Comparison of the Effect of Enhancing Dry Fermented Sausages with Salvia hispanica and Nigella sativa Seed on Selected Physicochemical Properties Related to Food Safety during Processing
Abstract
1. Introduction
2. Material and Methods
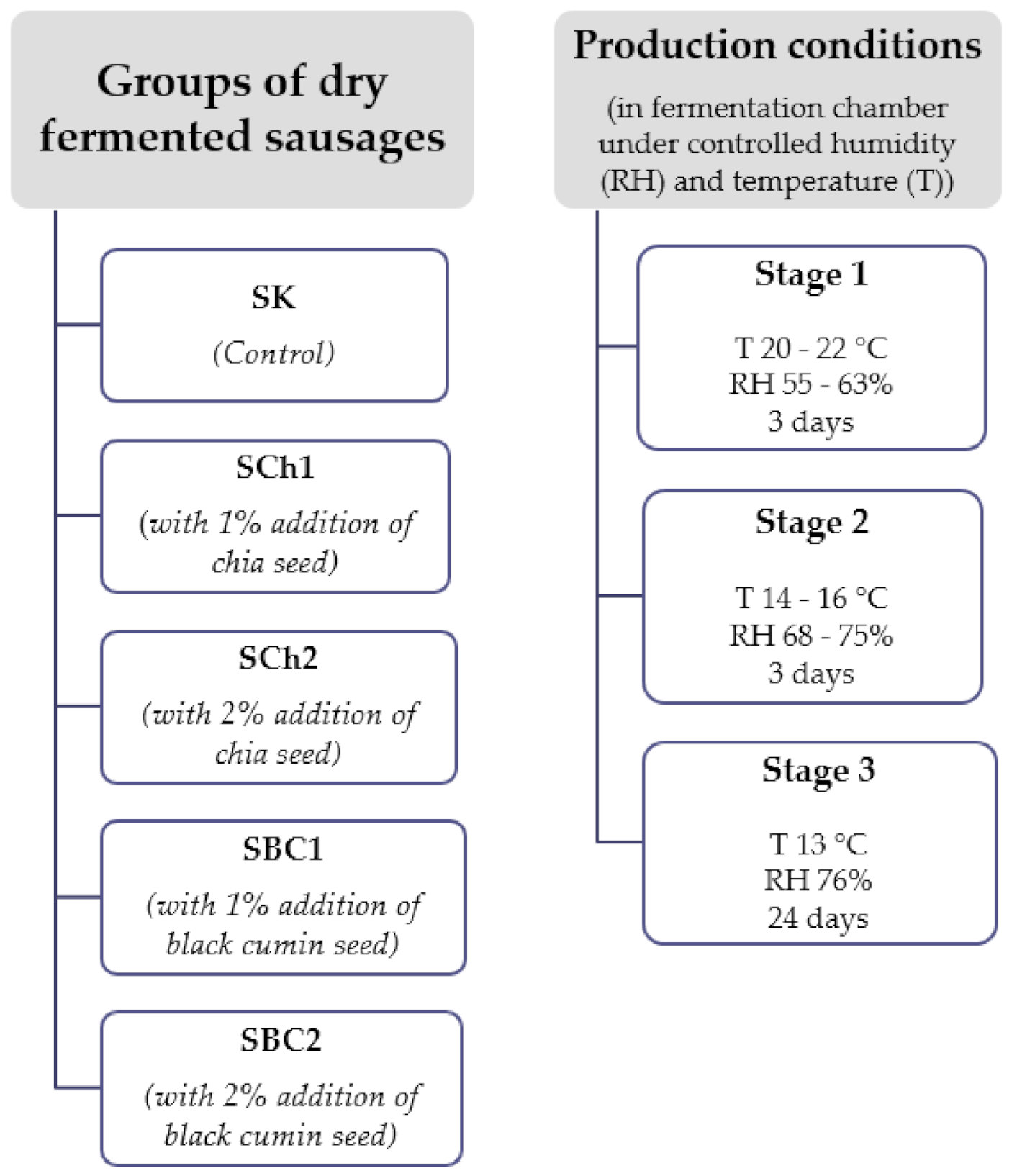
2.1. The Experimental Material Preparation
2.2. The Physicochemical Parameters (WC-Water Content, pH, and Water Activity)
2.3. Lipid Oxidation Analyzes
