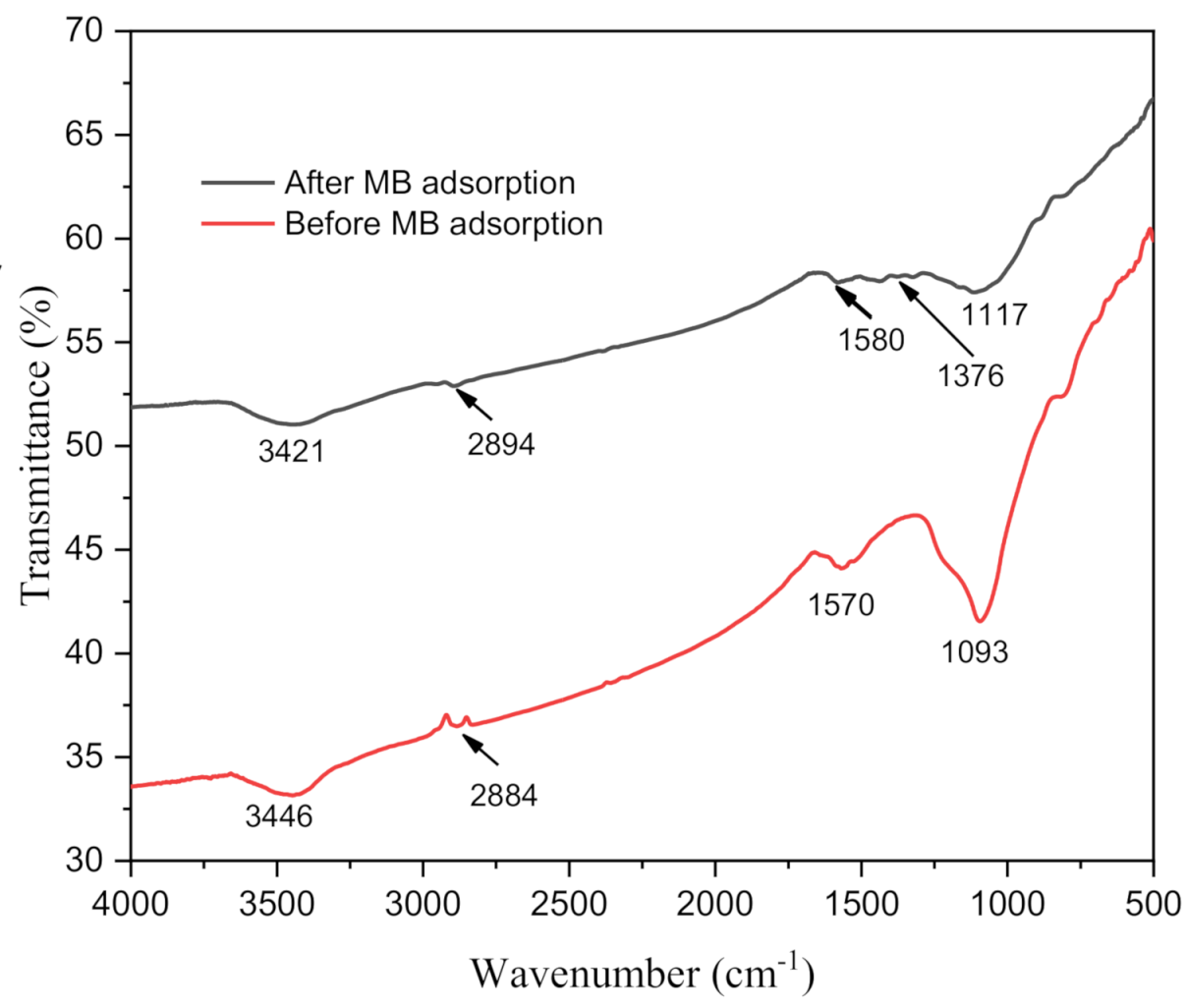
| Functional Group | Wavenumber (cm−1) | Source | References | |
|---|---|---|---|---|
| Bagasse | Char | |||
| C–O asymmetric stretching | 1053 | - | cellulose/hemicellulose | [48] |
| C–O–C asymmetric stretching | 1162 | - | cellulose/hemicellulose | [13,48] |
| C–O stretching | 1247 | 1223 | lignin | [48,49] |
| C=C aromatic | 1512, 1608, 1632 | 1592 | lignin | [49] |
| C=O stretching from vibration | 1733 | 1712 | cellulose, hemicellulose, and lignin | [44,45] |
| C–H stretching | 2856, 2921 | 2884 | polysaccharides | [13] |
| O–H stretching | 3421 | 3422 | polysaccharides/cellulose | [13,49] |
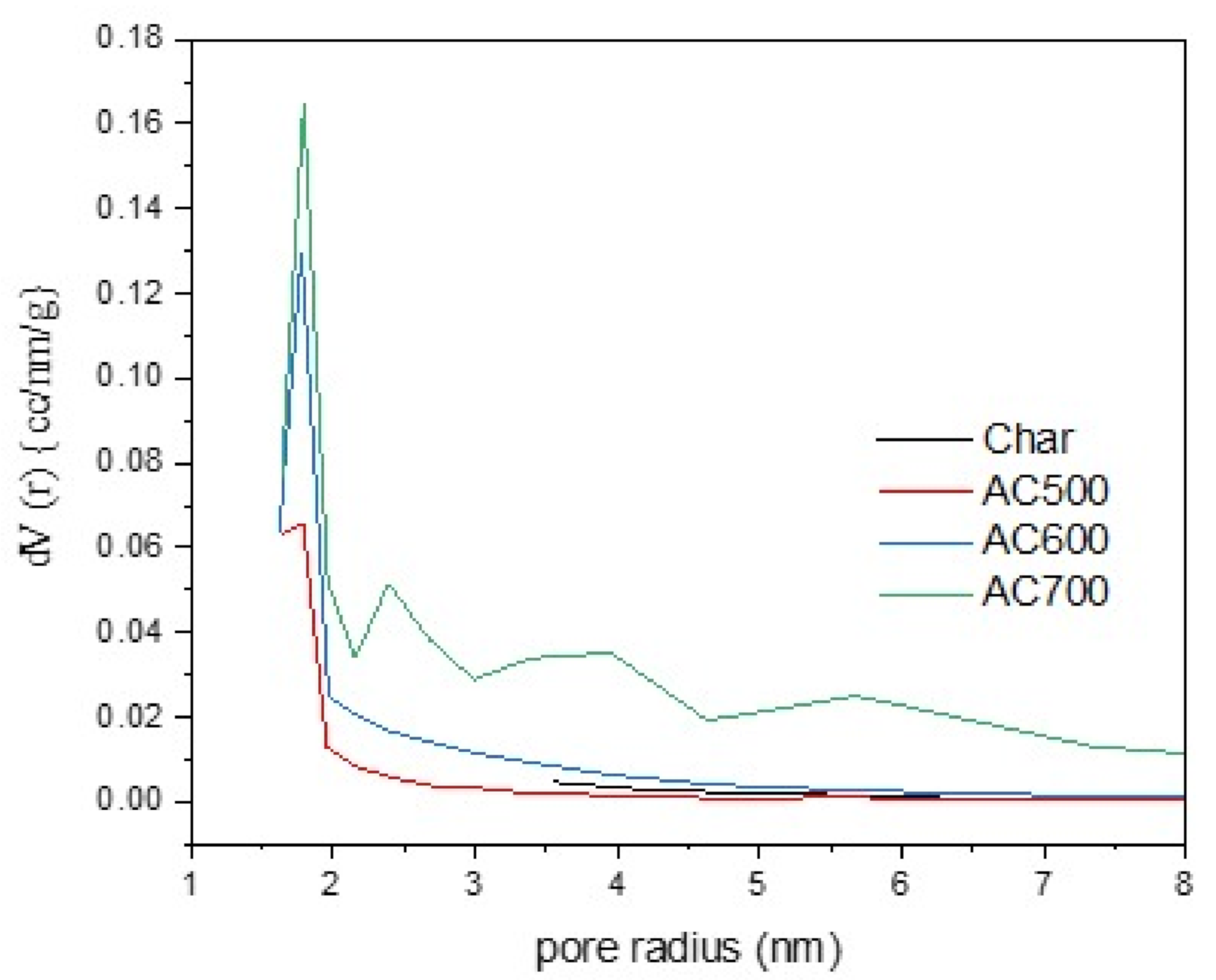
| The Prepared Materials | Surface Area Multi Point BET (m2/g) | Total Pore Volume (×10−1 cc/g) | Average Pore Size (nm) |
|---|---|---|---|
| Carbonized bagasse | 24.8 ± 0.0 | 0.4 | 3.2 |
| AC500 | 366.7 ± 0.1 | 2.1 | 1.1 |
| AC600 | 465.6 ± 0.1 | 2.9 | 1.2 |
| AC700 | 592.4 ± 0.2 | 4.7 | 1.6 |
| Isotherm | Parameters | Value |
|---|---|---|
| Langmuir | a (mg/g) | 21.008 ± 0.021 |
| b (L/mg) | 0.685 ± 0.001 | |
| R2 | 0.999 | |
| Freundlich | KF | 1.076 ± 0.327 |
| 1/n | 0.881 ± 0.268 | |
| R2 | 0.696 | |
| D–R | B (J/mol)−2 | (−4 ± 0.220) × 10−8 |
