Synthesis of Proposed Structure of Aaptoline B via Transition Metal-Catalyzed Cycloisomerization and Evaluation of Its Neuroprotective Properties in C. Elegans
Abstract
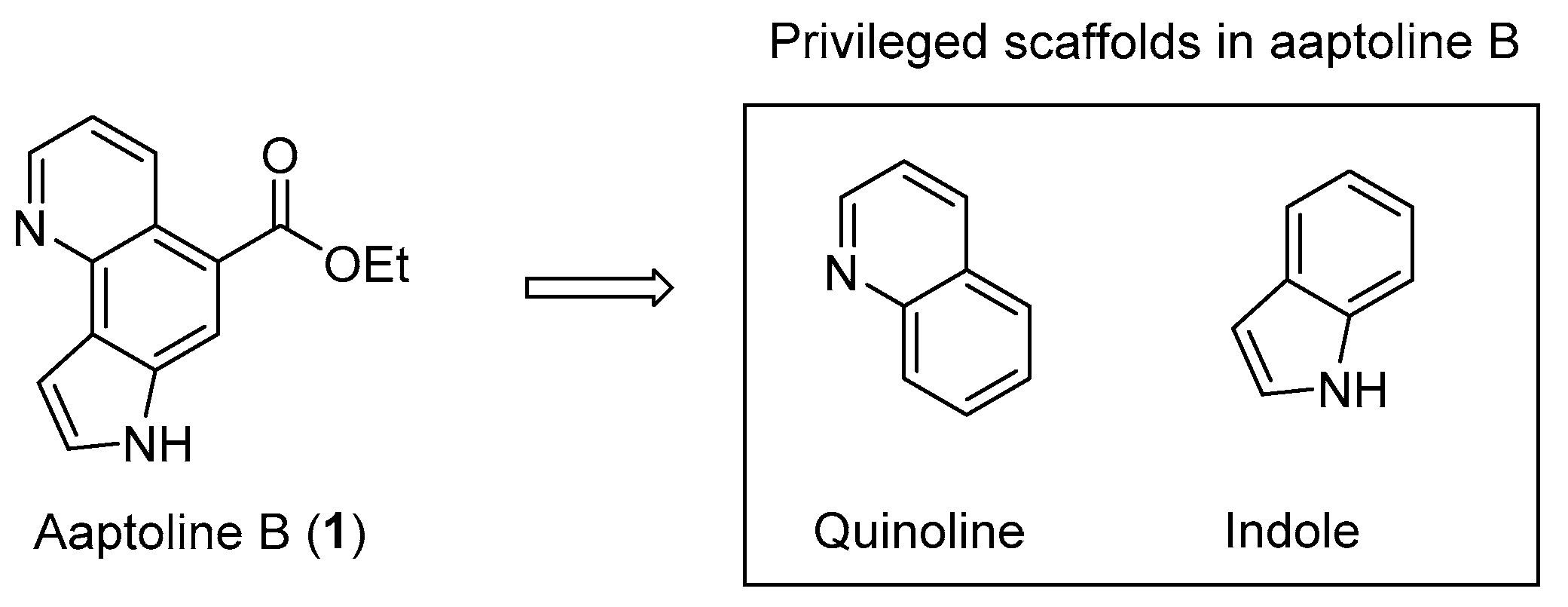
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Experimental
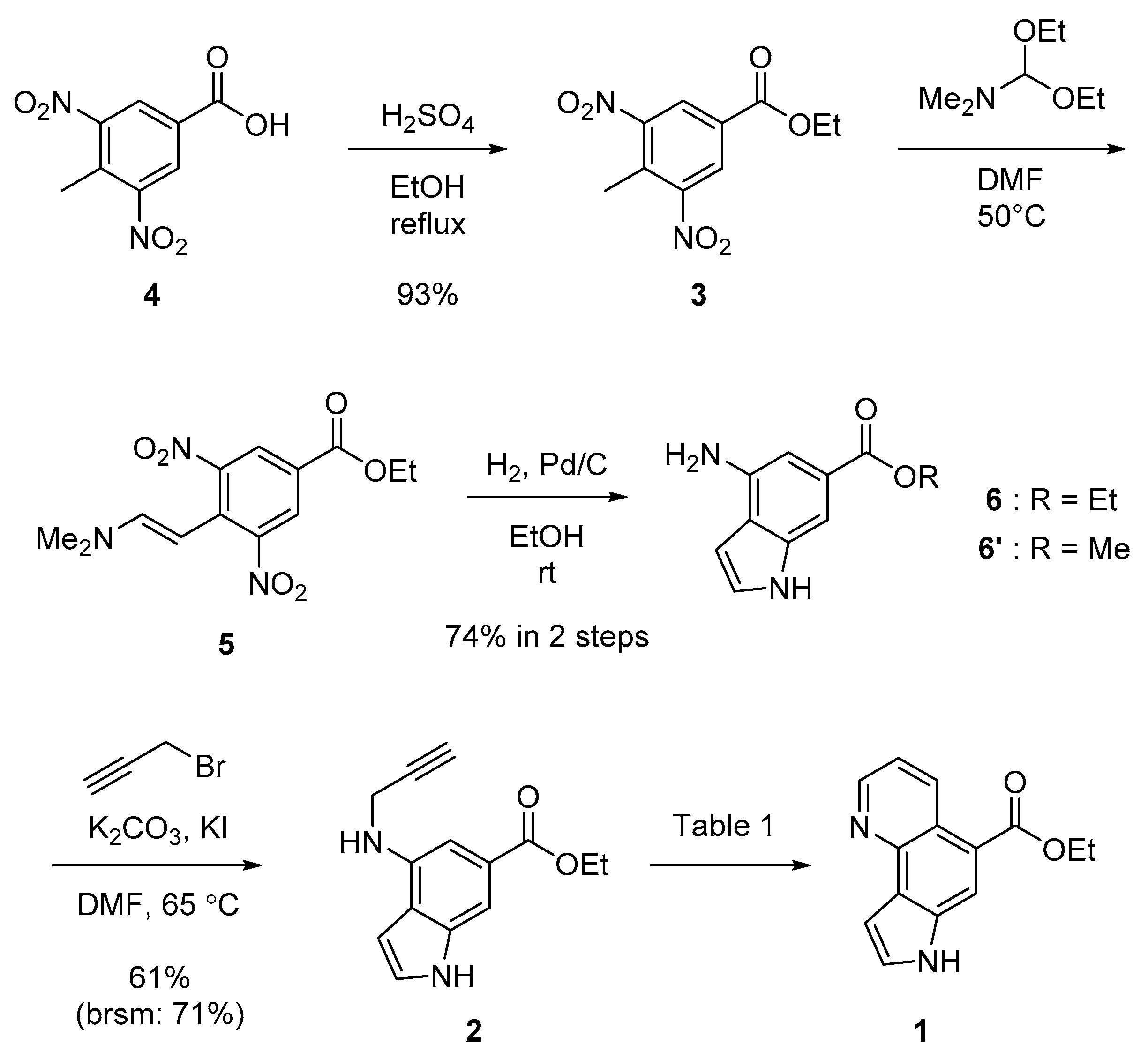
2.1.2. Ethyl 4-methyl-3,5-dinitrobenzoate (3)
2.1.3. Ethyl 4-amino-1H-indole-6-carboxylate (6)
2.1.4. Ethyl 4-(prop-2-yn-1-ylamino)-1H-indole-6-carboxylate (2)
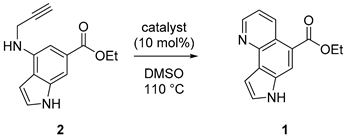
2.1.5. Representative Procedure of Transition Metal-Catalyzed Cycloisomerization for Ethyl 7H-pyrrolo [2,3-h]quinoline-5-carboxylate (Aaptoline B; 1)
2.2. Biology
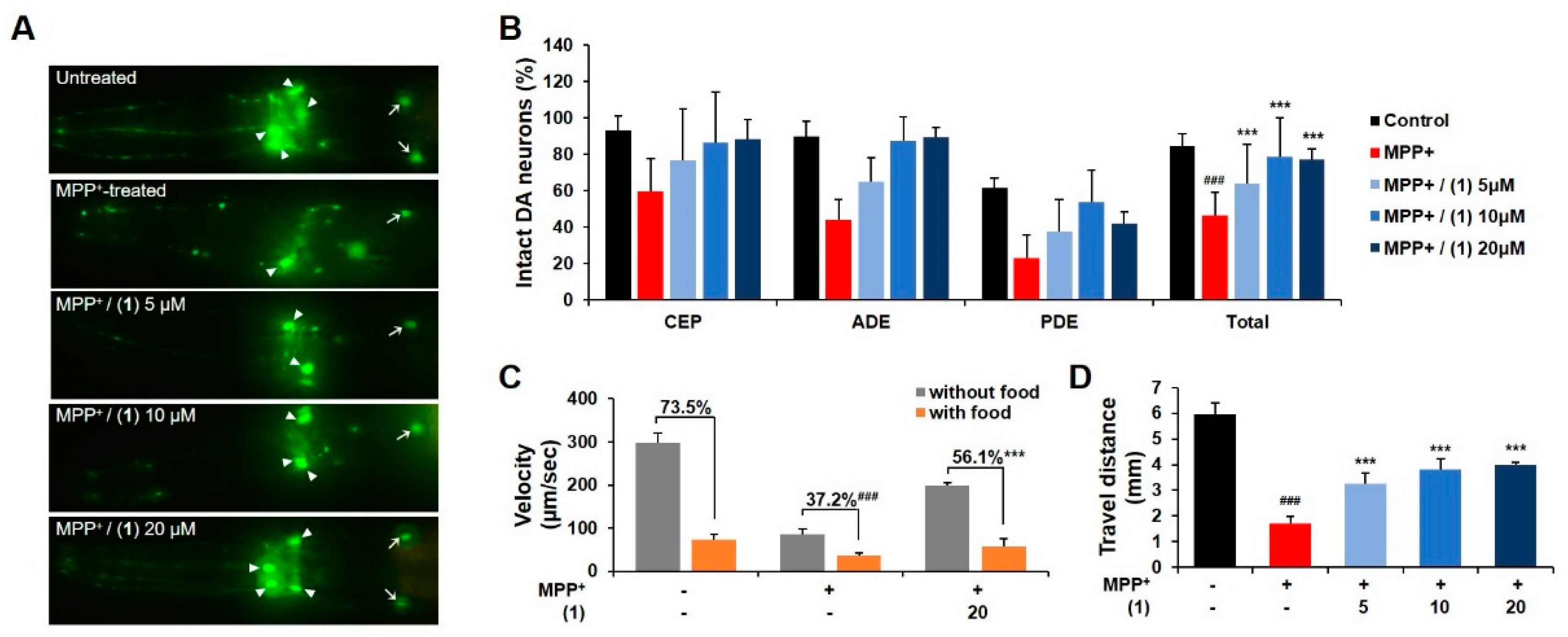
2.2.1. C. Elegans Maintenance and MPP+ Treatment
2.2.2. Fluorescence Microscopy and Visualization
2.2.3. Behavioral Assay
2.2.4. Statistical Analysis
3. Results and Discussion
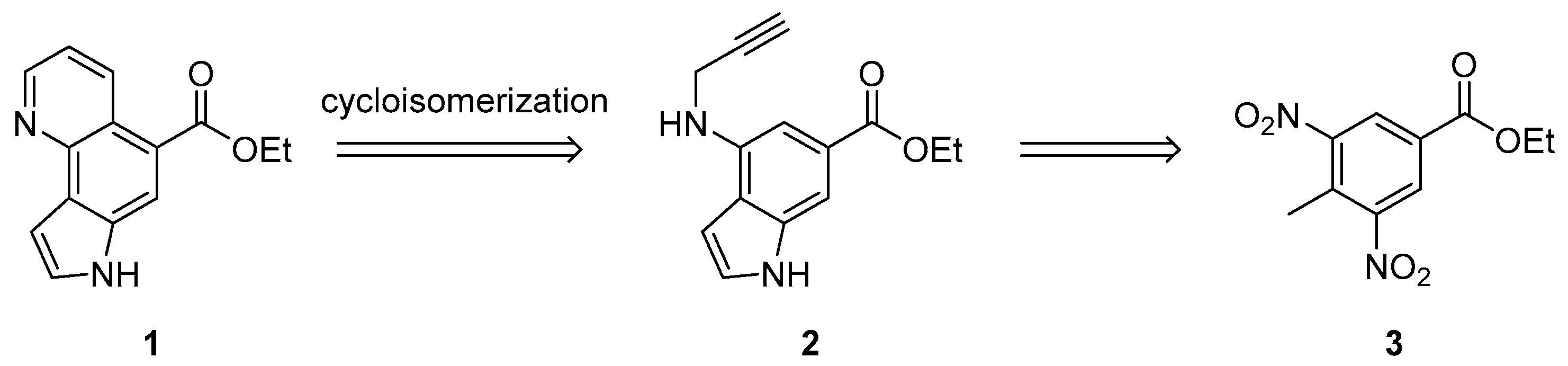
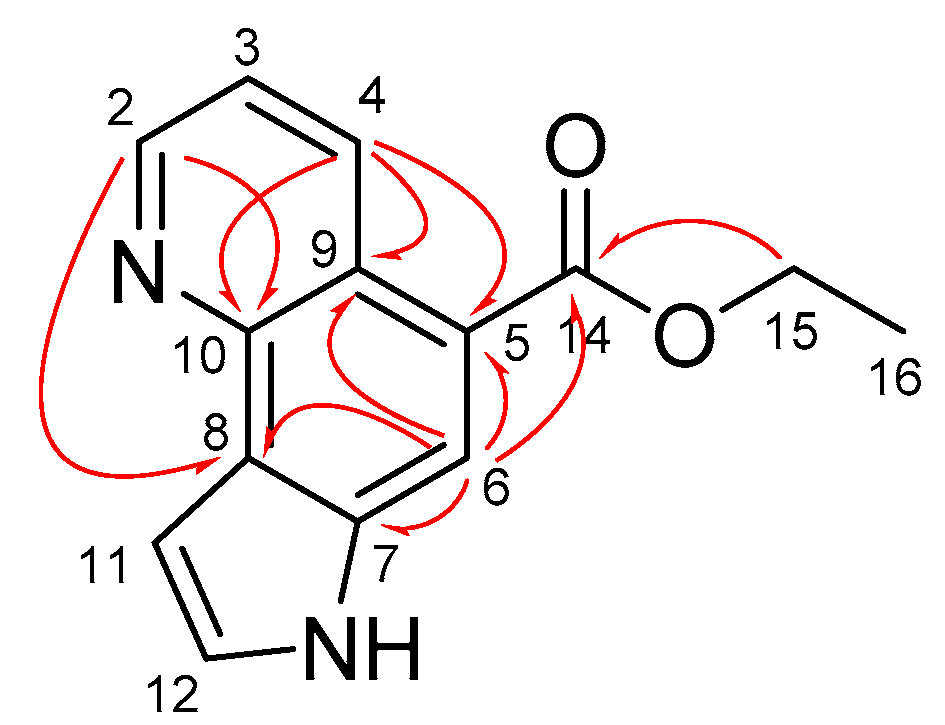
3.1. Synthesis and Structure Elucidation of Aaptoline B
3.2. Evaluation of Neuroprotective Potential of Aaptoline B
