FT-IR Transflection Micro-Spectroscopy Study on Normal Human Breast Cells after Exposure to a Proton Beam
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Irradiation and Fixation
2.3. FT-IR Spectra Measurements
2.4. Data Analysis
3. Results and Discussion
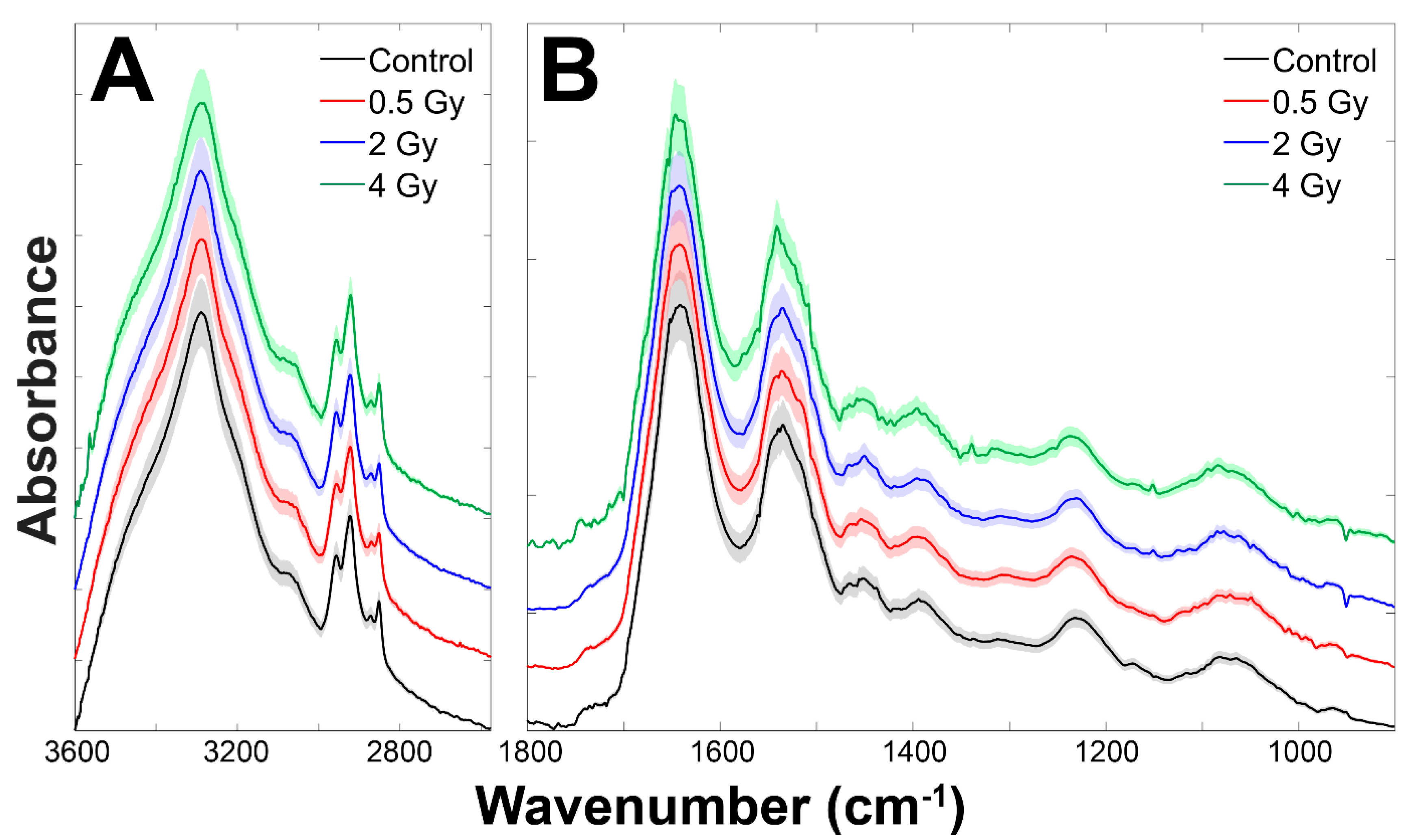
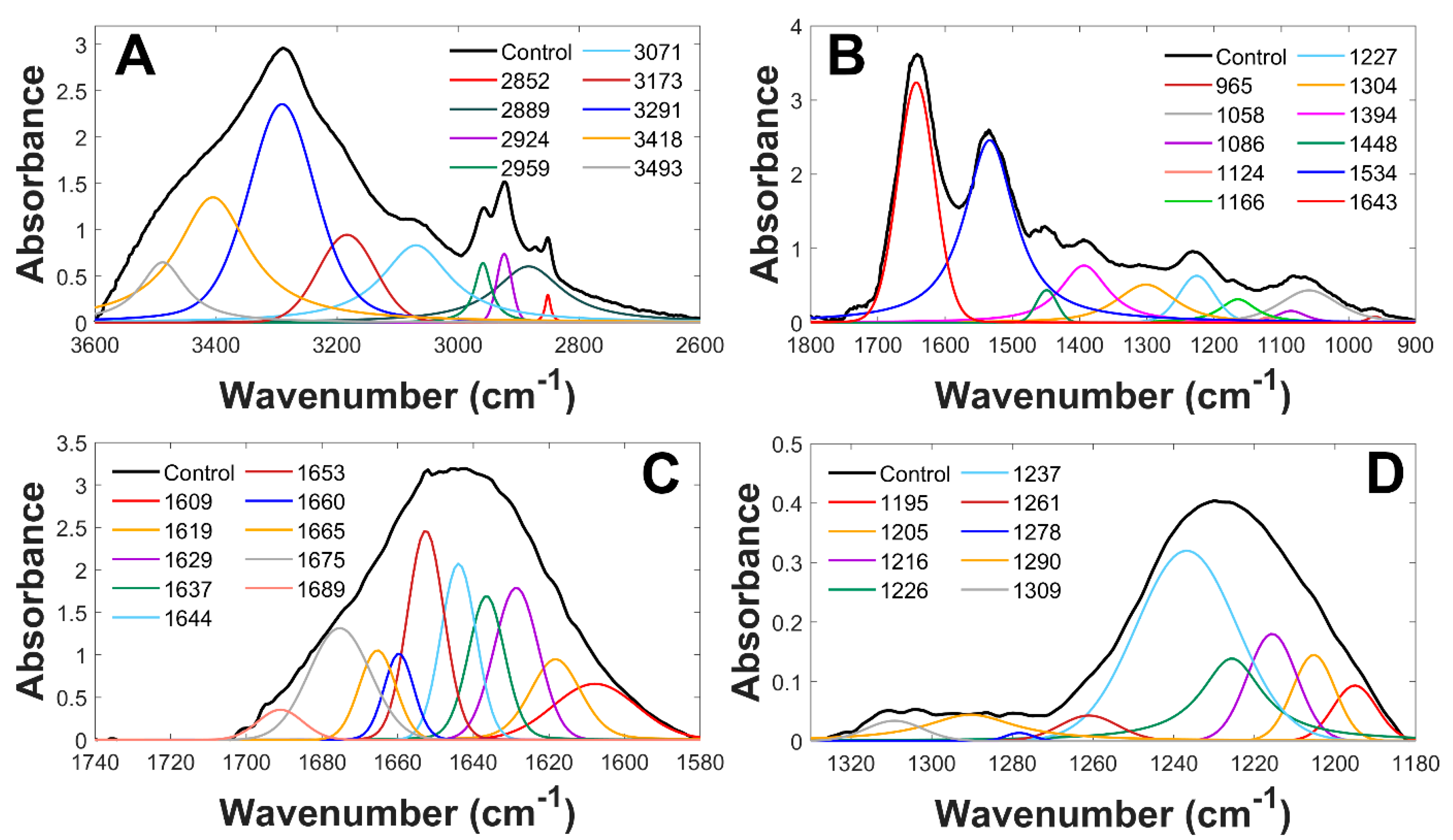
3.1. Analysis of Infrared Spectra from Control and Exposed Cells
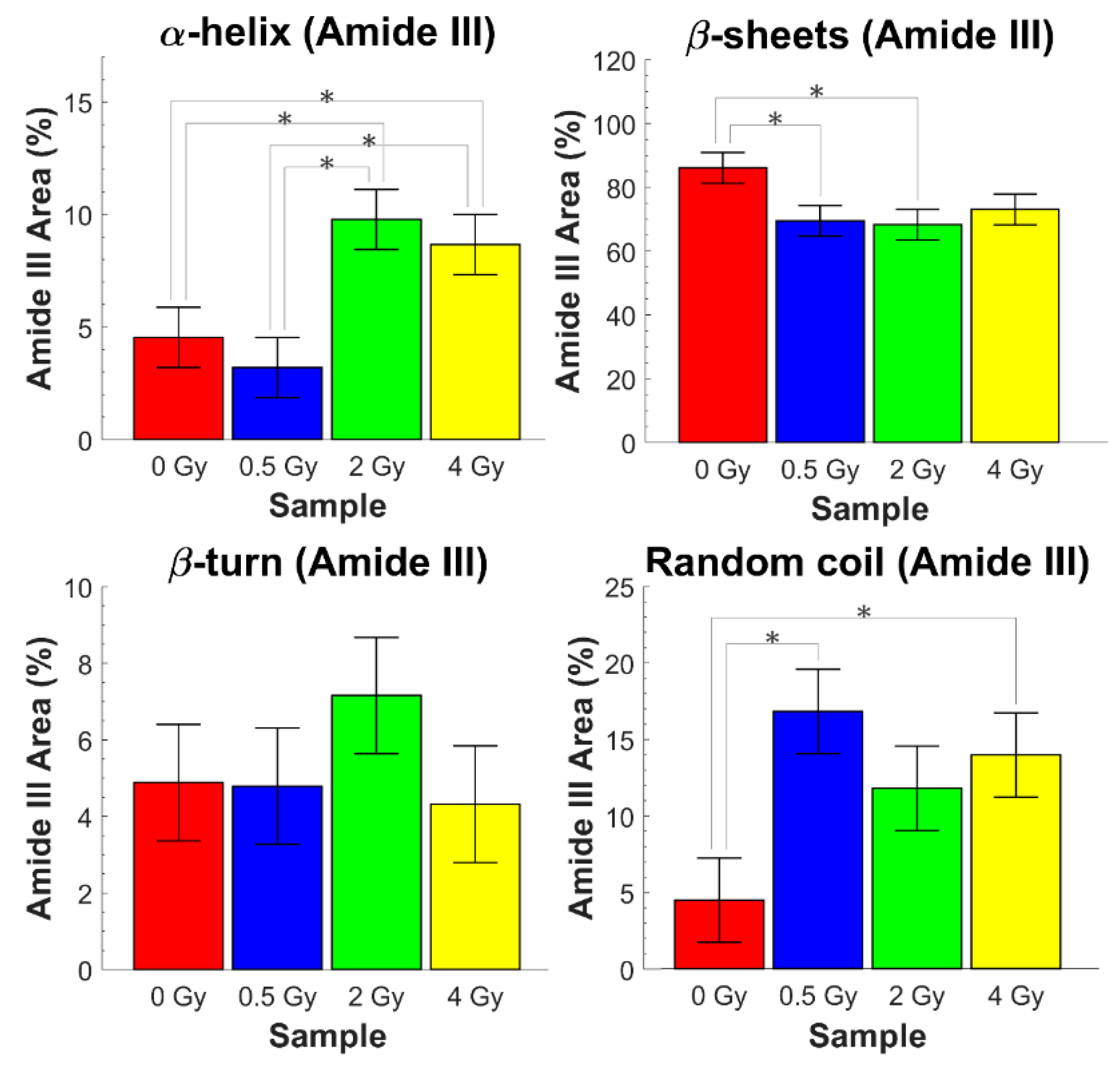
3.2. Analysis of Amide I and Amide III Bands
3.3. Analysis of Relative Absorbance Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gault, N.; Lefaix, J.L. Infrared microspectroscopic characteristics of radiation-induced apoptosis in human lymphocytes. Radiat. Res. 2003, 160, 238–250. [Google Scholar] [PubMed]
- Gasparri, F.; Muzio, M. Monitoring of apoptosis of HL60 cells by Fourier-transform infrared spectroscopy. Biochem. J. 2003, 369, 239–248. [Google Scholar] [PubMed]
- Gault, N.; Rigaud, O.; Poncy, J.L.; Lefaix, J.L. Infrared microspectroscopy study of γ-irradiated and H2O2-treated human cells. Int. J. Radiat. Biol. 2005, 81, 767–779. [Google Scholar] [PubMed]
- Meade, A.; Clarke, C.; Byrne, H.; Lyng, F. Fourier transform infrared microspectroscopy and multivariate methods for radiobiological dosimetry. Radiat. Res. 2010, 173, 225–237. [Google Scholar] [PubMed]
- Gianoncelli, A.; Vaccari, L.; Kourousias, G.; Cassese, D.; Bedolla, D.E.; Kenig, S.; Storici, P.; Lazzarino, M.; Kiskinova, M. Soft X-ray microscopy radiation damage on fixed cells investigated with synchrotron radiation FT-IR microscopy. Sci. Rep. 2015, 5, 10250. [Google Scholar]
- Ricciardi, V.; Portaccio, M.; Manti, L.; Lepore, M. An FT-IR microspectroscopy ratiometric approach for monitoring X-ray irradiation effects on SH-SY5Y human neuroblastoma cells. Appl. Sci. 2020, 10, 2974. [Google Scholar] [CrossRef]
- Paganetti, H. Proton Therapy Physics; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Goitein, M. Radiation Oncology: A Physicist’s Eye View; Springer: New York, NY, USA, 2007. [Google Scholar]