2.4. Instrumental Color Measurement and Nitrosylmyoglobin Content
2.5. Microbiological Analyzes
2.6. Biogenic Amines (BAs) Determination
2.7. Statistical Analysis
3. Results
3.1. The Results of Physicochemical Parameters (Water Content, pH, and Water Activity)
3.2. The TBARS Results
3.3. The Results of Instrumental Color Measurement and Nitrosylmyoglobin Content
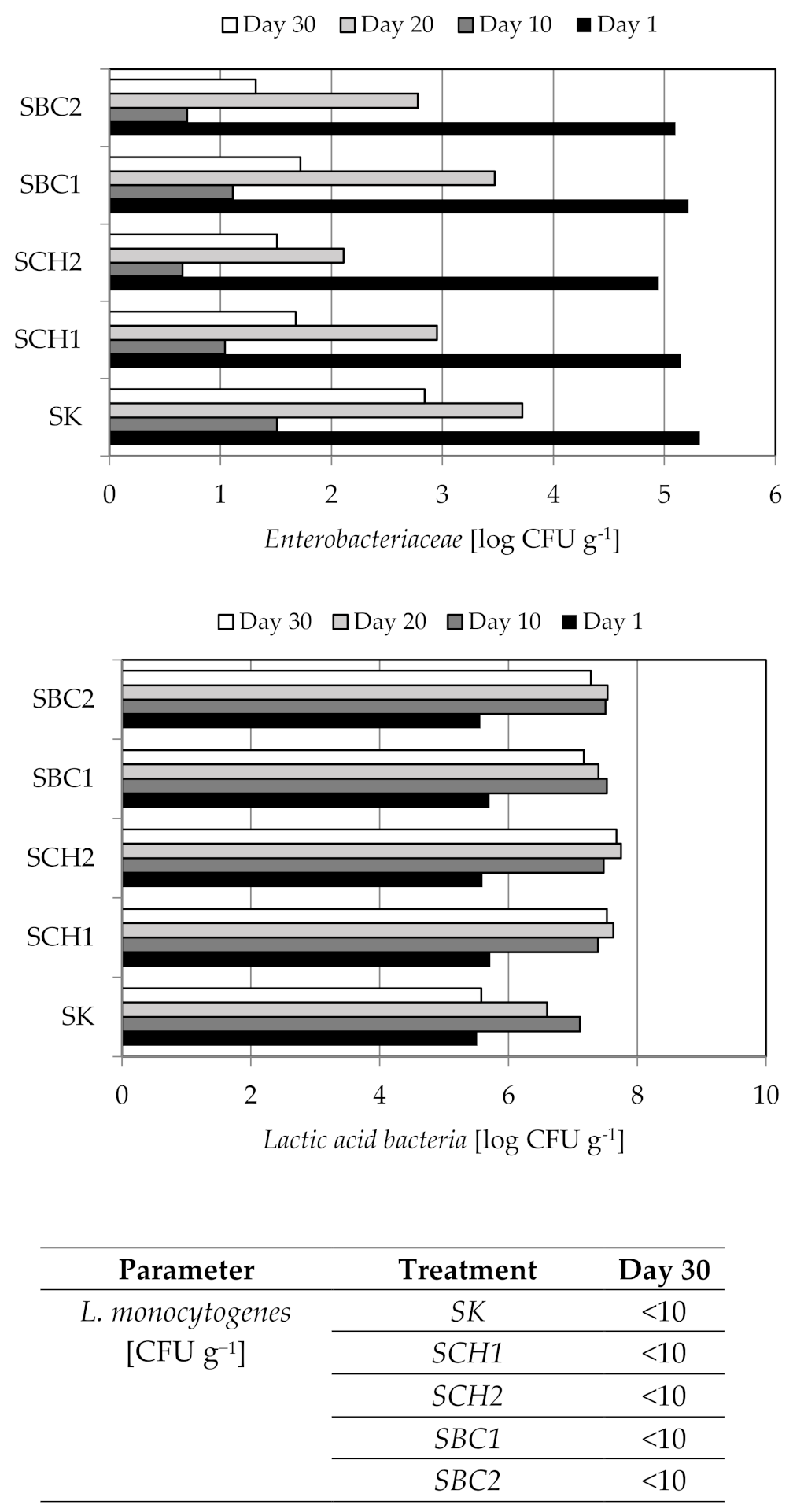
3.4. The Results of Microbiological Analyzes
