18 Years of Medication-Related Osteonecrosis of the Jaw (MRONJ) Research: Where Are We Now?—An Umbrella Review
Abstract
:1. Introduction
- (1)
- Current or previous treatment with antiresorptive or antiangiogenic agents; exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for longer than 8 weeks;
- (2)
- No history of radiation therapy to the jaws or obvious metastatic disease to the jaws [8].
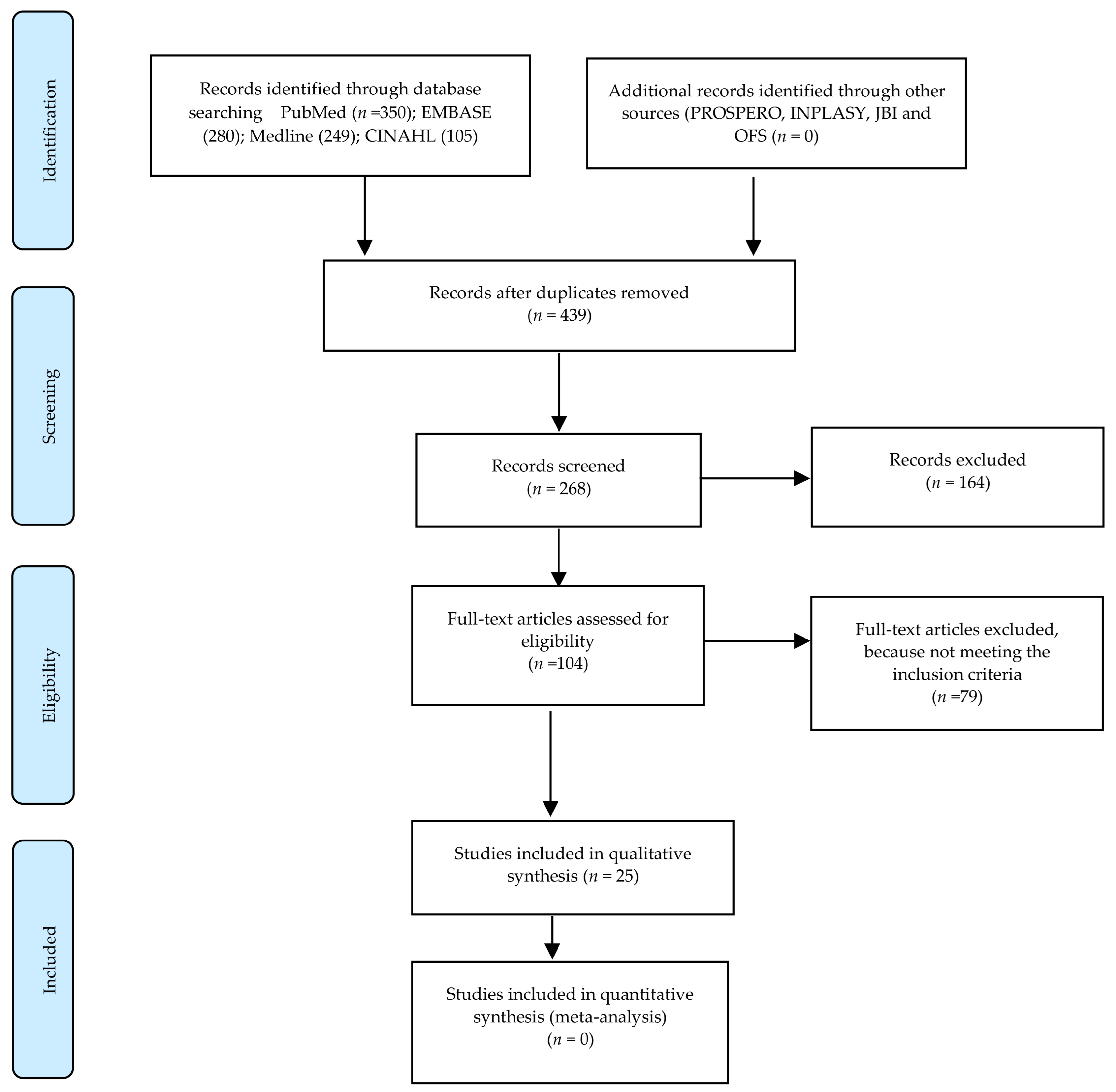
2. Materials and Methods
- Verify the study eligibility derived from the inclusion/exclusion criteria. Carry out the methodological quality assessment.
- Extract data on study characteristics and outcomes for the included studies (Figure 1).
- -
- Population (P): any (no limits of age) patients with MRONJ;
- -
- Interventions (I): any types;
- -
- Comparison (C): any types;
- -
- Outcome (O): state of knowledge based on the type of studies included in the reviews;
- -
- Study (S): systematic review (SR) or meta-analysis (MA).
- Osteonecrosis [MeSH Terms] OR Avascular osteonecrosis of the jaw [MeSH Terms] OR Osteonecrosis of the jaw [MeSH Terms] OR MRONJ [MeSH Terms] OR ONJ [MeSH Terms] OR BONJ [MeSH Terms] OR ARONJ [MeSH Terms] OR BRONJ Patients [MeSH Terms] OR Any patients [MeSH Terms] OR Oncology [MeSH Terms] OR Osteoporosis [MeSH Terms] OR Non-oncologic patients;
- Systematic review [MeSH Terms] OR Review [MeSH Terms] OR Meta-analysis;
- 1 and 2 and 3.
2.1. Criteria for Inclusion in This Review
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Outcomes Measured
- (a)
- Primary outcomeEvaluate the current state of knowledge regarding the medication-related osteonecrosis of the jaw as it relates with non-interventional type of studies and interventional type of studies, as well as the trends (number of SR and MA) per year.
- (b)
- Secondary outcomeEvaluate factors such as:
- Type of studies included in the reviews;
- Number of patients included in the review;
- Patients’ demographic;
- Type of patient groups (Oncology vs. Non-oncology).
