Abstract
Alveolar preservation can minimize bone resorption after tooth removal and additional topical antibiotics might also be considered. The goal of this study was to observe alveolar preservation with albumin and gentamycin-coated allograft compared to unfilled control sockets after mandibular third molar removal. Twenty-two patients were involved, 11 in the control group and 11 in the test group. CBCT analysis and micromorphometric analysis were performed. After one year, graft integration was observed with remaining graft particles. Micromorphometric analysis showed increased density and lower trabeculae formation in the grafted group. The buccal height reduction of the alveolar ridge was significantly lower when alveolar preservation was applied (control: 2.54 ± 2.01 mm, graft: 1.37 ± 1.04 mm, p < 0.05). Horizontal bone loss prevention was not significant. At the distal site of the second molar, the marginal bone level (MBL) was significantly lower in the control group. At the control group, five pockets persisted from the eight initial and all healed in the graft group. Alveolar preservation improves bone formation, helps to preserve the buccal bone crest, and minimizes MBL loss and pocket formation on the adjacent teeth. Thus, it needs to be also considered after third molar surgical removal.
Keywords:
albumin; allograft; clinical attachment loss; gentamycin; marginal bone level; third molar 1. Introduction
Inadequate alveolar bone poses great challenge when rehabilitation is planned with dental implants [1]. Bone resorbs progressively without loading in both horizontal and vertical dimensions. After tooth extraction, it might be prevented with alveolar preservation [2,3]. The presence of bacteria and inflammation often compromises bone grafting at the time of tooth removal. Therefore, topical antibiotics can be applied to prevent infection and graft sequestration.
The use of topical antibiotics is widespread both in orthopedics and oral surgery because it is difficult to reach a sufficient concentration of antibiotics in the bone due to its low metabolic rate. High local concentration and progressive elution might prevent the adhesion and proliferation of bacteria on the surface of grafts. Topical antibiotics have been used for preventing perioperative inflammation, treating chronic osteomyelitis or severe bone necrosis caused by compromised healing of patients with diabetes mellitus [4,5,6]. For topical application, gentamycin, vancomycin, and tobramycin are the first-choice antibiotics due to their broad antibacterial spectrum and low percent existence of resistant bacteria species [4,5,7]. In vitro experiments proved the antibiotic-coated allograft could provide sustained release of antibiotics after emplacement [8,9].The use of gentamycin or other topical antibiotic is indeed indicated in limited cases, however, the practice is more widespread than it should be. The reasoning that oral surgery is contaminated and therefore prevention of infection is of importance is reasonable, and one choice is the use of local antibiotics where the first choice is typically gentamycin. One would hypothesize that adding an extra layer of prevention would not do any harm so even if this is not specifically indicated it may be used as a cautionary measure [10]. One of the key observations of the current study is that this reasoning is downright wrong, and not just because of the often cited issue of promoting antibiotic resistance, but also that gentamycin interferes with bone remodelling and thus hinders the very aim of the surgery [11]. However, its use is not supported by conclusive evidence and clear surgical protocol in the literature. Several type of antibiotics can be used in different dilution, which might be standardized with a factory made bone graft coated with antibiotics [10,12,13]. The current study aimed to use the best available bone filler that is known to support bone regeneration and test the hypothesis that it can be further enhanced by the use of an antibiotic.
After third molar surgical removal, patients usually suffer from pain, swelling and trismus at the early postoperative healing time [14]. At the long-term follow-up gum recession or marginal bone level (MBL) loss at its distal site of second molar may cause tooth sensitivity and periodontal pocket formation [15,16]. The risk of pocket formation is bigger when the third molar was is a mesioangular position and preoperative bone loss developed [17]. Flap design, suturing, preoperative periodontal status and postoperative plaque control care can also affect pocket formation. To avoid it alveolar preservation with bone grafts or guided tissue regeneration may be considered, although MBL loss is not always significant after third molar removal [18,19]. Another study observed bone grafting has beneficial effect on MBL at the distal site of second molar, although MBL loss does not increase after six months when alveolar preservation was not applied [20]. It needs to be emphasized that periodontal pocket formation should be prevented when third molar removal is necessary. The secondary aim of this study was to examine when alveolar preservation is significantly beneficial to prevent MBL loss.
2. Materials and Methods
2.1. Study Protocol
This prospective randomized controlled trial was conducted in accordance with Helsinki guidelines and with the Consolidated Standards of Reporting Trials Statement [21]. The study was approved by the Regional and Institutional Committee of Science, and Research Ethics of Semmelweis University, Budapest, Hungary (IRBID: 7786-9/2014/EKU) and was conducted in accordance with Helsinki guidelines.
In the test group, gentamycin and albumin-coated allografts were used for alveolar preservation after mandibular third molar surgical removal. In the control group, the surgical sockets were left empty and filled by a blood clot. All patients were informed about the study protocol and signed a written consent.
2.2. Sample Size Calculation
To calculate the sample size G*Power 3.1. program was used [22]. Based on previous data, the clinical attachement level gain was calculated as the primary outcome with a mean difference of 1.4 mm and a standard deviation of 1.17 mm between the test and control groups [10]. For the expected effect size of 1.2 with an alpha level of 0.05 and a power of 80% at the 1:1 distribution ratio, 10 patients per group was selected as the minimum sample size.