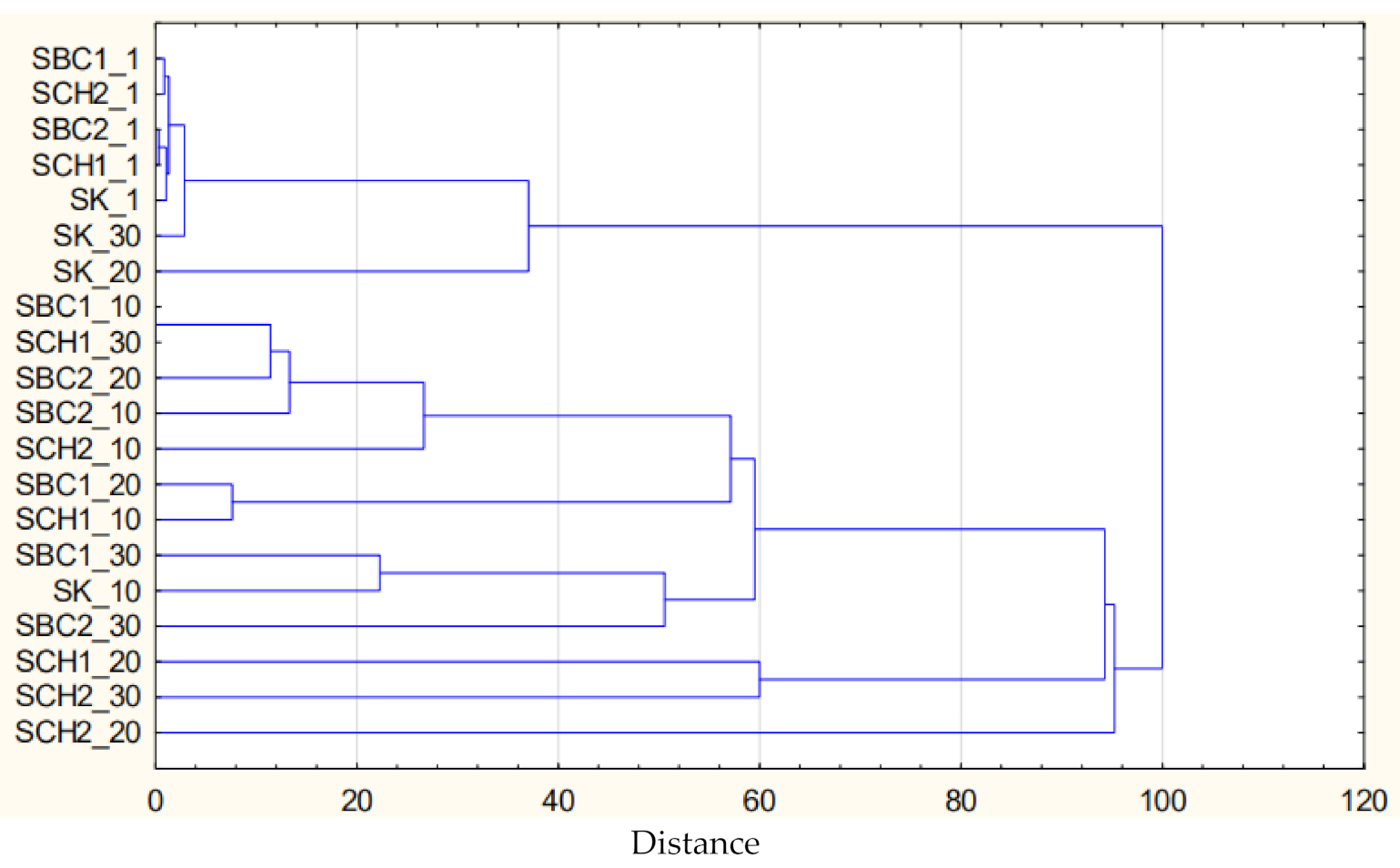
3.5. Results of Cluster and PCA Analysis
3.6. Biogenic Amines Content
4. Discussion
4.1. Physicochemical Parameters (Water Content, pH, Water Activity)
4.2. Lipid Oxidation Analyzes
4.3. Instrumental Color Parameters and Nitrosylmyoglobin Content
4.4. Microbiological Analyzes
4.5. Biogenic Amines Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT 2019. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 18 August 2021).