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nass, R.; Hall, D.H.; Miller, D.M.; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.Z.; Yu, H.B.; Lu, J.R.; Lin, H.W.; Sun, F.; Wang, S.P.; Yang, F. Aaptolines A and B, two new quinoline alkaloids from the marine sponge Aaptos aaptos. Chem. Biodivers. 2020, 17, e2000074. [Google Scholar] [CrossRef] [PubMed]
- de Sá Alves, F.R.; Barreiro, E.J.; Fraga, C.A. From nature to drug discovery: The indole scaffold as a ‘privileged structure’. Mini Rev. Med. Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Bongarzone, S.; Bolognesi, M.L. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Discov. 2011, 6, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Yoon, J.A.; Han, Y.T. Total synthesis of the natural pyridocoumarins goniothaline A and B. Synthesis 2019, 51, 552–556. [Google Scholar]
- Yoon, J.A.; Lim, C.; Han, Y.T. Preliminary study on novel expedient synthesis of 5-azaisocoumarins by transition metal-catalyzed cycloisomerization. Front. Chem. 2020, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.A.; Han, Y.T. Efficient synthesis of pyrido [3, 2-c] coumarins via silver nitrate catalyzed cycloisomerization and application to the first synthesis of polyneomarline C. Synthesis 2019, 51, 4611–4618. [Google Scholar] [CrossRef]