2.1.4. Data Extracted
2.1.5. Review Quality Assessment Criteria
- (1)
- The methodological limitations of the individual qualitative studies contributing to a review finding;
- (2)
- The coherence of the review finding;
- (3)
- The adequacy of data supporting a review finding;
- (4)
- The relevance of the data from the primary studies supporting a review finding to the context (perspective or population, phenomenon of interest and/or setting) specified in the review question [65].
3. Results
4. Review Quality Assessment
- Methodological limitations: The extent to which there are problems in the design or conduct of the primary studies that contributed evidence to a review finding.
- Relevance: The extent to which the body of evidence from the primary studies supporting a review finding is applicable to the context (perspective or population, phenomenon of interest, setting) specified in the review question.
- Coherence: The extent to which the review finding is well grounded in data from the contributing primary studies and provides a convincing explanation for the patterns found in these data.
- Adequacy of data: An overall determination of the degree of richness and quantity of data supporting a review finding.
- High confidence: It is highly likely that the review finding is a reasonable representation of the phenomenon of interest.
- Moderate confidence: It is likely that the review finding is a reasonable representation of the phenomenon of interest.
- Low confidence: It is possible that the review finding is a reasonable representation of the phenomenon of interest.
- Very low confidence: It is not clear whether the review finding is a reasonable representation of the phenomenon of interest.
- There were minor concerns with respect to the relevance and coherence of the epidemiological type of reviews in all studies. Moderate concerns were noted regarding the methodological limitations in eight studies and serious concerns were highlighted for similar limitations in four studies. Serious concerns were also noted for the adequacy of data of their results in all of the included studies. Due to the high number of serious concerns, particularly regarding methodology and result data, the overall assessment was assessed as very low confidence (lack of clarity whether the review finding is a reasonable representation of the phenomenon of interest).
- Minor concerns were highlighted with respect to the relevance and coherence of management types of reviews. Moderate concerns were raised regarding methodological limitations in three studies and serious concerns in methodological limitations in one study. Serious concerns were raised for one study regarding the adequacy of data in the results. Due to these concerns, the overall assessment regarding the management type was graded as very low confidence (lack of clarity whether the review finding is a reasonable representation of the phenomenon of interest).
- With respect to predicting markers, the reviews were graded as having minor concerns for relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding methodological limitations and adequacy of the result data. Due to these concerns, the overall assessment regarding predicting markers was rated as having very low confidence (lack of clarity whether the review finding is a reasonable representation of the phenomenon of interest).
- Relating to dental rehabilitation, reviews were graded with minor concerns for relevance, moderate concerns regarding methodological limitations and coherence, and serious concerns regarding adequacy of result data. This domain again was graded as having very low confidence (lack of clarity whether the review finding is a reasonable representation of the phenomenon of interest).
- Regarding preventive strategy, reviews demonstrated minor concerns for relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding adequacy of data results. The overall assessment for preventative strategy was rated as very low confidence (lack of clarity whether the review finding is a reasonable representation of the phenomenon of interest).
- Finally, when assessing the diagnostic investigations within the reviews, this review identified minor concerns regarding relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding adequacy of the data results. Therefore, with regard to the overall assessment, this domain was graded as very low confidence (lack of clarity whether the reviews found a reasonable representation of the phenomenon of interest).
5. Discussion
- Sample size calculation should be established and employed for all the RCTs. Large RCTs should be carried out and described in sufficient detail to allow precise assessment, management and/or epidemiological findings.
- Risk stratification should be applied for any clinical studies in order to minimize the effect modification and/or confounding factors that could potentially affect the final result/s.
- Common, quantifiable and clinically relevant endpoints (time to complete wound healing, pain, specific investigations, treatment acceptability and participant satisfaction) should be described in sufficient detail particularly in patients undergoing to any type of intervention including preventive strategies.
- An adequate follow-up period is essential if MRONJ treatment or preventive strategy is studied in order to evaluate the long-term effects on this group of patients.
- A predictable special investigation, such as CT, CBCT or MRI should be used for any of the observational and interventional studies at diagnosis and during the follow-up.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the international recommendations for management from the international task force on ONJ. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patología Oral y Cirugia Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25, iii124–iii137. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; McClung, M.R.; Davison, K.S.; Dian, L.; Harris, S.T.; Miller, P.D.; Lewiecki, E.M.; Kendler, D.L. Western Osteoporosis Alliance Clinical Practice Series: Evaluating the Balance of Benefits and Risks of Long-Term Osteoporosis Therapies. Am. J. Med. 2017, 130, 862.e1–862.e7. [Google Scholar] [CrossRef]