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Wei, Q. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, P.; Pateiro, M.; Gagaoua, M.; Franco, D.; Zhang, W.; Lorenzo, J.M. Evaluation of the antioxidant and antimicrobial activities of porcine liver protein hydrolysates obtained using alcalase, bromelain, and papain. Appl. Sci. 2020, 10, 2290. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Soyer, A. Effects of chitosan as a surface fungus inhibitor on microbiological, physicochemical, oxidative and sensory characteristics of dry fermented sausages. Meat Sci. 2018, 145, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Escudero, E.; Toldrá, F. Bioactive peptides and free amino acids profiles in different types of European dry-fermented sausages. Int. J. Food Microb. 2018, 276, 71–78. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.D.; Borrajo, P.; Lorenzo, J.M. Comparative Studies on the Fatty Acid Profile and Volatile Compounds of Fallow Deer and Beef Fermented Sausages without Nitrite Produced with the Addition of Acid Whey. Appl. Sci. 2021, 11, 1320. [Google Scholar] [CrossRef]
- Mikami, N.; Tsukada, Y.; Pelpolage, S.W.; Han, K.H.; Fukushima, M.; Shimada, K. Effects of Sake lees (Sake-kasu) supplementation on the quality characteristics of fermented dry sausages. Heliyon 2020, 6, e03379. [Google Scholar] [CrossRef]
- Balamurugan, S.; Gemmell, C.; Lau, A.T.Y.; Arvaj, L.; Strange, P.; Gao, A.; Barbut, S. High pressure processing during drying of fermented sausages can enhance safety and reduce time required to produce a dry fermented product. Food Contr. 2020, 113, 107224. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Penna, A.L.B.; da Silva Barretto, A.C. Volatile profile of fermented sausages with commercial probiotic strains and fructooligosaccharides. J. Food Sci. Technol. 2019, 56, 5465–5473. [Google Scholar] [CrossRef]
- Cao, C.C.; Feng, M.Q.; Sun, J.; Xu, X.L.; Zhou, G.H. Screening of lactic acid bacteria with high protease activity from fermented sausages and antioxidant activity assessment of its fermented sausages. CyTA-J. Food 2019, 17, 347–354. [Google Scholar] [CrossRef]
- Yu, D.; Feng, M.Q.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Contr. 2021, 123, 107743. [Google Scholar] [CrossRef]
- Zhao, B.; Zhou, H.; Zhang, S.; Pan, X.; Li, S.; Zhu, N.; Chen, W. Changes of protein oxidation, lipid oxidation and lipolysis in Chinese dry sausage with different sodium chloride curing salt content. Food Sci. Hum. Well. 2020, 9, 328–337. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Galanakis, C.M. Introduction in functional components for membrane separations. In Separation of Functional Molecules in Food by Membrane Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 31–77. [Google Scholar]
- Adegbeye, M.J.; Elghandour, M.M.; Faniyi, T.O.; Perez, N.R.; Barbabosa-Pilego, A.; Zaragoza-Bastida, A.; Salem, A.Z. Antimicrobial and antihelminthic impacts of black cumin, pawpaw and mustard seeds in livestock production and health. Agrofor. Syst. 2020, 94, 1255–1268. [Google Scholar] [CrossRef]
- Tashla, T.; Puvača, N.; Pelić, D.L.; Prodanović, R.; Bošković, J.; Ivanišević, D.; Lević, J. Dietary medicinal plants enhance the chemical composition and quality of broiler chicken meat. J. Hell. Vet. Med. Soc. 2019, 70, 1823–1832. [Google Scholar] [CrossRef][Green Version]