- Chowdhary, M.; Lee, A.; Gao, S.; Wang, D.; Barry, P.N.; Diaz, R.; Bagadiya, N.R.; Park, H.S.; Yu, J.B.; Wilson, L.D.; et al. Is Proton Therapy a “Pro” for Breast Cancer? A Comparison of Proton vs. Non-proton Radiotherapy Using the National Cancer Database. Front. Oncol. 2018, 8, 678. [Google Scholar]
- Cheng, Y.J.; Nie, X.Y.; Ji, C.C.; Lin, X.X.; Liu, L.J.; Chen, X.M.; Yao, N.; Wu, S.H. Long term cardiovascular risk after radiotherapy in women with breast cancer. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Jimenez, R.B.; Hickey, S.; DePauw, N.; Yeap, B.Y.; Batin, E.; Gadd, M.A.; Specht, M.; Isakoff, S.J.; Smith, B.L.; Liao, R.C.; et al. Phase II study of proton beam radiation therapy for patients with breast cancer requiring regional nodal irradiation. J. Clin. Oncol. 2019, 37, 2778–2785. [Google Scholar]
- Lipiec, E.; Birarda, G.; Kowalska, J.; Lekki, J.; Vaccari, L.; Wiecheć, A.; Wood, B.R.; Kwiatek, W.M. A new approach to studying the effects of ionising radiation on single cells using FT-IR synchrotron microspectroscopy. Radiat. Phys. Chem. 2013, 93, 135–141. [Google Scholar]
- Lipiec, E.; Bambery, K.R.; Heraud, P.; Hirschmugl, C.; Lekki, J.; Kwiatek, W.M.; Tobin, M.J.; Vogel, C.; Whelan, D.; Wood, B.R. Synchrotron FT-IR shows evidence of DNA damage and lipid accumulation in prostate adenocarcinoma PC-3 cells following proton irradiation. J. Mol. Struct. 2014, 1073, 134–141. [Google Scholar] [CrossRef]
- Lipiec, E.; Kowalska, J.; Lekki, J.; Wiechec, A.; Kwiatek, W.M. FT-IR Microspectroscopy in Studies of DNA Damage Induced by Proton Microbeam in Single PC-3 Cells. Acta Phys. Pol. A 2012, 121, 506–509. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological material. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Perna, G.; Capozzi, V.; Lasalvia, M. A comparison between FTIR spectra from HUKE and SH-SY5Y cell lines grown on different substrates. Appl. Sci. 2020, 10, 8825. [Google Scholar] [CrossRef]
- DeVetter, B.M.; Kenkel, S.; Mittal, S.; Bhargava, R.; Wrobel, T.P. Characterization of the structure of low-e-substrate and consequence for IR transflection measurements. Vib. Spectrosc. 2017, 91, 119–127. [Google Scholar] [CrossRef]
- Rutter, A.V.; Crees, J.; Wright, H.; Raseta, M.; van Pittius, D.G.; Roach, P.; Sulé-Suso, J. Identification of a glass substrate to study cells using Fourier Transform Infrared spectroscopy: Are we close to a spectral pathology? Appl. Spectrosc. 2020, 74, 178–186. [Google Scholar] [CrossRef]