- Marx, E.R. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral. Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Sacco, R.; Shah, S.; Leeson, R.; Moraschini, V.; Mourão, C.D.A.B.; Akintola, O.; Lalli, A. Osteonecrosis and osteomyelitis of the jaw associated with tumour necrosis factor-alpha (TNF-α) inhibitors: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Sacco, R.; Ball, R.; Barry, E.; Akintola, O. The role of illicit drugs in developing medication-related osteonecrosis (MRONJ): A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, 398–406. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Fedele, S.; Porter, S.; D’Aiuto, F.; Aljohani, S.; Vescovi, P.; Manfredi, M.; Arduino, P.G.; Broccoletti, R.; Musciotto, A.; di Fede, O.; et al. Nonexposed Variant of Bisphosphonate-associated Osteonecrosis of the Jaw: A Case Series. Am. J. Med. 2010, 123, 1060–1064. [Google Scholar] [CrossRef] [Green Version]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac. Surg. Clin. North Am. 2015, 27, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, J.S.; Zhou, J.; Kuo, Y.-F.; Baillargeon, J. Risk of Jaw Osteonecrosis after Intravenous Bisphosphonates in Cancer Patients and Patients without Cancer. Mayo Clin. Proc. 2017, 92, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of Osteonecrosis of the Jaw in Patients with Oral Bisphosphonate Exposure. J. Oral Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Brown, J.E.; Van Poznak, C.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef]
- Tennis, P.; Rothman, K.J.; Bohn, R.L.; Tan, H.; Zavras, A.; Laskarides, C.; Calingaert, B.; Anthony, M.S. Incidence of osteonecrosis of the jaw among users of bisphosphonates with selected cancers or osteoporosis. Pharmacoepidemiol. Drug Saf. 2012, 21, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Van den Wyngaert, T.; Wouters, K.; Huizing, M.T.; Vermorken, J.B. RANK ligand inhibition in bone metastatic cancer and risk of oste-onecrosis of the jaw (ONJ): Non bis in idem? Support Care Cancer 2011, 19, 2035–2040. [Google Scholar] [CrossRef]
- Christodoulou, C.; Pervena, A.; Klouvas, G.; Galani, E.; Falagas, M.E.; Tsakalos, G.; Visvikis, A.; Nikolakopoulou, A.; Acholos, V.; Karapanagiotidis, G.; et al. Combination of bisphosphonates and antiangiogenic factors induces oste-onecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009, 76, 209–211. [Google Scholar] [CrossRef]
- Al-Husein, B.; Abdalla, M.; Trepte, M.; DeRemer, D.L.; Somanath, P.R. Antiangiogenic Therapy for Cancer: An Update. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 1095–1111. [Google Scholar] [CrossRef] [Green Version]
- Diniz-Freitas, M.; Limeres, J. Prevention of medication-related osteonecrosis of the jaws secondary to tooth extractions. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e250–e259. [Google Scholar] [CrossRef]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Ficarra, G.; Beninati, F. Bisphosphonate—Related osteonecrosis of the jaws: The point of view of the oral pathologist. Clin. Cases Miner. Bone Metab. 2007, 4, 53–57. [Google Scholar] [PubMed]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Stürzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws—Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Cranio-Maxillofac. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef]
- Mena, A.C.; Pulido, E.G.; Ponce, C.G. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: Sunitinib. Anti-Cancer Drugs 2010, 21, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Fung, P.; Nicoletti, P.; Shen, Y.; Porter, S.; Fedele, S. Pharmacogenetics of Bisphosphonate-associated Osteonecrosis of the Jaw. Oral Maxillofac. Surg. Clin. North Am. 2015, 27, 537–546. [Google Scholar] [CrossRef]
- Khan, A.A.; Sándor, G.K.B.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Dempster, D.W.; et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2008, 35, 1391–1397. [Google Scholar]
- Dickinson, M.; Prince, H.M.; Kirsa, S.; Zannettino, A.; Gibbs, S.D.J.; Mileshkin, L.; O’Grady, J.; Seymour, J.F.; Szer, J.; Horvath, N.; et al. Osteonecrosis of the jaw complicating bisphosphonate treatment for bone disease in multiple myeloma: An overview with recommendations for prevention and treatment. Intern. Med. J. 2009, 39, 304–316. [Google Scholar] [CrossRef]
- Terpos, E.; Sezer, O.; Croucher, P.; Garcia-Sanz, R.; Boccadoro, M.; Miguel, J.S.; Ashcroft, J.; Bladé, J.; Cavo, M.; Delforge, M.; et al. The use of bisphosphonates in multiple myeloma: Recommendations of an expert panel on behalf of the European Myeloma Network. Ann. Oncol. 2009, 20, 1303–1317. [Google Scholar] [CrossRef]
- McLeod, N.; Davies, B.; Brennan, P. Management of patients at risk of bisphosphonate osteonecrosis in maxillofacial surgery units in the UK. Surg. 2009, 7, 18–23. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Adler, R.A.; Edwards, B.; Jacobsen, P.L.; Kalmar, J.R.; Koka, S.; Migliorati, C.A.; Ristic, H. American Dental Association Council on Scientific Affairs Expert Panel on Antiresorptive Agents. Managing the care of patients receiving an-tiresorptive therapy for prevention and treatment of osteoporosis: Executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2011, 142, 1243–1251. [Google Scholar]
- Snowden, J.A.; Ahmedzai, S.H.; Ashcroft, J.; D’Sa, S.; Littlewood, T.; Low, E.; Lucraft, H.; Maclean, R.; Feyler, S.; Pratt, G.; et al. Haemato-oncology Task Force of British Committee for Standards in Haematology and UK Myeloma Forum. Guidelines for supportive care in multiple myeloma. Br. J. Haematol. 2011, 154, 76–103. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Rhee, Y.; Kwon, Y.-D.; Kwon, T.-G.; Lee, J.K.; Kim, D.-Y. Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons. J. Bone Metab. 2015, 22, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Svejda, B.; Muschitz, C.; Gruber, R.; Brandtner, C.; Svejda, C.; Gasser, R.W.; Santler, G.; Dimai, H.P. Position paper on med-ication-related osteonecrosis of the jaw (MRONJ). Wien. Med. Wochenschr. 2016, 166, 68–74. [Google Scholar] [CrossRef]
- Japanese Allied Committee on Osteonecrosis of the Jaw; Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Nagata, T.; Urade, M.; et al. Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J. Bone Miner. Metab. 2016, 35, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Scottish Dental Clinical Effectiveness Programme. Oral health management of patients at risk of medication-related osteone-crosis of the jaw. Br. Dent. J. 2017, 222, 930. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Calasans-Maia, M.D.; Louro, R.S.; Arantes, E.B.; Calasans-Maia, J.D. Weak evidence for the management of medication-related osteone-crosis of the jaw: An overview of systematic reviews and meta-analyses. J. Oral. Pathol. Med. 2021, 50, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Aljohani, S.; Fliefel, R.; Ihbe, J.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: A systematic review. J. Cranio-Maxillofac. Surg. 2017, 45, 1493–1502. [Google Scholar] [CrossRef]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H. Interventions for managing medication-related osteonecrosis of the jaw (MRONJ). Cochrane Database Syst. Rev. 2016, 6, CD012432. [Google Scholar] [CrossRef]