2.3. Patients
Altogether 26 patients were recruited in this study from March 2015 through June 2015 at the Department of Oral Diagnostics of Semmelweis University. Inclusion criteria were general healthiness and impacted mandibular third molar covered by intact mucosa and indicated for removal due to orthodontic treatment. Patients ranged in age between 19 and 30 years, given that preventive removal of third molars were indicated [23]. Exclusion criteria were any medicament consumption and the common dentoalveolar surgical contraindications with special regard to excluding patients with acute oral inflammation.
The patients were randomly divided into two groups by using a software generated list. (www.randomizer.org). This was done by a clinical doctor, who was not involved in the surgeries or in the data evaluation. Four patients were lost during follow-up period. Thus, the final analysis included only 11 patients for both group (Figure 1).
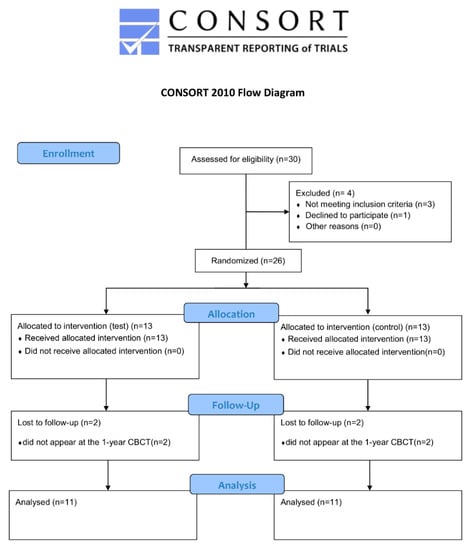
Figure 1.
CONSORT flow diagram.
2.4. Materials
BoneAlbumin (BA) (OrthoSera Dental Zrt., Győr, Hungary) is a human serum albumin-coated allograft that has an additional gentamycin coating in this study. During the sterilization and lyophilization of the bone material, the structure of the bone remains the original, but the proteins get denaturalized, and all cells are destroyed [24,25]. The lost osteoinductive and osteogenetic capacity can be compensated by the human serum albumin coating of the graft. In vitro experiments verified that albumin enhances stem cell migration from the bone marrow and increases osteogenic cell adhesion on the surface of the graft [26]. It also has an anti-inflammatory effect due to its antioxidant property, and it regulates the gene expression of immunomodulatory cytokines and growth factors [27]. Albumin coating allograft resulted in stronger bone formation and faster healing in rats compared to the uncoated one [28,29]. BA has been successfully used in orthopaedic surgery when severe inflammation indicates joint prosthesis revision [30,31]. In oral surgery, BA showed faster and more complete bone remodelling when used for sinus augmentation or alveolar preservation compared to xenografts [32,33].
2.5. Surgical Method
Each mandibular third molar was removed surgically by the same 10 years experienced oral surgeon. Preoperative CBCT was evaluated to avoid inferior alveolar nerve damage [34]. Anesthesia was provided by direct block anesthesia of the inferior alveolar nerve and local infiltration at the buccal site [35]. A three-cornered mucoperiosteal flap was created, extending from the retromolar area to the second molar with a vertical incision. The overlying bone was removed with a bur in a surgical handpiece with caution to remove as little buccal bone as possible. The lingual cortical was never affected. The tooth was delivered in multiple pieces when too much force would be necessary to remove it. After the tooth removal, the surgical socket was cleaned mechanically and by saline irrigation. In the graft group, the bone graft particles were placed into the socket to fill it completely. In the control group, only the blood clot filled the socket. Then, the flap was repositioned, and primary wound closure was performed with non-resorbable sutures.
Antibiotics (875 mg amoxicillin + 125 mg clavulanic acid two times a day for 7 days) and nonsteroidal anti-inflammatory drugs (275 mg naproxen maximum three times a day) were prescribed. The sutures were removed one week postoperatively.
2.6. Follow-Up Evaluation
One week after the surgery, the patients scored their postoperative pain from zero to 10 at a visual analog scale (VAS) and registered their painkiller consumption.
Ultra-low dose cone beam computer tomography (CBCT) images were taken preoperatively and 1 year after the surgery with 100-micrometer voxel sizes (Promax 3D Mid, PlanmecaOy, Helsinki, Finland). The CBCT images were examined with DataViewer software (Bruker, Kontik, Belgium) by two investigators experienced in radiology blinded toward the groups and their results were averaged.
The qualitative analysis included the examination of the structure of trabecular and cortical bone formation at the surgical sites and the presence of graft remnants or sequestration. The density of the newly formed bone and graft remnants were measured in Hounsfield Units (HU).
Micromorphometric analysis was performed at the 1-year images with CTAn software (Bruker, Kontik, Belgium). Bone surface/volume ratio (BS/BV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and total porosity (Po(tot)) were compared.
To follow the changes of the vertical and horizontal dimensions of the alveolar ridge, the pre-and postoperative CBCT images were set to the same position with the help of anatomical lines as reference. The vertical bone height was measured from the highest point of the buccal ridge to the lowest point of the mandibular base at the coronal image, 5 mm distally from the cemento-enamel junction (CEJ) at the midline of the second molar. The horizontal bone width was measured between the buccal and lingual cortical at the axial image, 5 mm distally from the second molar, 3 and 5 mm lower than the CEJ of the second molar.