- Yao, J.; Li, Q.; Zhou, B.; Wang, D.; Wu, R. Advantages of infrared transflection micro spectroscopy and paraffin-embedded sample preparation for biological studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 25–30. [Google Scholar] [CrossRef]
- Filik, J.; Frogley, M.D.; Pijanka, J.K.; Wehbe, K.; Cinque, G. Electric field standing wave artefacts in FT-IR micro-spectroscopy of biological materials. Analyst 2012, 137, 853–861. [Google Scholar] [CrossRef]
- Staniszewska-Slezak, E.; Rygula, A.; Malek, K.; Baranska, M. Transmission versus transflection mode in FT-IR analysis of blood plasma: Is the electric field standing wave effect the only reason for observed spectral distortions? Analyst 2015, 140, 2412–2421. [Google Scholar] [CrossRef]
- Wrobel, T.P.; Wajnchold, B.; Byrne, H.J.; Baranska, M. Corrigendum. Electric field standing wave effects in FT-IR transflection spectra of biological tissue sections: Simulated models of experimental variability. Vib. Spectrosc. 2014, 71, 115–117. [Google Scholar] [CrossRef]
- Cao, J.; Ng, E.S.; McNaughton, D.; Stanley, E.G.; Elefanty, A.G.; Tobin, M.J.; Heraud, P. Fourier transform infrared microspectroscopy reveals that tissue culture conditions affect the macromolecular phenotype of human embryonic stem cells. Analyst 2013, 138, 4147–4160. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Heraud, P.; Marzec, K.M.; De La Guardia, M.; Kiupel, M.; Wood, B.R. Comparison of transflection and transmission FT-IR imaging measurements performed on differentially fixed tissue sections. Analyst 2015, 140, 2376–2382. [Google Scholar] [CrossRef]
- Lee, J. On the non-existence of the so-called “electric field standing wave effect in transflection FTIR spectra. Vib. Spectrosc. 2017, 90, 104–111. [Google Scholar] [CrossRef]
- Kim, B.; Bae, H.; Lee, H.; Lee, S.; Park, J.C.; Kim, K.R.; Kim, S.J. Proton Beams Inhibit Proliferation of Breast Cancer Cells by Altering DNA Methylation Status. J. Cancer 2016, 7, 344–352. [Google Scholar] [CrossRef]