- Breda, S.G.; Mathijs, K.; Sági-Kiss, V.; Kuhnle, G.G.; van der Weer, B.; Jones, R.R.; Sinha, R.; Ward, M.H.; de Kok, T.M. Impact of high drinking water nitrate levels on the endogenous formation of apparent N-nitroso compounds in combination with meat intake in healthy volunteers. Environ. Health 2019, 18, 1–12. [Google Scholar]
- Chauhan, P.; Das, A.K.; Nanda, P.K.; Kumbhar, V.; Yadav, J.P. Effect of Nigella sativa seed extract on lipid and protein oxidation in raw ground pork during refrigerated storage. Nutr. Food Sci. 2018, 48, 1. [Google Scholar] [CrossRef]
- Mahgoub, S.A.M.; Osman, A.; Ramadan, M.F. Inhibitory effect of Nigella sativa oil against Listeria monocytogenes and Salmonella Enteritidis inoculated in minced beef meat. J. Food Meas. Charact. 2017, 11, 2043–2051. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalisz, S.; Wirkowska-Wojdyła, M.; Oszmiański, J. Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage. LWT 2020, 130, 109718. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (black cumin): A promising natural remedy for wide range of illnesses. Evid. -Based Compl. Altern. Med. 2019. [Google Scholar] [CrossRef]
- Topcagic, A.; Zeljkovic, S.C.; Karalija, E.; Galijasevic, S.; Sofic, E. Evaluation of phenolic profile, enzyme inhibitory and antimicrobial activities of Nigella sativa L. seed extracts. Bosn. J. Basic Med. Sci. 2017, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Kumar, S.; Yadav, S. Pumpkin and chia seed as dietary fibre source in meat products: A review. Pharma Inn. J. 2021, 10, 477–485. [Google Scholar]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro, C.; Haros, C.M.; Pérez-Álvarez, J.A. Chia (Salvia hispanica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Sci. 2019, 156, 139–145. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and chia products as ingredients for healthier processed meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Santillán-Álvarez, A.; Dublán-García, O.; López-Martínez, L.X.; Quintero-Salazar, B.; Gómez-Oliván, L.; Díaz-Bandera, D.; Hernández-Navarro, M.D. Effect of chia seed on physicochemical and sensory characteristics of common carp restructured as functional food. J. Food Sci. Eng. 2017, 7, 115–126. [Google Scholar] [CrossRef][Green Version]
- Zaki, E.F. Impact of adding chia seeds (Salvia hispanica) on the quality properties of camel burger ”Camburger” during cold storage. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1356–1363. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No. 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177. [Google Scholar]
- PN; ISO 1442: 2000. Meat and Meat Products-Determination of Moisture Content (Reference Method); Polish Committee for Standardization: Warsaw, Poland, 2000. (In Polish) [Google Scholar]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurements Guidelines; American Meat Science Association: Savoy, IL, USA, 2012. [Google Scholar]
- Mokrzycki, W.S.; Tatol, M. Color difference ∆E—A survey. In Proceedings of the Machine Graphic & Vision, Warsaw, Poland, 8 October 2012. [Google Scholar]
- Hornsey, H.C. The colour of cooked cured pork. I.—Estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agric. 1959, 7, 534–540. [Google Scholar] [CrossRef]
- ISO. ISO 11290-2:1998—Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes—Part 2—Enumeration Method; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Safaa, A.L.; Rewaa, A.A.M. Quality characteristics of chicken sausage formulated with chia seeds. Suez Canal Univ. J. Food Sci. 2019, 6, 87–96. [Google Scholar]