MBL was measured at the distal site of the second molar, from the CEJ to the deepest point of the surrounding bone, both in the pre-and postoperative CBCT.
Statistical analysis was performed with IBM SPSS Statistics software (IBM Corporation, New York, NY, USA). All data were expressed as mean ± standard deviation. Shapiro–Wilk test was used to access the normality of data distribution. For pairwise comparison the data were analysed by One-way ANOVA test in case of normal distribution and independent-samples Mann–Whitney-U test was used in case of non-normal distribution.
3. Results
The one-year follow-up period was completed by 11–11 patients in both groups. Regarding the sex and age of patients and the angulation and depth of impaction of third molars, the two groups were statistically identical (Table 1).

Table 1.
Data of patients and the position of the third molars. Depth of impaction was classified according to Winter (1926) and Pell-Gregory (1942). The meaning of numbers: (I) third molar reaches occlusion; (II) third molar is between the occlusion and the neck of the second molar; and (III) third molar is below the neck of the second molar.
The VAS scores (control, 5.55 ± 2.11; graft, 4.73 ± 2.10) and the painkiller consumption of the two groups did not show significant differences.
The macromorphology of the regenerated bone was different in the two groups (Figure 2, Table 2). In the control group, the homogenous trabecular bone formation could be seen in all cases. Continuous cortical bone covered the surgical sockets in 68.19%, and it was fenestrated in 31.82%. The density of the newly formed bone was 176.41 ± 107.98 HU. In the graft group, the socket was filled with homogenous trabecular bone in 36.37%, graft remnants could be seen in 50% of patients, and demarcated bone graft particles occurred in 13.64%. The overlying cortical bone was continuous in 4.55% and fenestrated in 54.55%. The augmented area was not covered by cortical bone in 40.91%. The density of the newly formed bone was 370.14 ± 184.39 HU and 718.67 ± 306.41 HU for the graft remnants.
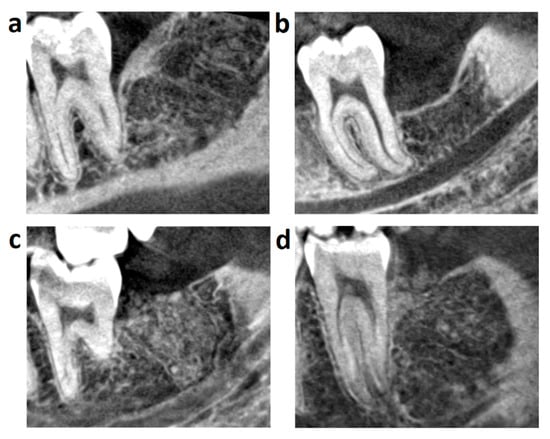
Figure 2.
One year follow-up CBCT images: (a) Control healed without significant MBL loss, new bone is homogenous, layered by continuous cortical; (b) Control healed with more than 3 mm MBL loss, new bone is homogenous, layered by fenestrated cortical; (c) Grafted socket regenerated by the homogenous bone formation and lack of cortical layer; (d) Grafted socket regenerated by homogenous bone formation with visible graft remnants and layered by fenestrated cortical.

Table 2.
Bone morphometric characteristics were analysed by CTAn software according to the manual Bruker MicroCT Morphometric parameters measured by CT-analyzer software 1.15.4.0 by Bruker microCT. Abbreviations: One-way ANOVA test (#), independent-samples Mann-Whitney-U test (##).
The result of the micromorphometric analysis can be seen in Figure 3. BS/BV and Po(tot) were significantly higher at the control group showing the high volume and number of resorption lacunae. Tb.Th and Tb.N were significantly higher at the graft group, referring to a denser bone structure and also to the graft remnants. BV/TV of the grafted and test groups were 28.01 ± 7.43% and 9.49 ± 6.12%, respectively. BS/BV, Tb.Th, and Tb.N showed normal distribution with Shapiro–Wilk test, while the one-way ANOVA test was used to validate significance. Po(tot) was analysed by a Mann–Whitney-U test since it showed a non-normal distribution.
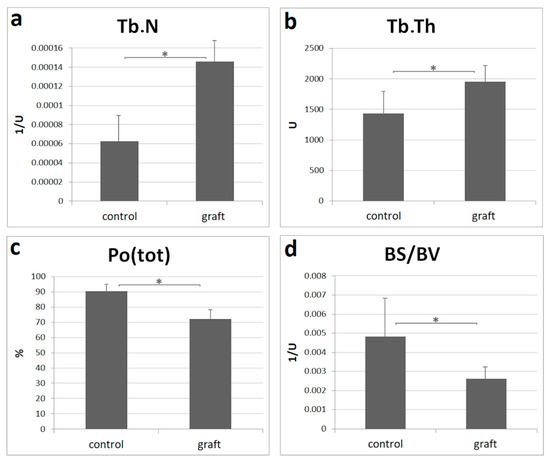
Figure 3.
One year micromorphometry analysis: (a) trabecular number (Tb.N) is the total number of trabeculae per unit of length; (b) trabecular thickness (Tb.Th) indicates the mean thickness of trabeculae; (c) total porosity (Po(tot)) shows the total number of open and closed pores; (d) bone surface/volume ratio (BS/BV) refers to the volume ratio of calcified tissues; control: n = 11, graft: n = 11. All data are presented as mean ± SD. Statistical significance (p < 0.05) was marked by an asterisk.