- Bravatà, V.; Minafra, L.; Cammarata, F.P.; Pisciotta, P.; Lamia, D.; Marchese, V.; Petringa, G.; Manti, L.; Cirrone, G.A.P.; Gilardi, M.C.; et al. Gene expression profiling of breast cancer cell lines treated with proton and electron radiations. Br. J. Radiol. 2018, 91, 20170934. [Google Scholar] [CrossRef]
- Bravatà, V.; Cammarata, F.P.; Minafra, L.; Pisciotta, P.; Scazzone, C.; Manti, L.; Savoca, G.; Petringa, G.; Cirrone, G.A.P.; Cuttone, G.; et al. Proton-irradiated breast cells: Molecular points of view. J. Radiat. Res. 2019, 60, 451–465. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Cuttone, G.; Lojacono, P.A.; Lo Nigro, S.; Mongelli, V.; Patti, I.V.; Privitera, G.; Raffaele, L.; Rifuggiato, D.; Sabini, M.G.; et al. A 62-MeV proton beam for the treatment of ocular melanoma at Laboratori Nazionali del Sud-INFN. IEEE Trans. Nucl. Sci. 2004, 51, 3568–3662. [Google Scholar] [CrossRef]
- Bassan, P.; Byrne, H.J.; Bonnier, F.; Lee, J.; Dumas, P.; Gardner, P. Resonant Mie scattering in infrared spectroscopy of biological materials–understanding the ‘dispersion artefact’. Analyst 2009, 134, 1586–1593. [Google Scholar] [CrossRef]
- Bassan, P.; Kohler, A.; Martens, H.; Lee, J.; Byrne, H.J.; Dumas, P.; Gazi, E.; Brown, M.; Clarke, N.; Gardner, P. Resonant Mie scattering (RMieS) correction of infrared spectra from highly scattering biological samples. Analyst 2010, 135, 268–277. [Google Scholar] [CrossRef]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chem. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef]
- Lipiec, E.; Bambery, K.R.; Lekki, J.; Tobin, M.J.; Vogel, C.; Whelan, D.R.; Wood, B.R.; Kwiatek, W.M. SR-FT-IR coupled with principal component analysis shows evidence for the cellular bystander effect. Radiat. Res. 2015, 184, 73–82. [Google Scholar] [CrossRef]
- Pelton, J.T.; McLean, L.R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Coe, J.V.; Nystrom, S.V.; Chen, Z.; Li, R.; Verreault, D.; Hitchcock, C.L.; Allen, H.C. Extracting Infrared Spectra of Protein Secondary Structures Using a Library of Protein Spectra, and the Ramachandran Plot. J. Phys. Chem. B 2015, 119, 13079–13092. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Miller, L.; Gao, W.; Gross, R.A. Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules 2003, 4, 70–74. [Google Scholar] [CrossRef]