- Pickaert, G.; Ziessel, R. Synthesis of oligopyridinic scaffolds from amido substituted phenyl rings for extended hydrogen bonding. Synthesis 2004, 2004, 2716–2726. [Google Scholar]
- Li, J.J. Name Reactions in Heterocyclic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 104–109. [Google Scholar]
- Demont, H.E.; Faller, A.; MacPherson, D.T.; Milner, P.H.; Naylor, A.; Redshaw, S.; Stanway, S.J.; Vesey, R.D.; Walter, D.S. Preparation of Hydroxyethylamine Derivatives for the Treatment of Alzheimer’s Disease. WO 2004050619A1, 17 June 2004. [Google Scholar]
- Zhao, Y.; Cai, L.; Huang, T.; Meng, S.; Chan, A.S.; Zhao, J. Solvent-mediated C3/C7 regioselective switch in chiral phosphoric acid-catalyzed enantioselective Friedel-Crafts alkylation of indoles with α-ketiminoesters. Adv. Synth. Catal. 2020, 362, 1309–1316. [Google Scholar] [CrossRef]
- Joule, J.A. Product Class 13: Indole and Its Derivatives; Thieme: Stuttgart, Germany, 2001; Volume 10, pp. 361–652. [Google Scholar]
- da Rosa Monte Machado, G.; Diedrich, D.; Ruaro, T.C.; Zimmer, A.R.; Lettieri Teixeira, M.; de Oliveira, L.F.; Jean, M.; Van de Weghe, P.; de Andrade, S.F.; Baggio Gnoatto, S.C.; et al. Quinolines derivatives as promising new antifungal candidates for the treatment of candidiasis and dermatophytosis. Braz. J. Microbiol. 2020, 51, 1691–1701. [Google Scholar] [CrossRef]
- Lloyd, L.S.; Adams, R.W.; Bernstein, M.; Coombes, S.; Duckett, S.B.; Green, G.G.; Lewis, R.J.; Mewis, R.E.; Sleigh, C.J. Utilization of SABRE-derived hyperpolarization to detect low-concentration analytes via 1D and 2D NMR methods. J. Am. Chem. Soc. 2012, 134, 12904–12907. [Google Scholar] [CrossRef]
- Krzymiński, K.; Malecha, P.; Zadykowicz, B.; Wróblewska, A.; Błażejowski, J. 1H and 13C NMR spectra, structure and physicochemical features of phenyl acridine-9-carboxylates and 10-methyl-9-(phenoxycarbonyl)acridinium trifluoromethanesulphonates–alkyl substituted in the phenyl fragment. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 78, 401–409. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Entry 1 | Catalyst | Time (h) | Yield 2 (%) |
| 1 | CuI | 2 | 56 |
| 2 | InCl3 | 27 | 18 |
| 3 | AuCl | 8 | 27 |
| 4 | AgSbF6 | 4.5 | 73 |
| 5 | AgNO3 | 5 | 61 |