- Pérez-Álvarez, J.Á.; García-Martín, J.; Roldán-Verdú, A.; Martínez-Mayoral, A.; de Vera, C.N.R.; Sayas-Barberá, E.; Fernández-López, J. Application of chia seed coproduct in dry-cured sausages: Effect upon its physicochemical properties. Multidiscip. Digit. Publ. Inst. Proc. 2020, 70, 87. [Google Scholar]
- Liu, Y.; Wan, Z.; Yohannes, K.W.; Yu, Q.; Yang, Z.; Li, H.; Liu, J.; Wang, J. Functional characteristics of Lactobacillus and yeast single starter cultures in the ripening process of dry fermented sausage. Front. Microbiol. 2021, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Agüero, N.D.L.; Frizzo, L.S.; Ouwehand, A.C.; Aleu, G.; Rosmini, M.R. Technological characterization of probiotic lactic acid bacteria as starter cultures for dry fermented sausages. Foods 2020, 9, 596. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Q. Isolation of Antibacterial, Nitrosylmyoglobin Forming Lactic Acid Bacteria and Their Potential Use in Meat Processing. Front. Microbiol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Deraz, S.F.; Khalil, A.A. A model system for conversion of metmyoglobin to bright red myoglobin derivatives in organic sausages using potential probiotic lactic acid bacteria. S. Asian J. Life Sci. 2018, 6, 22–35. [Google Scholar]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Haouet, M.N. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef]
- Łaszkiewicz, B.; Szymański, P.; Kołożyn-Krajewska, D. The effect of selected lactic acid bacterial strains on the technological and microbiological quality of mechanically separated poultry meat cured with a reduced amount of sodium nitrite. Poul. Sci. 2021, 100, 263–272. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Meloni, D. High-hydrostatic-pressure (HHP) processing technology as a novel control method for Listeria monocytogenes occurrence in Mediterranean-style dry-fermented sausages. Foods 2019, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Akan, S.; Ocak, Ö.Ö. Evaluation of storage time and grape seed extract addition on biogenic amines content of tarhana: A cereal-based fermented food. LWT 2019, 111, 861–868. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Świder, O.; Roszko, M.Ł.; Wójcicki, M.; Szymczyk, K. Biogenic amines and free amino acids in traditional fermented vegetables—Dietary risk evaluation. J. Agric. Food Chem. 2019, 68, 856–868. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Control of biogenic amines in fermented sausages: Role of starter cultures. Front. Microbiol. 2012, 3, 169. [Google Scholar] [CrossRef]
- Kononiuk, A.D.; Karwowska, M. Comparison of selected parameters related to food safety of fallow deer and beef uncured fermented sausages with freeze-dried acid whey addition. Meat Sci. 2020, 161, 108015. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Galgano, F.; Caruso, M.; Condelli, N.; Favati, F. Focused review: Agmatine in fermented foods. Front. Microbiol. 2012, 3, 199. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kim, J.Y.; Kim, J.Y.; Kim, J.H.; Lee, J.E. Therapeutic effect of agmatine on neurological disease: Focus on ion channels and receptors. Neurochem. Res. 2019, 44, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Kononiuk, A.D.; Karwowska, M. Influence of freeze-dried acid whey addition on biogenic amines formation in a beef and deer dry fermented sausages without added nitrite. Asian-Austral. J. Anim. Sci. 2020, 33, 332. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F. Chapter 1: Biogenic amines formation, toxicity, regulations in food. In Biogenic Amines in Food. Analysis, Occurrence and Toxicity; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–17. [Google Scholar]