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Mita, D.G.; Lepore, M. Enzyme distribution and secondary structure of sol-gel immobilized glucose oxidase by micro-attenuated total reflection FT-IR spectroscopy. Mater. Sci. Eng. C 2013, 33, 304–310. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Gault, N.; Rigaud, O.; Poncy, J.L.; Lefaix, J.L. Biochemical alterations in human cells irradiated with alpha particles delivered by macro- or microbeams. Radiat. Res. 2007, 167, 551–562. [Google Scholar] [CrossRef]
- Li, J.Y.; Ying, G.G.; Jones, K.C.; Martin, F.L. Real-world carbon nanoparticle exposures induce brain and gonadal alterations in zebrafish (Danio rerio) as determined by biospectroscopy techniques. Analyst 2015, 140, 2687–2695. [Google Scholar] [CrossRef]
- Dovbeshko, G.; Gridina, N.Y.; Kruglova, E.B.; Pashchuk, O.P. FT-IR Spectroscopy studies of nucleic acid damage. Talanta 2000, 53, 233–246. [Google Scholar] [CrossRef]
- Lasalvia, M.; Perna, G.; Pisciotta, P.; Cammarata, F.P.; Manti, L.; Capozzi, V. Raman spectroscopy for the evaluation of the radiobiological sensitivity of normal human breast cells at different time points after irradiation by a clinical proton beam. Analyst 2019, 144, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y cancer cell response to treatment with polyphenol extracts using FT-IR spectroscopy. Biosensors 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Koike, K. Lipid and Membrane Dynamics in Biological Tissues—Infrared Spectroscopic Studies. Adv. Planar Lipid Bilayers Liposomes 2011, 13, 1–32. [Google Scholar]
- Sailer, K.; Viaggi, S.; Nusse, M. Radiation–induced structural modifications in dsDNA analysed by FT–Raman spectroscopy. Int. J. Radiat. Biol. 1996, 69, 601–613. [Google Scholar] [CrossRef]
- Barraza-Garza, G.; Castillo-Michel, H.; de la Rosa, L.A.; Martinez-Martinez, A.; Pérez-León, J.A.; Cotte, M.; Alvarez-Parrilla, E. Infrared spectroscopy as a tool to study the antioxidant activity of polyphenolic compounds in isolated rat enterocytes. Oxidative Med. Cell. Longev. 2016, 2016, 9245150. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, T.; Mukherjee, R.; Ariese, F.; Somasundaram, K.; Umapathy, S. Raman and infra-red microspectroscopy: Towards quantitative evaluation for clinical research by ratiometric analysis. Chem. Soc. Rev. 2016, 45, 1879–1900. [Google Scholar] [CrossRef]