- Cabras, M.; Gambino, A.; Broccoletti, R.; Sciascia, S.; Arduino, P.G. Lack of evidence in reducing risk of MRONJ after teeth extractions with systemic antibiotics. J. Oral Sci. 2021, 63, 217–226. [Google Scholar] [CrossRef]
- Prá, K.D.; Lemos, C.; Okamoto, R.; Soubhia, A.; Pellizzer, E. Efficacy of the C-terminal telopeptide test in predicting the development of bisphosphonate-related osteonecrosis of the jaw: A systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Duarte, N.T.; Rech, B.D.O.; Martins, I.G.; Franco, J.B.; Ortega, K.L. Can children be affected by bisphosphonate-related osteonecrosis of the jaw? A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 183–191. [Google Scholar] [CrossRef]
- Gelazius, R.; Poskevicius, L.; Sakavicius, D.; Grimuta, V.; Juodzbalys, G. Dental implant placement in patients on bisphosphonate therapy: A systematic review. J. Oral. Maxillofac. Res. 2018, 9, e2. [Google Scholar] [CrossRef]
- Govaerts, D.; Piccart, F.; Ockerman, A.; Coropciuc, R.; Politis, C.; Jacobs, R. Adjuvant therapies for MRONJ: A systematic review. Bone 2020, 141, 115676. [Google Scholar] [CrossRef]
- Hennedige, A.A.; Jayasinghe, J.; Khajeh, J.; Macfarlane, T.V. Systematic Review on the Incidence of Bisphosphonate Related Osteonecrosis of the Jaw in Children Diagnosed with Osteogenesis Imperfecta. J. Oral Maxillofac. Res. 2013, 4, e1. [Google Scholar] [CrossRef]
- Hess, L.M.; Jeter, J.M.; Benham-Hutchins, M.; Alberts, D.S. Factors Associated with Osteonecrosis of the Jaw among Bisphosphonate Users. Am. J. Med. 2008, 121, 475–483.e3. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Chamorro-Petronacci, C.; Vila, P.G.; López-Jornet, P.; Carballo, J.; García-García, A. Association between periodontitis and medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Oral Pathol. Med. 2020, 49, 190–200. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; González-Palanca, S.; Chamorro-Petronacci, C.; Bagán, J.; García-García, A. Bi-omarkers to predict the onset of biphosphonate-related osteonecrosis of the jaw: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2019, 24, e26–e36. [Google Scholar] [PubMed]
- Madrid, C.; Sanz, M. What impact do systemically administrated bisphosphonates have on oral implant therapy? A system-atic review. Clin. Oral. Implants Res. 2009, 4, 87–95. [Google Scholar] [CrossRef]
- Mauri, D.; Valachis, A.; Polyzos, I.P.; Polyzos, N.P.; Kamposioras, K.; Pesce, L.L. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: A meta-analysis. Breast Cancer Res. Treat. 2009, 116, 433–439. [Google Scholar] [CrossRef] [Green Version]
- McGowan, K.; McGowan, T.; Ivanovski, S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018, 24, 527–536. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Woo, S.B.; Hewson, I.; Barasch, A.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T. Bisphosphonate Osteonecrosis Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of bisphosphonate osteonecrosis (BON) in cancer. Support Care Cancer 2010, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, C.; Schiodt, M.; Gotfredsen, K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medica-tion-related osteonecrosis of the jaw (MRONJ): A systematic review. Heliyon 2020, 6, e03795. [Google Scholar] [CrossRef]
- Palaska, P.K.; Cartsos, V.; Zavras, A.I. Bisphosphonates and time to osteonecrosis development. Oncologist 2009, 14, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Querrer, R.; Ferrare, N.; Melo, N.; Stefani, C.M.; Dos Reis, P.E.D.; Mesquita, C.R.M.; Borges, G.A. Differ-ences between bisphosphonate-related and denosumab-related osteonecrosis of the jaws: A systematic review. Support Care Cancer 2021, 29, 2811–2820. [Google Scholar] [CrossRef]
- Rollason, V.; Laverrière, A.; MacDonald, L.C.; Walsh, T.; Tramèr, M.R.; Vogt-Ferrier, N.B. Interven-tions for treating bisphosphonate-related osteonecrosis of the jaw (BRONJ). Cochrane Database Syst. Rev. 2016, 2, CD008455. [Google Scholar]
- Sacco, R.; Woolley, J.; Yates, J.; Calasans-Maia, M.D.; Akintola, O.; Patel, V. The role of antiresorptive drugs and medica-tion-related osteonecrosis of the jaw in nononcologic immunosuppressed patients: A systematic review. J. Res. Med. Sci. 2021, 26, 23. [Google Scholar] [CrossRef]
- Sacco, R.; Woolley, J.; Yates, J.; Calasans-Maia, M.D.; Akintola, O.; Patel, V. A systematic review of metastatic cancer pre-senting in osteonecrosis of the jaws (MC-ONJ) in patients undergoing antiresorptive and/or antiangiogenic therapy for skele-tal-related adverse events. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 131, 650–659. [Google Scholar] [CrossRef]
- Sacco, R.; Woolley, J.; Patel, G.; Calasans-Maia, M.D.; Yates, J. A systematic review of medication related osteonecrosis of the jaw (MRONJ) in patients undergoing only antiangiogenic drug therapy: Surgery or conservative therapy? Br. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- de Souza Tolentino, E.; de Castro, T.F.; Michellon, F.C.; Passoni, A.C.C.; Ortega, L.J.A.; Iwaki, L.C.V.; da Silva, M.C. Adju-vant therapies in the management of medication-related osteonecrosis of the jaws: Systematic review. Head Neck 2019, 41, 4209–4228. [Google Scholar] [CrossRef]
- Woo, S.-B.; Hellstein, J.W.; Kalmar, J.R. Systematic Review: Bisphosphonates and Osteonecrosis of the Jaws. Ann. Intern. Med. 2006, 144, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolley, J.; Akintola, O.; Yates, J.; Calasans-Maia, M.D.; Calasans-Maia, J.D.A.; Kocherhina, I.; Sacco, R. The risk of osteonecrosis of the jaw and adverse outcomes in patients using antiresorptive drugs undergoing orthodontic treatment: A systematic review. Heliyon 2021, 7, e05914. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Glenton, C.; Munthe-Kaas, H.; Carlsen, B.; Colvin, C.J.; Gülmezoglu, M.; Noyes, J.; Booth, A.; Garside, R.; Ra-shidian, A. Using qualitative evidence in decision making for health and social interventions: An approach to assess confi-dence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med. 2015, 12, e1001895. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Tanna, N.; Patel, V. Medication-related osteonecrosis of the jaw unrelated to bisphosphonates and denosumab—A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 289–299. [Google Scholar] [CrossRef]
- Wat, W.Z.M. Current Controversies on the Pathogenesis of Medication-Related Osteonecrosis of the Jaw. Dent. J. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxford Centre for Evidence-Based Medicine. 2009. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 1 June 2021).