After one year, the vertical bone loss at the buccal site of the removed third molar was significantly higher when alveolar preservation was not applied. The Shapiro–Wilk test showed non-normal distribution, and thus the Mann–Whitney-U test was used to validate data significance. Horizontal bone reduction data showed normal distribution. One-way ANOVA analysis did not provide significant differences, although higher bone loss could be observed when bone grafting was not applied (Figure 4, Table 3).
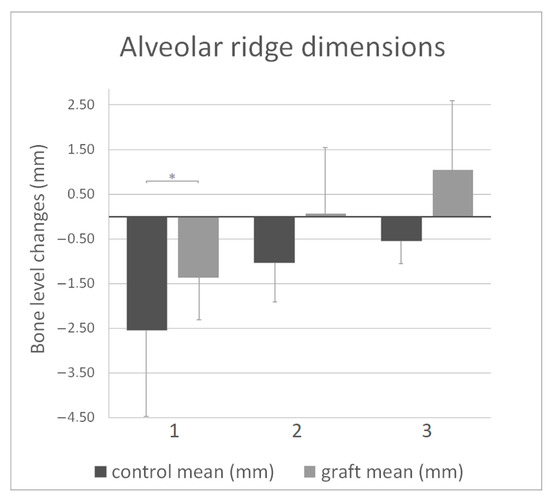
Figure 4.
Alveolar ridge dimension changes at 1-year follow-up: (1) vertical buccal bone reduction; (2) horizontal bone reduction (control) and gain (graft) at 3 mm depth; (3) horizontal bone reduction (control) and gain (graft) at 5 mm depth. All data are presented as mean ± SD, Statistical significance (p < 0.05) was marked by an asterisk.

Table 3.
Alveolar ridge dimension changes at 1-year follow-up. Abbreviations: One-way ANOVA test (#), independent-samples Mann-Whitney-U test (##).
The MBL loss was significantly higher in the control group at the distal site of the second molar (control: 2.85 ± 1.27 mm, graft: 1.24 ± 0.55, p < 0.05) (Figure 5A, Table 4). The MBL gain was examined from the aspect of the preoperative MBL and the proximity of the third molar to the second molar. When the preoperative MBL loss was less than 3 mm, both control and graft groups showed similar slight MBL gain. It was significantly different when preoperative MBL loss was more than 3 mm, and thus resulted in a pocket. When bone graft was applied, all eight pockets healed, and the MBL loss reduced to less than 3 mm. Without grating, the MBL did not change remarkably, and five from eight pockets persisted 1 year postoperatively. A similar tendency could be seen when the proximity of the second and third molar was observed. When they were in direct contact without persisted interalveolar septum, grafting resulted in spectacular MBL gain compared to control. When the interalveolar septum was preserved between the second and third molar, there was a non-significant difference in the MBL gain comparing the two groups. The difference between the pre-and postoperative MBL is visualized in Figure 5b,c. All MBL data showed normal distribution with Shapiro-Wilk test, thus significance between groups was validated by One-way ANOVA test. The angulation and depth of impaction of the third molar did not show correlation with the postoperative MBL.
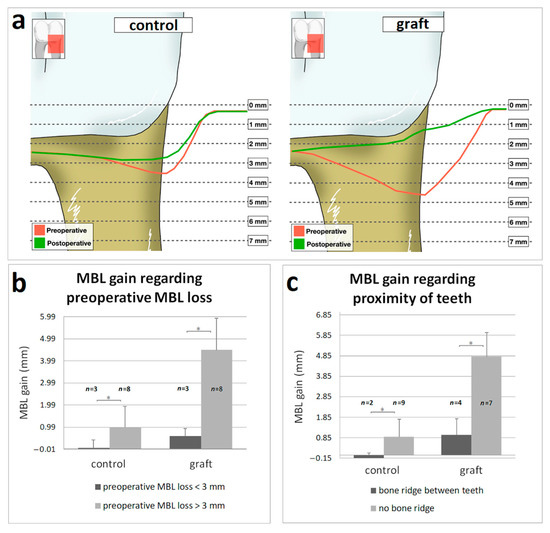
Figure 5.
Changes of the marginal bone level (MBL) at the distal site of second molar after 1 year follow-up: (a) The results presented regarding to preoperative MBL loss (b) and to the proximity of teeth (c). Data are presented as mean (a) and mean ± SD (b,c). Statistical significance (p < 0.05) was marked by an asterisk.

Table 4.
Changes of the marginal bone level (MBL) at the distal site of second molar. For statistical analysis One-way ANOVA test (#) was used.
The reliability of interobserver data was verified with a 0.968 intraclass correlation coefficient (ICC) in the case of vertical buccal bone loss and MBL loss.
Raw data of all analysis can be find as Supplementary Material Files online.
4. Discussion
After removal of the third molar, swelling causes the most discomfort for patients and is the main reason for postoperative pain [36]. Graft application did not influence the length or intensity of postoperative pain significantly, according to VAS.