- Gautam, R.; Chandrasekar, B.; Deobagkar-Lele, M.; Rakshit, S.; Umapathy, S.; Nandi, D. Identification of early biomarkers during acetaminophen-induced hepatotoxicity by Fourier transform infrared microspectroscopy. PLoS ONE 2012, 7, e45521. [Google Scholar] [CrossRef][Green Version]
- Zelig, U.; Kapelushnik, J.; Moreh, R.; Mordechai, S.; Nathan, I. Diagnosis of cell death by means of Infrared Spectroscopy. Biophys. J. 2009, 97, 2107–2114. [Google Scholar] [CrossRef]
- Singh, J.K.; Dasgupta, A.; Adayen, T.; Shahmehdi, S.A.; Hammond, D.; Banerjee, P. Apoptosis is associated with an increase in saturated fatty acid containing phospholipids in the neuronal cell line, HN2–5. Biochim. Biophys. Acta 1996, 1304, 171–178. [Google Scholar] [CrossRef]
- Delfino, I.; Perna, G.; Ricciardi, V.; Lasalvia, M.; Manti, L.; Capozzi, V.; Lepore, M. X-ray irradiation effects on nuclear and membrane regions of single SH-SY5Y human neuroblastoma cells investigated by Raman micro-spectroscopy. J. Of Pharm. Biomed. Anal. 2019, 164, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Vileno, B.; Jeney, S.; Sienkiewicz, A.; Marcoux, P.R.; Miller, L.M.; Forró, L. Evidence of lipid peroxidation and protein phosphorylation in cells upon oxidative stress photo-generated by fullerols. Biophys. Chem. 2010, 152, 164–169. [Google Scholar] [CrossRef] [PubMed]
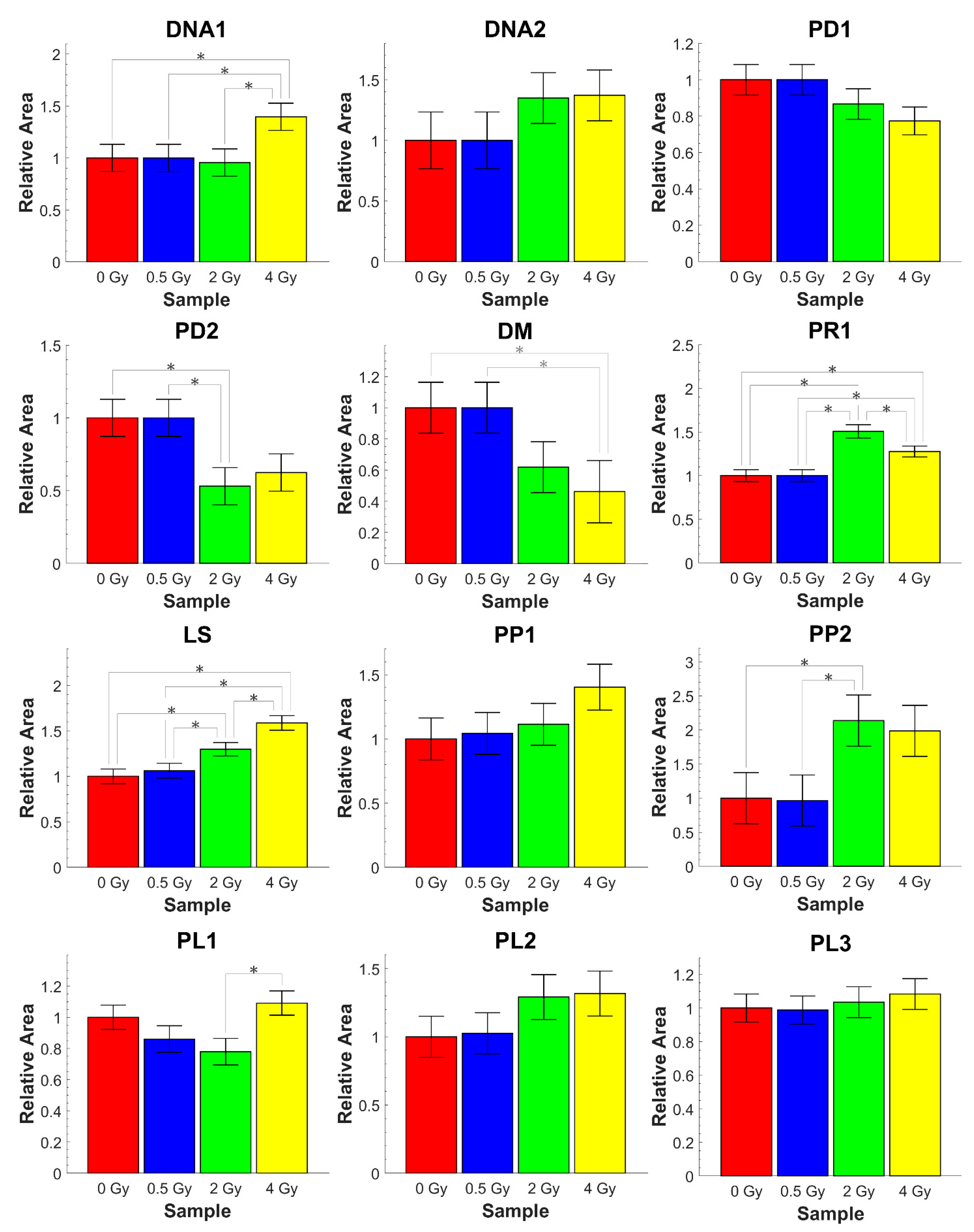
| Ratio | Biomolecular Origin | Indication |
|---|---|---|
| AX/AY | ||
| A1227/A1448 | PO2− as. ν /CH3 as. δ, CH2 sc. | DNA modification (DNA 1) |
| A1086/A1448 | PO2− s. ν /CH3 as. δ, CH2 sc. | DNA modification (DNA 2) |
| A1643/A1227 | Amide I/PO2− as. ν | Protein/DNA content (PD1) |
| A1643/A1086 | Amide I/PO2− s. ν | Protein/DNA content (PD2) |
| A1227/A1086 | PO2− as. ν, C-O-P ν/ PO2−s. ν, C-O-P ν | DNA modification (DM) |
| A1643/A1534 | Amide I/Amide II | Protein rearrangement (PR1) |
| A1534/A2960 | Amide II/CH3 as. ν | Protein/Lipid content (PL1) |