- Grootveld, M.; Percival, B.C.; Zhang, J. Extensive chemometric investigations of distinctive patterns and levels of biogenic amines in fermented foods: Human health implications. Foods 2020, 9, 1807. [Google Scholar] [CrossRef]
- EFSA. EFSA Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA. Panel Biol. Hazards (BIOHAZ) EFSA J. 2011, 9, 2393. [Google Scholar]

| Parameter | Treatment | Processing Day | |||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 20 | Day 30 | ||
| Water content (%) | SK | 67.09 ± 0.70 aC | 49.66 ± 2.34 aB | 36.28 ± 3.02 aA | 32.87 ± 1.73 aA |
| SCH1 | 66.33 ± 1.37 aC | 50.48 ± 2.22 aB | 35.72 ± 1.39 aA | 33.44 ±1.11 aA | |
| SCH2 | 64.41 ± 1.04 aC | 48.65 ± 1.50 aB | 34.21 ± 0.33 aA | 32.93 ± 1.55 aA | |
| SBC1 | 65.37 ± 0.84 aC | 50.06 ± 1.40 aB | 36.61 ± 0.85 aA | 34.33 ± 0.96 aA | |
| SBC2 | 65.69 ± 1.86 aC | 50.01 ± 0.72 aB | 34.75 ± 0.35 aA | 32.36 ± 1.12 aA | |
| pH | SK | 5.75± 0.03 aC | 5.56 ± 0.03 dB | 5.27 ± 0.08 dA | 5.92 ± 0.04 cD |
| SCH1 | 5.78 ± 0.01 aD | 4.92 ± 0.02 bB | 4.72 ± 0.03 abA | 5.29 ± 0.03 aC | |
| SCH2 | 5.69 ± 0.01 aD | 4.81 ± 0.05 aB | 4.62 ± 0.01 aA | 5.21 ± 0.02 aC | |
| SBC1 | 5.72 ± 0.05 aD | 5.04 ± 0.02 cB | 4.89 ± 0.06 bA | 5.49 ±0.04 bC | |
| SBC2 | 5.74 ± 0.05 aD | 4.94 ± 0.03 bcB | 4.81 ± 0.07 bcA | 5.47 ± 0.11 bC | |
| Water activity | SK | 0.968 ± 0.005 aD | 0.913 ± 0.010 aC | 0.829 ±0.017 aB | 0.801 ± 0.010 aA |
| SCH1 | 0.973 ± 0.003 aD | 0.917 ±0.006 aC | 0.835 ±0.010 aB | 0.802 ±0.001 aA | |
| SCH2 | 0.972 ± 0.006 aD | 0.915 ± 0.002 aC | 0.830 ±0.003 aB | 0.801 ± 0.005 aA | |
| SBC1 | 0.968 ± 0.005 aD | 0.910 ± 0.005 aC | 0.830 ± 0.005 aB | 0.813 ± 0.002 aA | |
| SBC2 | 0.969 ± 0.004 aD | 0.910 ±0.001 aC | 0.831 ±0.001 aB | 0.798 ± 0.012 aA | |
| Parameter | Treatment | Processing Day | |||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 20 | Day 30 | ||
| L* color parameter | SK | 49.24 ± 1.73 aB | 45.58 ± 1.88 aB | 37.44 ± 1.56 aA | 35.61 ± 3.87 aA |
| SCH1 | 47.43 ± 1.53 aAB | 51.99 ± 2.22 bB | 43.41 ± 1.29 abA | 43.08 ± 4.72 bA | |
| SCH2 | 47.95 ± 2.34 aAB | 51.74 ± 0.88 bB | 48.31 ± 2.64 bAB | 43.01 ±3.26 bA | |
| SBC1 | 45.17 ± 1.87 aB | 48.27 ± 2.69 abB | 42.96 ± 3.41 abB | 35.76 ± 1.74 aA | |
| SBC2 | 44.61 ± 1.80 aB | 48.32 ± 1.16 abB | 42.40 ± 6.51 abAB | 37.07 ± 3.59 abA | |
| a* color parameter | SK | 7.78 ± 1.05 abA | 9.34 ± 0.84 bA | 9.32 ± 2.75 bA | 8.03 ± 1.55 bA |
| SCH1 | 8.08 ± 0.80 bA | 9.85 ± 1.11 bA | 9.97 ± 0.57 bA | 9.06 ± 1.16 bA | |
| SCH2 | 9.08 ± 1.35 bA | 10.09 ± 0.75 bA | 7.73 ± 0.54bA | 8.18 ± 0.61bA | |
| SBC1 | 5.85 ± 0.96 aA | 7.69 ± 0.80 abA | 5.73 ± 0.81 abA | 6.61 ± 1.17 abA | |
| SBC2 | 5.55 ± 0.69 aA | 6.47 ± 0.10 aA | 4.42 ± 0.40 aA | 4.45 ± 1.02 aA | |
| b* color parameter | SK | 9.27 ± 0.43 abC | 6.98 ± 0.55 abB | 6.77 ± 1.14 bB | 5.01 ± 0.84 abA |
| SCH1 | 10.13 ± 0.78 bC | 8.08 ± 0.60 bcB | 7.00 ± 1.53 bAB | 5.48 ± 1.07 bA | |
| SCH2 | 10.64 ± 0.46 bC | 9.07 ± 0.46 cB | 5.90 ± 0.60 bA | 6.05 ± 1.32 bA | |