| E (kJ/mol) | 3.536 ± 0.194 | |
| R2 | 0.945 |
| Kinetics of Adsorption | |||
| Order | Rate Constant (min−1) | R2 | Sum of Square (χ2) |
| 1st | 0.031 ± 0.003 | 0.947 | 0.031 |
| 2nd | 0.020 ± 0.002 | 0.969 | 0.006 |
| Thermodynamics of Adsorption | |||
| T (K) | ΔG (kJ/mol.K) | ΔH (kJ/mol) | ΔS (kJ/mol.K) |
| 298 | −1.105 | 1.48 × 10−4 | 0.041 |
| 313 | −1.750 | ||
| 318 | −1.543 | ||
| 323 | −2.660 | ||
| Precursors | Activating Agent | Isotherm | Kinetic of Adsorption | Thermodynamics of Adsorption | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Langmuir qmax (mg/g) | Freundlich 1/n | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (kJ/mol) | ||||
| Citrullus lanatus rind | ZnCl2 | 231.480 | 0.136 | Pseudo-second-order | 23.83 | −3.07 | 90.23 | [57] |
| Viscose fibers | Water vapor pretreated by diammonium phosphate | 256.13 | 0.0669 | Pseudo-second-order | - | - | - | [62] |
| Tea seed shells | ZnCl2 | 324.7 | 0.1010 | Pseudo-second-order | −26.80 | −1.720 | −84.64 | [63] |
| Ficus carica | H2SO4 | 47.62 | 0.398 | Pseudo-second-order | 21.550 | −1.55 | 76.24 | [32] |
| Ackee apple pods | ZnCl2 | 46.95 | 0.2624 | Pseudo-second-order | - | - | - | [64] |