Compared to the control group, bone grafting resulted in a denser bone structure but impaired cortical bone formation. Bone macromorphology seems to be similar when compared to simple albumin coated allograft in other studies, although more graft remnants or sequester can be noticed when it was coated with additional gentamycin [32,33]. Bone grafting also affected cortical bone formation. One possible explanation is that the gentamycin might have a deceleration effect on bone remodeling in the present study. When calcium sulfate is used as a carrier for gentamycin, it prolonged the hydration of calcium sulfate and adhered to the whole crystal-forming process [11]. In vivo experiments proved that gentamycin or vancomycin-coated human bone graft application in a bone defect model caused decreased healing rate and less mineralization [37,38]. Although a study combining in vivo and in vitro approaches claimed that there is no adverse effect of gentamycin on bone-forming cells and remodeling when human bone graft is coated [39]. When other types of grafts are applied, like bovine xenograft or synthetic materials, graft remnants also occurred in the regenerated area, and its volume did not correlate with the healing time [2,40]. It seems that regardless of whether we use a bone allograft, some particles of the graft cannot be resorbed.
Micromorphometry analysis of CBCT images are reliable indicators of bone morphology, and its measurements correlate positively with microCT analysis of bone core biopsy samples [41]. BS/BV refers to the volume ratio of calcified tissues Po(tot) shows the total volume of open and closed pores [42]. The high value of BS/BV and Po(tot) indices reflected a higher volume and number of resorption lacunae in the control group. Tb.Th indicates the mean thickness of trabeculae. Tb.N is the total number of trabeculae per unit of length. When bone graft was applied, these indicators proved the presence of more and thicker trabeculae formation with fewer pores resulting in a denser bone structure. These results are consistent with the results of a previous CBCT study based on T.bTh values, however the observed Po(tot) values seem to be different, which might be due to different voxel sizes were used in the two studies. [41]. The microCT data of Kivovics et al. are positively correlated with our CBCT results in regard of Po(tot) values, which might be caused by the higher resolution of CBCT imaging in the present investigation. Albumin-coated allograft was previously investigated under similar test conditions. The values of BS/BV, Tb.Th, Tb.N and Po(tot) of that study correlate with our results, which suggests that the gentamycin component of the double-coating does not influence the microstructure of the augmented area. [33]. It seems that the double-coated allograft forms a denser new alveolar bone, like the single albumin coated allograft.
Alveolar preservation is a well-known technique after tooth extraction when the further dental restoration is planned [43,44]. Although third molars do not tend to be replaced prosthetically, the vertical ridge height protecting effect of the graft can be remarkable. In the present study, vertical ridge reduction was significantly less at the buccal cortical when the alveolar was filled in with graft. Similar results have already been observed when other teeth were removed. The changes of the alveolar bone dimensions can be measured manually with a periodontal probe or radiographically [45,46,47,48]. Some previous studies used simple periapical radiography with custom made film holders, while other CBCT studies also used the same measurement points as we have [49,50,51]. Both methods were used for the analyses, and the same tendencies were observed after alveolar preservation compared to the control groups. CBCT was used in the present study for evaluation due to its low radiation exposure compared to its high resolution that allowed accurate measurements. Previous study used the same anatomical lines as reference at CBCT images [49]. The results of the vertical bone loss are nearly identical with the present study (Walker: control—2.6 mm, graft—1.12 mm; present study: control—2.54 mm, graft—1.37 mm). Horizontal bone changes differ which can be the consequence that simple tooth extractions were performed contrary to surgical third molar removals. Several studies observed the same tendency: the main effect of alveolar preservation can be seen at the buccal site of the alveolar ridge, and it has a slight effect on horizontal ridge dimensions [45,48,49,50,51]. The exact result data might vary due to the used material and the location of the extracted tooth. The present study has justified previous results, and this fact might have significance for third molar surgeries when the tooth is deeply impacted.
The most significant result of this study is the MBL gain at the distal site of the second molar when bone grafting was applied. CBCT measurements are reliable; they correlate with the manual periodontal probe measurement results [52].
The most obvious result is that bone grafting maintained the MBL. The interesting part was to observe if other factors had an impact on postoperative MBL or not. According to our result, in case of more than 3 mm loss on the distal site of the second molar or direct contact between the second and third molar without persisted interalveolar septum bone grafting should be considered, otherwise it is not required. It needs to be clear that more than 3 mm preoperative MBL loss can be measured only on radiographic image, but not by periodontal probing. The presence of impacted third molar hinders the real bone loss. The significance of preoperative measured MBL loss can be revealed during surgery.
When the alveolar was left empty, the MBL remained at almost the same level behind the second molar. In the control group, eight patients had more than 3 mm MBL loss preoperatively, meaning high risk of postoperative pocket formation. Periodontal pocket formed in five patients at the one-year follow-up, meaning 62.5% pocket formation rate without alveolar preservation when preoperatively MBL loss was more than 3 mm. Previous study examined the effect of preoperative periodontal status and oral hygiene [17]. The MBL loss at the distal site of second molar was 5.4 ± 0.8 mm 6–36 months after third molar surgical removal without alveolar preservation. Mesioangular third molar position, preoperative radiolucency around the tooth and inadequate plaque control increased MBL loss. This value is remarkably higher than the control data of present study, further emphasizing the importance of bone augmentation when preoperative bone loss occurs. In the present study third molar position did not show significant correlation with MBL loss, which might be the result of low sample size.