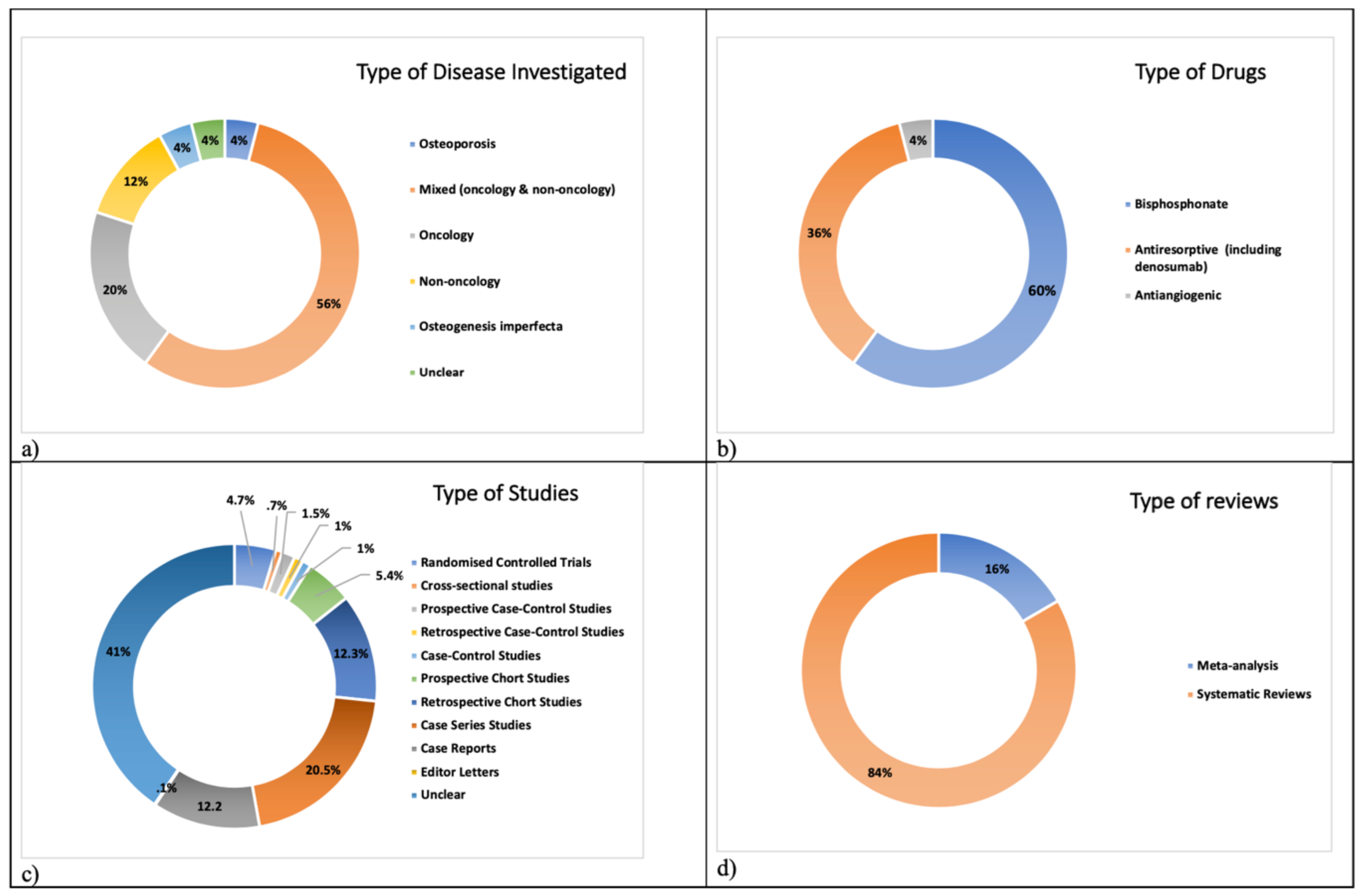
| Guideline/Position Paper/Recommendation | Country | Year |
|---|---|---|
| Canadian Consensus of Practice Guidelines for Bisphosphonate Associated Osteonecrosis of the Jaw [26] | Canada | 2008 |
| Osteonecrosis of the Jaw Complicating Bisphosphonate Treatment for Bone Disease in Multiple Myeloma: An Overview with Recommendations for Prevention and Treatment [27] | Australia | 2009 |
| The Use of Bisphosphonates in Multiple Myeloma: Recommendations of an Expert Panel on Behalf of the European Myeloma Network [28] | Europe | 2009 |
| Management of Patients at Risk of Bisphosphonate Osteonecrosis in Maxillofacial Surgery Units in the UK [29] | UK | 2009 |
| Managing the Care of Patients Receiving Antiresorptive Therapy for Prevention and Treatment of Osteonecrosis. Executive Summary of Recommendations from the American Dental Association Council on Scientific Affairs [30] | USA | 2011 |
| Guidelines for Supportive Care in Multiple Myeloma 2011 [31] | UK | 2011 |
| Medication-Related Osteonecrosis of the Jaw-2014 Update [8] | USA | 2014 |
| Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus [32] | Canada | 2014 |
| Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons [33] | Korea | 2015 |
| “Position paper on medication-related osteonecrosis of the jaw (MRONJ)” [34] | Germany | 2016 |
| Antiresorptive Agent-Related Osteonecrosis of the Jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw [35] | Japan | 2016 |
| Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management from the International Task Force on ONJ [1] | Canada | 2017 |
| Oral Health Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw [36] | Scotland | 2017 |
| Authors | Focused Question | Type of Patients | Type of Reviews |
|---|---|---|---|
| Aljohani et al. (2017) [40] | What is the effect of ARDs on MRONJ development in osteoporosis patients? and what are the risk factors, demographical and clinical characteristics associated with MRONJ in this particular group of patients? | Osteoporosis | SR |
| Beth-tasdogan et al. (2017) [41] | What are the effects of different interventions to either prevent or treat medication-related osteonecrosis of the jaw compared with each other or compared with no treatment or an inactive intervention (’placebo’)? | Mixed (Oncology & Non-Oncology) | MA |
| Cabras et al. (2021) [42] | In populations of patients treated with antiresorptive agents undergoing tooth extraction, which antibiotic is more effective in reducing risk of MRONJ, compared to other antibiotics or placebo? | Mixed (Oncology & Non-Oncology) | SR |
| Dal Prá et al. (2017) [43] | Is the C-terminal telopeptide test effective in predicting the development of bisphosphonate-related osteonecrosis of the jaw? | Mixed (Oncology & Non-Oncology) | SR |
| Duarte et al. (2020) [44] | Can children be affected by bisphosphonate-related osteonecrosis of the jaw? | Mixed (Oncology & Non-Oncology) | SR |
| Gelazius et al. (2018) [45] | Is dental implant placement purposeful for patients using bisphosphonates? | Mixed (Oncology & Non-Oncology) | SR |
| Govaerts et al. (2020) [46] | NR | Mixed (Oncology & Non-Oncology) | SR |
| Hennedige et al. (2014) [47] | NR | Osteogenesis Imperfecta | SR |
| Hess et al. (2008) [48] | NR | Non-Oncology | SR |
| Lorenzo pouso et al. (2020) [49] | NR | Mixed (Oncology & Non-Oncology) | MA |
| Lorenzo-pouso et al. (2019) [50] | What are the most effective biomarkers for the risk assessment of developing BRONJ? | Mixed (Oncology & Non-Oncology) | SR |
| Madrid & Sanz (2009) [51] | In patients on IV or orally administered BPs, what is the risk of developing BRONJ when dental implants are placed and what is the impact of BP therapy on implant outcome? | Unclear | SR |
| Mauri et al. (2009) [52] | NR | Oncology | MA |
| McGowan et al. (2018) [53] | NR | Mixed (Oncology & Non-Oncology) | SR |
| Migliorati et al. (2010) [54] | NR | Oncology | SR |
| Ottesen et al. (2020) [55] | Is a high-dose AR drug holiday at the time of tooth extraction, or other dentoalveolar surgery, necessary to prevent the development of MRONJ in patients with cancer? | Mixed (Oncology & Non-Oncology) | SR |
| Palaska et al. (2009) [56] | NR | Mixed (Oncology & Non-Oncology) | SR |
| Querrer et al. (2021) [57] | Are bisphosphonate-related ONJ and denosumab-related ONJ any different, regarding clinical and imaging aspects? | Mixed (Oncology & Non-Oncology) | SR |
| Rollason et al. (2016) [58] | NR | Oncology | SR |
| Sacco et al. (2021a) [59] | Is there any sufficient evidence that non-oncological immunosuppressed patients are at higher risk of developing ONJ due to antiresorptive drug therapy? | Non-Oncology | SR |