| 6 | AgOTf | 5 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Yang, W.; Han, Y.-T.; Cha, D.-S. Synthesis of Proposed Structure of Aaptoline B via Transition Metal-Catalyzed Cycloisomerization and Evaluation of Its Neuroprotective Properties in C. Elegans. Appl. Sci. 2021, 11, 9125. https://doi.org/10.3390/app11199125
Kim S, Yang W, Han Y-T, Cha D-S. Synthesis of Proposed Structure of Aaptoline B via Transition Metal-Catalyzed Cycloisomerization and Evaluation of Its Neuroprotective Properties in C. Elegans. Applied Sciences. 2021; 11(19):9125. https://doi.org/10.3390/app11199125
Chicago/Turabian StyleKim, Soobin, Wooin Yang, Young-Taek Han, and Dong-Seok Cha. 2021. "Synthesis of Proposed Structure of Aaptoline B via Transition Metal-Catalyzed Cycloisomerization and Evaluation of Its Neuroprotective Properties in C. Elegans" Applied Sciences 11, no. 19: 9125. https://doi.org/10.3390/app11199125
APA StyleKim, S., Yang, W., Han, Y.-T., & Cha, D.-S. (2021). Synthesis of Proposed Structure of Aaptoline B via Transition Metal-Catalyzed Cycloisomerization and Evaluation of Its Neuroprotective Properties in C. Elegans. Applied Sciences, 11(19), 9125. https://doi.org/10.3390/app11199125