| Nuchar WWH (commercial) | - | 21.50 | 0.370 | - | - | - | - | [65] |
| Bagasse | Water vapor | 21.008 ± 0.021 | 0.881 ± 0.268 | Pseudo-second-order | (−4 ± 0.220) × 10−8 | −1.105 | 0.0408 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmawati, F.; Ridassepri, A.F.; Chairunnisa; Wijayanta, A.T.; Nakabayashi, K.; Miyawaki, J.; Miyazaki, T. Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue. Appl. Sci. 2021, 11, 678. https://doi.org/10.3390/app11020678
Rahmawati F, Ridassepri AF, Chairunnisa, Wijayanta AT, Nakabayashi K, Miyawaki J, Miyazaki T. Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue. Applied Sciences. 2021; 11(2):678. https://doi.org/10.3390/app11020678
Chicago/Turabian StyleRahmawati, Fitria, Arikasuci Fitonna Ridassepri, Chairunnisa, Agung Tri Wijayanta, Koji Nakabayashi, Jin Miyawaki, and Takahiko Miyazaki. 2021. "Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue" Applied Sciences 11, no. 2: 678. https://doi.org/10.3390/app11020678
APA StyleRahmawati, F., Ridassepri, A. F., Chairunnisa, Wijayanta, A. T., Nakabayashi, K., Miyawaki, J., & Miyazaki, T. (2021). Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue. Applied Sciences, 11(2), 678. https://doi.org/10.3390/app11020678