| A1394/A2960 | COO− s. ν/CH3 as. ν | Protein/Lipid content (PL2) |
| A1643/A2960 | Amide I/CH3 as. ν | Protein/Lipid content (PL3) |
| A2924/A2960 | CH2 as. ν/CH3 as. ν | Lipid saturation (LS) |
| A1227/A2960 | PO2− as. ν, C-O-P ν/CH3 as. ν | Protein phosphorylation (PP1) |
| A1086/A2960 | PO2− s. ν, C-O-P ν/CH3 as. ν | Protein phosphorylation (PP2) |
| Peak | Assignment | |||
|---|---|---|---|---|
| cm−1 | DNA/RNA | Protein | Lipid | Carbohydrate |
| 3500–3300 | O-H ν | |||
| 3291 | Amide A (-N-H ν) | O-H ν | ||
| 3173 | -NH3+ as. ν (a. a.) | |||
| 3071 | Amide B(-N-H ν, δ) | O-H ν | ||
| 2959 | CH3 as. Ν | CH3 as. ν | ||
| 2924 | CH2 as. ν | |||
| 2889 | CH3 s. ν | CH3 s. ν | ||
| 2852 | CH2 s. ν | |||
| 1643 | Amide I (C=O ν, C-N ν) | |||
| 1534 | Amide II (C-Nν,C-NH δ) | |||
| 1448 | CH3 as. δ, CH2 sc. | CH3 as. δ, CH2 sc. | ||
| 1394 | COO− s. ν | |||
| 1304 | Amide III(-N-Hδ,-C-N ν) | |||
| 1291 | Amide III(-N-Hδ, -C-N ν) | |||
| 1227 | PO2− as. ν | C-O-P ν | ||
| 1207 | C-H ring δ | |||
| 1171 | Sugar -phosphate backbone vbr | |||
| 1166 | CO-O-C s. as. ν | |||
| 1143 | Ribose C-O ν | |||
| 1124 | ν -C-O | |||
| 1100 | P-O-C s. ν | |||
| 1086 | PO2− s. ν | C-O-P ν | ||
| 1058 | ν -C-O | |||
| 965 | PO4− s. ν | C-0 ν, C=C ν (a. a.) | ||
| 0 Gy | Assignment | 0.5 Gy | 2 Gy | 4 Gy |
|---|---|---|---|---|
| Peak (cm−1) | Peak (cm−1) | Peak (cm−1) | Peak (cm−1) | |
| 3493 | c | 3490 (−3) | 3492 (−1) | 3493 |
| 3418 | c | 3411 (−7) | 3417 (−1) | 3421 (+3) |
| 3291 | p, c | 3291 | 3291 | 3295 (+4) |
| 3173 | p | 3179 (+6) | 3178 (+5) | 3186 (+13) |
| 3071 | p, c | 3078 (+7) | 3081 (+10) | 3071 |
| 2959 | p, l | 2960 (+1) | 2960 (+1) | 2960 (+1) |
| 2924 | l | 2924 | 2924 | 2923 (−1) |
| 2889 | p, l | 2880 (−9) | 2880 (−9) | 2893 (+4) |
| 2852 | l | 2855 (+3) | 2858 (+6) | 2859 (+7) |
| 1643 | p | 1643 | 1645 (+2) | 1644 (+1) |
| 1534 | p | 1534 | 1534 | 1535 (+1) |
| 1448 | p, l | 1448 | 1451 (+3) | 1451 (+3) |
| 1394 | p | 1394 | 1395 (+1) | 1396 (+2) |
| 1304 | p, l | 1304 | 1303 (−1) | 1311 (+7) |
| 1291 | p | 1286 (−5) | 1295 (+4) | 1287 (−4) |
| 1227 | DNA, c | 1227 | 1231 (+4) | 1233 (+5) |
| 1207 | DNA, RNA | 1203 (−4) | 1202 (−5) | |
| 1171 | DNA | 1171 | 1171 | 1169 (−2) |