| Sacco et al. (2021b) [60] | Is there any evidence that malignant cells or metastatic cancer is present within osteonecrosis of the jaws in patients treated with antiresorptive and/or antiangiogenic medications? | Oncology | SR |
| Sacco et al. (2021c) [61] | Which is the best available treatment option for managing antiangiogenic related MRONJ in oncology patients? | Oncology | SR |
| de Souza Tolentino et al. (2019) [62] | Does hyperbaric oxygenation have positive effects in the treatment of medication-related osteonecrosis of the jaws? Does low-intensity laser therapy have positive effects in the treatment of medication-related osteonecrosis of the jaws? Additionally, does platelet-rich plasma (PRP) have positive effects in the treatment of medication-related osteonecrosis of the jaws? | Mixed (Oncology & Non-Oncology) | MA |
| Woo et al. (2006) [63] | NR | Mixed (Oncology & Non-Oncology) | SR |
| Woolley et al. (2021) [64] | Is there any evidence that orthodontic treatment induces ONJ or other adverse outcomes inpatients treated with antiresorptive drug therapy? | Non-Oncology | SR |
| Authors | Number and Study Types Included in the Analysis | Number of Patients Included in the Research | Type of Drug Therapy Used |
|---|---|---|---|
| Aljohani et al. (2017) [40] | 1 PR; 20 RE; 20 CS; 3 CR | 587 | Antiresorptive (including denosumab) |
| Beth-tasdogan et al. (2017) [41] | 5 RCT | 1218 | Antiresorptive (including denosumab) |
| Cabras et al. (2021) [42] | 1 RCT; 7 PR; 4 RE; 5 CS | 1888 | Antiresorptive (including denosumab) |
| Dal Prá et al. (2017) [43] | 8 PR | 1442 | Antiresorptive (including denosumab) |
| Duarte et al. (2020) [44] | 2 PR; 5 RE | 538 | Bisphosphonate |
| Gelazius et al. (2018) [45] | 1 RCT; 1 PR; 2 RE; 2 CS; 3 CR | 514 | Bisphosphonate |
| Govaerts et al. (2020) [46] | 4 RCT; 4 P-CCS; 7 R-CCS; 7 PR; 7 RE | 1513 | Antiresorptive (including denosumab) |
| Hennedige et al. (2014) [47] | 4 RE; 1 CS | 501 | Bisphosphonate |
| Hess et al. (2008) [48] | 1 RCT; 8 RE; 18 CS; 3 CR | 99 | Bisphosphonate |
| Lorenzo pouso et al. (2020) [49] | 7 CCS; 5 RE | 2995 | Bisphosphonate |
| Lorenzo-pouso et al. (2019) [50] | 6 P-CCS; 1 LE | 2623 | Bisphosphonate |
| Madrid & Sanz (2009) [51] | 1 P-CCS; 3 RE | 1561 | Bisphosphonate |
| Mauri et al. (2009) [52] | 15 RCT | 10,707 | Bisphosphonate |
| McGowan et al. (2018) [53] | Unclear | 4106 | Antiresorptive (including denosumab) |
| Migliorati et al. (2010) [54] | Unclear | 39,124 | Bisphosphonate |
| Ottesen et al. (2020) [55] | 3 PR; 10 RE; 1 CS | 2100 | Antiresorptive (including denosumab) |
| Palaska et al. (2009) [56] | 72 CS; 33 CR | 656 | Bisphosphonate |
| Querrer et al. (2021) [57] | 2 RCT; 5 CSS | 7755 | Antiresorptive |
| Rollason et al. (2016) [58] | 1 RCT | 49 | Bisphosphonate |
| Sacco et al. (2021a) [59] | 9 RE; 8 CS; 10 CR | 206 | Bisphosphonate and anti-TNF inhibitors |
| Sacco et al. (2021b) [60] | 3 PR; 2 RE; 2 CS; 6 CR | 37 | Bisphosphonate |
| Sacco et al. (2021c) [61] | 1 RCT; 7 CS; 20 CR | 36 | Antiangiogenic |
| de Souza Tolentino et al. (2019) [62] | 1 RCT; 5 PR; 4 RE; 3 CS | 188 | Antiresorptive |
| Woo et al. 2006 [63] | Unclear | 368 | Bisphosphonate |
| Woolley et al. (2021) [64] | 1 RE; 1 CS; 5 CR | 29 | Bisphosphonate |
| Non-Interventional Reviews | |
| Author | Result |
| Aljohani et al. (2017) [40] | The mean age of MRONJ osteoporosis patients in our study was 69.7 ± 5.2 years. The mandible was the most common site (394, 70.6%), followed by maxilla (152 case, 27.2%) and then in both of them (only 12 cases, 2.2%). The ratio of mandible to maxilla and both jaws involvement was 2.4:1. There was variability in the duration of BPs therapy, which ranged from 2 weeks to 93 months, with a mean duration of 51.9 ± 18 months. Extraction was the most frequently reported preceding event (244 patients, 48.5%). |
| Cabras et al. (2021) [42] | The data acquired from the moderate/high risk of bias studies suggested that 2–3 g of amoxicillin daily, either alone or in combination with clavulanate, for 6–7 days is the most commonly used antibiotic treatment to minimize risk of MRONJ in patients requiring a dental extraction, which could provide reduction of MRONJ risk. |
| Dal Prá et al. (2017) [43] | All eight of the selected studies found that CTX levels were not predictive of the development of BRONJ. In conclusion, this systematic review indicates that the CTX test has no predictive value in determining the risk of osteonecrosis in patients taking bisphosphonates. |