When the alveolar was filled with bone graft, the mean MBL loss was reduced to 1.24 ± 0.55 mm from 4.66 ± 2.3 mm. Preoperatively, eight patients had more than 3 mm MBL loss, equal to the control group. At the 1-year follow-up none of them had pocket at the distal site of second molar. Other study claimed bone grafting did not have effect on MBL loss after two years, however, preoperative MBL loss was less than 2 mm both in control and grafted groups. [20]. It may enforce the observation that alveolar preservation is not necessary when preoperative MBL loss is less than 3 mm. In the everyday dental practice when exact measurement of the MBL loss on CBCT is not possible, the proximity of the two neighbour teeth can also help to decide if the patient needs bone grafting after third molar removal or not. The data of MBL correlated when preoperative MBL or present of bone ridge was examined. The angulation and depth of impaction did not show any correlation with the results, only the bony wall behind the second molar affected the postoperative MBL.
Our results are consistent with another previous study claiming 4.7 mm MBL gain when bone defect was treated with guided tissue regeneration combined with gentamycin impregnated xenograft [10]. The additional gentamycin resulted in higher MBL gain compared to other methods. This study found positive correlation between probing depth and radiological bone loss measurement, thus CBCT data shall also be adequate for MBL observation.
The importance of topical antibiotics shall be further investigated due to the controversial literature. These results may claim attention on the importance of alveolar preservation after surgical third molar removal, although it has not been considered as a standard protocol yet. Due to the small sample size, further investigations may be needed to validate preliminary findings.
5. Conclusions
Although alveolar preservation is not widespread in clinical practice after third molar removal, the use of bone substitutes shall be considered when MBL loss was more than 3 mm at the distal site of second molars, or if there was direct contact between the second and third molar without persistent interalveolar septum. According to the literature several bone substitutes are used successfully for alveolar preservation. The novel gentamycin and albumin double-coated allograft seems to be suitable with predictable clinical outcome.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/2/586/s1, Data is contained within the article or supplementary material.
Author Contributions
Conceptualization, Z.L., L.S. and S.G.-G.; methodology, C.D.-N., L.S. and S.G.-G.; software, F.M.; validation, C.D.-N.; formal analysis, F.M. and C.D.-N.; investigation, F.M., B.T. and C.D.-N.; resources, Z.L., L.S. and S.G.-G.; data curation, F.M., B.T. and C.D.-N.; writing—original draft preparation, F.M.; writing—review and editing, C.D.-N. and B.T.; visualization, F.M.; supervision, C.D.-N.; project administration, C.D.-N. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Regional and Institutional Committee of Science, and Research Ethics of Semmelweis University (IRBID: 7786-9/2014/EKU).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
The applied bone graft was donated by OrthoSera Dental Zrt., Győr, Hungary. Daniel Palkovics visualized Figure 5A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakhshalian, N.; Freire, M.; Min, S.; Wu, I.; Zadeh, H.H. Retrospective Analysis of the Outcome of Ridge Preservation with Anorganic Bovine Bone Minerals: Microcomputed Tomographic Assessment of Wound Healing in Grafted Extraction Sockets. Int. J. Periodontics Restor. Dent. 2018, 38, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, M.; Al-Nawas, B.; Berres, M.; Kämmerer, P.W.; Schiegnitz, E. The Effects of Alveolar Ridge Preservation: A Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2016, 18, 1248–1268. [Google Scholar] [CrossRef] [PubMed]
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-extraction Dimensional Changes: A Systematic Review & Meta-analysis. J. Clin. Periodontol. 2020. [Google Scholar] [CrossRef]
- Ferguson, J.Y.; Dudareva, M.; Riley, N.D.; Stubbs, D.; Atkins, B.L.; McNally, M.A. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: A series of 195 cases. Bone Jt. J. 2014, 96-B, 829–836. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Wu, C.-C.; Fan, F.-Y.; Cheng, H.-C.; Liaw, Y.-C.; Huang, Y.-K.; Hsu, L.-H.; Yang, K.-C. Effects of the addition of vancomycin on the physical and handling properties of calcium sulfate bone cement. Process. Biochem. 2014, 49, 2285–2291. [Google Scholar] [CrossRef]
- Morley, R.; Lopez, F.; Webb, F. Calcium sulphate as a drug delivery system in a deep diabetic foot infection. Foot 2016, 27, 36–40. [Google Scholar] [CrossRef]
- Thomas, D.B.; Brooks, D.E.; Bice, T.G.; DeJong, E.S.; Lonergan, K.T.; Wenke, J.C. Tobramycin-impregnated calcium sulfate prevents infection in contaminated wounds. Clin. Orthop. Relat. Res. 2005, 441, 366–371. [Google Scholar] [CrossRef]