| 1166 | l | 1166 | 1170 (+4) | 1167 (+1) |
| 1143 | DNA | 1149 (+5) | ||
| 1124 | DNA | 1124 | 1121 (−3) | 1116 (−8) |
| 1100 | DNA | 1098 (−2) | 1097 (−3) | |
| 1086 | DNA, p | 1086 | 1085 (−1) | 1084 (−2) |
| 1058 | DNA, p | 1058 | 1051 (−7) | 1058 |
| 958 | DNA, p | 965 (+7) | 966 (+8) |
| t0 Cells | ||||
|---|---|---|---|---|
| Control | Assignments | 0.5 Gy | 2 Gy | 4 Gy |
| Peak (cm−1) | ||||
| 1180–1240 | β-sheets | - | - | - |
| %A = 86 ± 8 | %A = 74 ± 15 | %A = 74 ± 20 | %A = 76 ± 15 | |
| 1240–1270 | Random coil | - | - | - |
| %A = 4 ± 2 | %A = 23 ± 7 | %A = 22 ± 7 | %A = 16 ± 7 | |
| 1270–1290 | β-turn | - | - | - |
| %A = 5 ± 2 | %A = 7 ± 3 | %A = 7 ± 5 | %A = 7 ± 3 | |
| 1290–1330 | α-helix | - | - | - |
| %A = 5 ± 2 | %A = 10 ± 4 | %A = 10 ± 4 | %A = 13 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, V.; Portaccio, M.; Perna, G.; Lasalvia, M.; Capozzi, V.; Cammarata, F.P.; Pisciotta, P.; Petringa, G.; Delfino, I.; Manti, L.; et al. FT-IR Transflection Micro-Spectroscopy Study on Normal Human Breast Cells after Exposure to a Proton Beam. Appl. Sci. 2021, 11, 540. https://doi.org/10.3390/app11020540
Ricciardi V, Portaccio M, Perna G, Lasalvia M, Capozzi V, Cammarata FP, Pisciotta P, Petringa G, Delfino I, Manti L, et al. FT-IR Transflection Micro-Spectroscopy Study on Normal Human Breast Cells after Exposure to a Proton Beam. Applied Sciences. 2021; 11(2):540. https://doi.org/10.3390/app11020540
Chicago/Turabian StyleRicciardi, Valerio, Marianna Portaccio, Giuseppe Perna, Maria Lasalvia, Vito Capozzi, Francesco Paolo Cammarata, Pietro Pisciotta, Giada Petringa, Ines Delfino, Lorenzo Manti, and et al. 2021. "FT-IR Transflection Micro-Spectroscopy Study on Normal Human Breast Cells after Exposure to a Proton Beam" Applied Sciences 11, no. 2: 540. https://doi.org/10.3390/app11020540
APA StyleRicciardi, V., Portaccio, M., Perna, G., Lasalvia, M., Capozzi, V., Cammarata, F. P., Pisciotta, P., Petringa, G., Delfino, I., Manti, L., & Lepore, M. (2021). FT-IR Transflection Micro-Spectroscopy Study on Normal Human Breast Cells after Exposure to a Proton Beam. Applied Sciences, 11(2), 540. https://doi.org/10.3390/app11020540