| Duarte et al. (2020) [44] | Although no cases of osteonecrosis were identified, all studies had weaknesses such as a limited sample size or the absence of risk factors for the development of osteonecrosis. However, it is believed that patients with secondary osteoporosis who use bisphosphonates continuously should be followed up during adulthood, since bone turnover decreases over the years. |
| Hennedige et al. (2014) [47] | Currently, osteogenesis imperfecta patients are treated as high-risk candidates for developing osteonecrosis of the jaw after dental extractions. However, there is no evidence to support hypothesis of causal relationship between bisphosphonates and osteonecrosis of the jaw in children and adolescents with osteogenesis imperfecta. |
| Hess et al. (2008) [48] | Common risk factors, which were associated with 88.9% of all non-cancer cases of osteonecrosis of the jaw among bisphosphonate users were:
|
| Lorenzo pouso et al. (2020) [49] | MRONJ appears to be associated with an increase in prevalence of periodontal disease. However, the lack of scientific evidence in this matter does not allow clear conclusions to be drawn. |
| Lorenzo-pouso et al. (2019) [50] | A total of seven biomarkers were identified and classified into three groups: bone turnover, angiogenesis and endocrine markers. Conflicting results were found in relation to most biomarkers, which suggest that no useful markers are currently available to evaluate BRONJ risk. |
| Mauri et al. (2009) [52] | Overall, osteonecrosis of the jaw was a rare event, occurring in 13 (0.24%) of the 5312 patients receiving bisphosphonates. Treatment with zoledronic acid was significantly associated with the occurrence of osteonecrosis of the jaw (OR = 3.23, 95% CI = 1.7–8) compared with no use. |
| McGowan et al. (2018) [53] | A total of 4106 patients with MRONJ were identified, 39 different systemic diseases were implicated, and 14 medical and 11 dental risk factors were reported, although no statistical analysis of the significance of each of these factors was possible. However, the most reported dental risk factor was tooth extraction (45%), followed by periodontal disease (10%). |
| Migliorati et al. (2010) [54] |
|
| Palaska et al. (2009) [56] |
|
| Querrer et al. (2021) [57] | An increase in bone sequestra, cortical bone lysis, and bone density was observed in bisphosphonate-related ONJ, while larger bone sequestra, more frequent periosteal reactions, and mandibular canal enhancement were noted in denosumab-related ONJ. |
| Sacco et al. (2021a) [59] | The data reviewed have confirmed that an invasive procedure is the most common trigger of MRONJ with relatively high frequency of postoperative complications or recurrence following management. However, due to low-quality research available in the literature, it is difficult to ascertain quantitatively the susceptibility of immunosuppressed patients in the development of MRONJ in non-oncology patients. |
| Sacco et al. (2021b) [60] | Based on the limited data available in literature, it is plausible that not histologically analyzing all ONJ specimens could result in a small amount of undiagnosed and untreated malignant diseases (4.64% based on large cohort studies). |
| Woolley et al. (2021) [64] | Lack of evidence whether orthodontic treatment can precipitate MRONJ. Moreover, antiresorptive drug therapy has been associated with a sub-optimal treatment outcome. The review reported adverse outcomes including: difficulty achieving root parallelism, difficulty achieving complete space closure, exaggerated mobility post-debond, increased duration of orthodontic treatment beyond expected completion, sclerotic alveolar bone changes seen on post-op radiographic images, an increased amount of root resorption and one case of ONJ. |
| Interventional Reviews | |
| Author | Result |
| Beth-tasdogan et al. (2017) [41] | Available evidence is insufficient to either claim or refute a benefit for hyperbaric oxygen therapy as an adjunct to conventional therapy. There is also insufficient evidence to draw conclusions about autofluorescence-guided versus tetracycline fluorescence-guided bone surgery. Moreover, there is insufficient evidence to conclude that the use of the other interventions investigated would reduce the risk of MRONJ or would improve healing of MRONJ. |
| Gelazius et al. (2018) [45] | Patients with intraoral therapy appeared to have a better implant survival (5 implants failed out of 423) rate at 98.8% vs. patients treated intravenously (6 implants failed out of 68) at 91%. |