- Hernandez-Soria, A.; Yang, X.; Grosso, M.; Reinhart, J.; Ricciardi, B.; Bostrom, M. In vitro elution characteristics of antibiotic laden BoneSourceTM, hydroxyapatite bone cement. J. Biomater. Sci. Polym. Ed. 2013, 24, 797–806. [Google Scholar] [CrossRef]
- Hornyák, I.; Madácsi, E.; Kalugyer, P.; Vácz, G.; Horváthy, D.B.; Szendrői, M.; Han, W.; Lacza, Z. Increased release time of antibiotics from bone allografts through a novel biodegradable coating. Biomed. Res. Int. 2014, 2014, 459867. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Karring, E.S.; Kostopoulos, L.; Karring, T. Deproteinized bovine bone and gentamicin as an adjunct to GTR in the treatment of intrabony defects: A randomized controlled clinical study. J. Clin. Periodontol. 2003, 30, 486–495. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.K.; Chang, W.J.; Wu, Y.C.; Wang, C.C.; Yang, K.C. Limitation of the antibiotic-eluting bone graft substitute: An example of gentamycin-impregnated calcium sulfate. J. Biomed. Mater. Res. B Appl. Biomater 2018, 106, 80–87. [Google Scholar] [CrossRef]
- Choukroun, J.; Simonpieri, A.; Del Corso, M.; Mazor, Z.; Sammartino, G.; Dohan Ehrenfest, D.M. Controlling systematic perioperative anaerobic contamination during sinus-lift procedures by using metronidazole: An innovative approach. Implant. Dent. 2008, 17, 257–270. [Google Scholar] [CrossRef]
- Nagarjuna Reddy, Y.V.; Deepika, P.C.; Venkatesh, M.P.; Rajeshwari, K.G. Evaluation of moxifloxacin-hydroxyapatite composite graft in the regeneration of intrabony defects: A clinical, radiographic, and microbiological study. Contemp. Clin. Dent. 2016, 7, 357–365. [Google Scholar] [CrossRef]
- Pitros, P.; O’Connor, N.; Tryfonos, A.; Lopes, V. A systematic review of the complications of high-risk third molar removal and coronectomy: Development of a decision tree model and preliminary health economic analysis to assist in treatment planning. Br. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.T.; Dodson, T.B. Risk of periodontal defects after third molar surgery: An exercise in evidence-based clinical decision-making. Oral Surg. Oral Med. Oral. Pathol. Oral Radiol. Endod. 2005, 100, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Pigella, E.; Romano, F. Clinical and radiographic evaluation of the effects of guided tissue regeneration using resorbable membranes after extraction of impacted mandibular third molars. Int. J. Periodontics Restorative Dent. 2007, 27, 51–59. [Google Scholar] [PubMed]
- Kan, K.W.; Liu, J.K.; Lo, E.C.; Corbet, E.F.; Leung, W.K. Residual periodontal defects distal to the mandibular second molar 6-36 months after impacted third molar extraction. J. Clin. Periodontol. 2002, 29, 1004–1011. [Google Scholar] [CrossRef]
- Barbato, L.; Kalemaj, Z.; Buti, J.; Baccini, M.; La Marca, M.; Duvina, M.; Tonelli, P. Effect of Surgical Intervention for Removal of Mandibular Third Molar on Periodontal Healing of Adjacent Mandibular Second Molar: A Systematic Review and Bayesian Network Meta-Analysis. J. Periodontol. 2016, 87, 291–302. [Google Scholar] [CrossRef]
- Throndson, R.R.; Sexton, S.B. Grafting mandibular third molar extraction sites: A comparison of bioactive glass to a nongrafted site. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 94, 413–419. [Google Scholar] [CrossRef]
- Andrade Munhoz, E.; Bodanezi, A.; Ferreira Junior, O.; Mauro Granjeiro, J. Bone crestal height and bone density after third-molar extraction and grafting: A long-term follow-up study. Clin. Oral Investig. 2011, 15, 123–126. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Staderini, E.; Patini, R.; Guglielmi, F.; Camodeca, A.; Gallenzi, P. How to Manage Impacted Third Molars: Germectomy or Delayed Removal? A Systematic Literature Review. Medicina 2019, 55, 79. [Google Scholar] [CrossRef] [PubMed]
- Kyyak, S.; Blatt, S.; Pabst, A.; Thiem, D.; Al-Nawas, B.; Kämmerer, P.W. Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin—A comparative in vitro study. J. Biomater. Appl. 2020, 35, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.; Ackermann, M.; Thiem, D.; Kämmerer, P. Influence of Different Rehydration Protocols on Biomechanical Properties of Allogeneic Cortical Bone Plates: A Combined. J. Invest. Surg. 2020, 1–7. [Google Scholar] [CrossRef]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognár, E.; Stadler, G.; Peterbauer, A.; Csönge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Gardin, C.; Ferroni, L.; Lacza, Z.; Zavan, B. Albumin-impregnated bone granules modulate the interactions between mesenchymal stem cells and monocytes under in vitro inflammatory conditions. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110678. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Szabó, T.; Szigyártó, I.C.; Toró, I.; Vámos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabó, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Toró, I.; Szabó, T.; May, Z.; Duarte, M.; Hornyák, I.; Szabó, B.T.; Dobó-Nagy, C.; Doros, A.; et al. Remineralization of demineralized bone matrix in critical size cranial defects in rats: A 6-month follow-up study. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1336–1342. [Google Scholar] [CrossRef]