| SBC1 | 7.95 ± 0.75 aC | 6.38 ± 0.74 aB | 3.70 ± 0.31 aA | 4.29 ±0.91 aA | |
| SBC2 | 7.55 ± 0.51 aC | 6.22 ± 0.77 aB | 3.46 ± 0.20 aA | 3.91 ± 1.07 aA | |
| ∆E | SK | ||||
| SCH1 | 3.14 ± 1.12 a | 7.30 ± 2.35 a | 6.91 ± 2.07 a | 9.85 ± 2.13 b | |
| SCH2 | 3.68 ± 1.70 a | 6.66 ± 2.17 a | 11.37 ± 2.64 a | 7.79 ±4.44 ab | |
| SBC1 | 5.21 ± 2.26 a | 4.35 ± 2.03 a | 8.30 ± 2.46 a | 4.67 ± 1.73 a | |
| SBC2 | 5.75 ± 1.14 a | 4.69 ± 1.63 a | 10.63 ± 2.24 a | 5.54 ± 2.63 ab | |
| Nitrosylmyoglobin (mg kg−1) | SK | 11.74 ± 3.56 aA | 13.97 ± 0.85 aA | 25.81 ± 3.97 aB | 28.56 ± 5.04 aB |
| SCH1 | 14.93 ± 2.20 aA | 38.47 ± 0.54 bB | 48.57 ± 2.54 bcC | 56.07 ± 4.65 bC | |
| SCH2 | 15.32 ± 1.19 aA | 40.07 ± 1.01 bBC | 42.68 ± 6.51 bC | 33.54 ± 12.61 aB | |
| SBC1 | 16.57 ± 1.77 aA | 39.34 ± 1.66 bB | 52.49 ± 3.59 cC | 58.24 ± 1.74 bcC | |
| SBC2 | 18.17 ± 1.65 aA | 44.03 ± 1.80 bB | 62.35 ±2.72 dC | 66.55 ±2.63 cC | |
| TBARS (mg kg−1) | SK | 0.64 ± 0.05 aA | 0.77 ± 0.22 aA | 0.87 ± 0.29 aA | 0.95 ± 0.09 aA |
| SCH1 | 0.68 ± 0.06 aA | 0.77 ± 0.14 aAB | 1.38 ±0.10 aC | 1.31 ± 0.14 aBC | |
| SCH2 | 0.70 ± 0.08 aA | 0.98 ± 0.18 aA | 2.24 ± 0.89 bB | 2.53 ± 1.00 bB | |
| SBC1 | 0.74 ± 0.08 aA | 0.90 ± 0.14 aA | 1.15 ± 0.08 aA | 1.03 ± 0.08 aA | |
| SBC2 | 0.79 ± 0.06 aA | 1.11 ± 0.19 aA | 1.11 ± 0.04 aA | 1.19 ± 0.11 aA | |
| Tyramine | Putrescine | Cadaverine | Spermidine | Agmatine | Spermine | |
|---|---|---|---|---|---|---|
| SK | 0.094 ± 0.066 a | 0.048 ± 0.023 a | 0.099 ± 0.051 a | 0.010 ± 0.003 a | 0.105 ± 0.012 | 0.089 ± 0.036 c |
| SCH1 | 0.194 ± 0.080 ab | 0.114 ± 0.058 c | 0.103 ± 0.107 a | 0.010 ± 0.005 a | nd | 0.055 ± 0.028 abc |
| SCH2 | 0.223 ± 0.053 b | 0.147 ±0.009 c | 0.144 ± 0.043 a | 0.016 ± 0.001 b | nd | 0.070 ± 0.003 bc |
| SBC1 | 0.147 ± 0.028 ab | 0.104 ± 0.005 b | 0.120 ± 0.046 a | 1.010 ± 0.001 a | nd | 0.060 ± 0.003 ab |
| SBC2 | 0.166 ± 0.056 ab | 0.108 ± 0.004 b | 0.148 ± 0.066 a | 0.011 ± 0.001 a | nd | 0.059 ± 0.003 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrajo, P.; Karwowska, M.; Stasiak, D.M.; Lorenzo, J.M.; Żyśko, M.; Solska, E. Comparison of the Effect of Enhancing Dry Fermented Sausages with Salvia hispanica and Nigella sativa Seed on Selected Physicochemical Properties Related to Food Safety during Processing. Appl. Sci. 2021, 11, 9181. https://doi.org/10.3390/app11199181
Borrajo P, Karwowska M, Stasiak DM, Lorenzo JM, Żyśko M, Solska E. Comparison of the Effect of Enhancing Dry Fermented Sausages with Salvia hispanica and Nigella sativa Seed on Selected Physicochemical Properties Related to Food Safety during Processing. Applied Sciences. 2021; 11(19):9181. https://doi.org/10.3390/app11199181
Chicago/Turabian StyleBorrajo, Paula, Małgorzata Karwowska, Dariusz M. Stasiak, Jose M. Lorenzo, Marlena Żyśko, and Elżbieta Solska. 2021. "Comparison of the Effect of Enhancing Dry Fermented Sausages with Salvia hispanica and Nigella sativa Seed on Selected Physicochemical Properties Related to Food Safety during Processing" Applied Sciences 11, no. 19: 9181. https://doi.org/10.3390/app11199181
APA StyleBorrajo, P., Karwowska, M., Stasiak, D. M., Lorenzo, J. M., Żyśko, M., & Solska, E. (2021). Comparison of the Effect of Enhancing Dry Fermented Sausages with Salvia hispanica and Nigella sativa Seed on Selected Physicochemical Properties Related to Food Safety during Processing. Applied Sciences, 11(19), 9181. https://doi.org/10.3390/app11199181