- Klára, T.; Csönge, L.; Janositz, G.; Pap, K.; Lacza, Z. The use of structural proximal tibial allografts coated with human albumin in treating extensive periprosthetic knee-joint bone deficiency and averting late complications. Case report. Orv. Hetil 2015, 156, 67–70. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Márton, K.; Tamás, S.B.; Orsolya, N.; Béla, C.; Ferenc, D.; Péter, N.; Csaba, D.N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Com. Materials 2018, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Simonffy, L.; Minya, F.; Trimmel, B.; Lacza, Z.; Dobo-Nagy, C. Albumin-Impregnated Allograft Filling of Surgical Extraction Sockets Achieves Better Bone Remodeling Than Filling with Either Blood Clot or Bovine Xenograft. Int. J. Oral Maxillofac. Implant. 2020, 35, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Szalma, J.; Vajta, L.; Lovász, B.V.; Kiss, C.; Soós, B.; Lempel, E. Identification of Specific Panoramic High-Risk Signs in Impacted Third Molar Cases in Which Cone Beam Computed Tomography Changes the Treatment Decision. J. Oral Maxillofac. Surg. 2020, 78, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Thiem, D.G.E.; Schnaith, F.; Van Aken, C.M.E.; Köntges, A.; Kumar, V.V.; Al-Nawas, B.; Kämmerer, P.W. Extraction of mandibular premolars and molars: Comparison between local infiltration via pressure syringe and inferior alveolar nerve block anesthesia. Clin. Oral Investig. 2018, 22, 1523–1530. [Google Scholar] [CrossRef]
- Katz, M.S.; Peters, F.; Elvers, D.; Winterhalder, P.; Kniha, K.; Möhlhenrich, S.C.; Hölzle, F.; Modabber, A. Effect of drain application on postoperative complaints after surgical removal of impacted wisdom teeth-a randomized observer-blinded split-mouth clinical trial. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef]
- Shiels, S.M.; Raut, V.P.; Patterson, P.B.; Barnes, B.R.; Wenke, J.C. Antibiotic-loaded bone graft for reduction of surgical site infection in spinal fusion. Spine J. 2017, 17, 1917–1925. [Google Scholar] [CrossRef]
- Beuttel, E.; Bormann, N.; Pobloth, A.M.; Duda, G.N.; Wildemann, B. Impact of Gentamicin-Loaded Bone Graft on Defect Healing in a Sheep Model. Materials 2019, 12, 1116. [Google Scholar] [CrossRef]
- Lewis, C.S.; Supronowicz, P.R.; Zhukauskas, R.M.; Gill, E.; Cobb, R.R. Local antibiotic delivery with demineralized bone matrix. Cell Tissue Bank 2012, 13, 119–127. [Google Scholar] [CrossRef]
- Molly, L.; Vandromme, H.; Quirynen, M.; Schepers, E.; Adams, J.L.; van Steenberghe, D. Bone formation following implantation of bone biomaterials into extraction sites. J. Periodontol. 2008, 79, 1108–1115. [Google Scholar] [CrossRef]
- Kivovics, M.; Szabó, B.T.; Németh, O.; Iványi, D.; Trimmel, B.; Szmirnova, I.; Orhan, K.; Mijiritsky, E.; Szabó, G.; Dobó-Nagy, C. Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft. J. Clin. Med. 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Iocca, O.; Farcomeni, A.; Pardiñas Lopez, S.; Talib, H.S. Alveolar ridge preservation after tooth extraction: A Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J. Clin. Periodontol. 2017, 44, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 2), 195–223. [Google Scholar] [CrossRef]
- Camargo, P.M.; Lekovic, V.; Weinlaender, M.; Klokkevold, P.R.; Kenney, E.B.; Dimitrijevic, B.; Nedic, M.; Jancovic, S.; Orsini, M. Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 581–586. [Google Scholar] [CrossRef]
- Barone, A.; Aldini, N.N.; Fini, M.; Giardino, R.; Calvo Guirado, J.L.; Covani, U. Xenograft versus extraction alone for ridge preservation after tooth removal: A clinical and histomorphometric study. J. Periodontol. 2008, 79, 1370–1377. [Google Scholar] [CrossRef]
- Festa, V.M.; Addabbo, F.; Laino, L.; Femiano, F.; Rullo, R. Porcine-derived xenograft combined with a soft cortical membrane versus extraction alone for implant site development: A clinical study in humans. Clin. Implant. Dent. Relat. Res. 2013, 15, 707–713. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Walker, C.J.; Prihoda, T.J.; Mealey, B.L.; Lasho, D.J.; Noujeim, M.; Huynh-Ba, G. Evaluation of Healing at Molar Extraction Sites With and Without Ridge Preservation: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 241–249. [Google Scholar] [CrossRef]
- Maiorana, C.; Poli, P.P.; Deflorian, M.; Testori, T.; Mandelli, F.; Nagursky, H.; Vinci, R. Alveolar socket preservation with demineralised bovine bone mineral and a collagen matrix. J. Periodontal. Implant. Sci. 2017, 47, 194–210. [Google Scholar] [CrossRef]
- Manavella, V.; Romano, F.; Corano, L.; Bignardi, C.; Aimetti, M. Three-Dimensional Volumetric Changes in Severely Resorbed Alveolar Sockets After Ridge Augmentation with Bovine-Derived Xenograft and Resorbable Barrier: A Preliminary Study on CBCT Imaging. Int. J. Oral Maxillofac. Implant. 2018, 33, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, J.; Hannig, C.; Schulze, D.; Stricker, A.; Jacobs, R. Digital method for quantification of circumferential periodontal bone level using cone beam CT. Clin. Oral Investig. 2013, 17, 389–396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).