| Govaerts et al. (2020) [46] | Laser ablation had a success of 60–95% for complete healing. The controlled trials of leukocyte- and platelet-rich fibrin (LPRF) showed 60–100% success for the same outcome. Fluorescence-guided surgery had a complete healing percentage of 85–90%. |
| Madrid & Sanz (2009) [51] | From the analysis yield in this study, the placement of an implant may be considered a safe procedure in patients taking oral BPs for 5 years with regard to the occurrence of BRONJ since in these studies no BRONJ has been reported. |
| Ottesen et al. (2020) [55] | There is no evidence for using a drug holiday, but it is also clear that caused by a limited numbers of eligible patients, and a great variation in between these patients, high-level evidence for using an AR drug holiday is almost impossible to obtain this data. |
| Rollason et al. (2016) [58] | There is a lack of evidence from randomized controlled trials to guide treatment of bisphosphonate-related osteonecrosis of the jaw (BRONJ). |
| Sacco et al. (2021c) [61] | The data reviewed confirmed that an invasive procedure is the most common trigger of MRONJ. The overall MRONJ disease recurrence was identified in n = 6 (16.6%) cases. Two (n = 2) recurrences were observed in the conservative treatment group, n = 3 recurrences were recognized in the conservative and antibiotics treatment group of patients and n = 1 was observed in the surgical treatment group. However, due to the low quality of available research in literature, it is difficult to draw a definitive conclusion on the validity of the presented treatment to manage patients affected by MRONJ associated with angiogenic therapy. |
| de Souza Tolentino et al. (2019) [62] | Adjuvant therapies hyperbaric oxygen (HBO), low-intensity laser (LIL), and platelet-rich plasma (PRP) have positive effects on MRONJ treatment, being safe and well-tolerated.
|
| Woo et al. (2006) [63] | Over suppression of bone turnover is probably the primary mechanism for the development of this condition, although there may be contributing comorbid factors. All sites of potential jaw infection should be eliminated before bisphosphonate therapy is initiated in these patients to reduce the necessity of subsequent dentoalveolar surgery. Conservative debridement of necrotic bone, pain control, infection management, use of antimicrobial oral rinses, and withdrawal of bisphosphonates are preferable to aggressive surgical measures for treating this condition. |
| Systematic Review Category | Study/Studies | Assessment of Methodological Limitations * | Assessment of Relevance * | Assessment of Coherence * | Assessment of Adequacy * | Overall CERQual Assessment of Confidence ** | Explanation of Judgement |
|---|---|---|---|---|---|---|---|
| Epidemiological type of reviews (non-interventional) | Aljohani et al. (2017) [40]; Duarte et al. (2020) [44]; Hennedige et al. (2014) [47]; Hess et al. (2008) [48]; Lorenzo pouso et al. (2020) [49]; Mauri et al. (2009) [52]; McGowan et al. (2018) [53]; Migliorati et al. (2010) [54]; Palaska et al. (2009) [56]; Sacco et al. (2021a) [59]; Sacco et al. (2021b) [60]; de Souza Tolentino et al. (2019) [62]; Woolley et al. (2021) [64] | Serious concerns regarding methodological limitations in four studies and moderate concerns regarding methodological limitations in eight studies | Minor concerns regarding relevance in all studies | Minor concerns regarding coherence in all studies | Serious Concerns regarding adequacy in all studies | Very low confidence | This finding was graded as: minor concerns for relevance and coherence in all studies, moderate concerns regarding methodological limitations in eight studies and serious concerns regarding methodological limitations four studies and adequacy of data results in all studies |
| Management type of reviews (interventional) | Beth-tasdogan et al. (2017) [41]; Govaerts et al. (2020) [46]; Rollason et al. (2016) [58]; Sacco et al. (2021c) [61]; Woo et al. 2006 [63] | Serious concerns regarding methodological limitations in one study, moderate concerns regarding methodological limitations in three studies and minor concerns regarding methodological limitations in two studies and | Minor concerns regarding relevance in all studies | Minor concerns regarding coherence in all studies | Serious concerns regarding adequacy in all studies | Very low confidence | This finding was graded as: minor concerns for relevance and coherence, moderate concerns regarding methodological limitations in three studies and serious concerns regarding methodological limitations (one study) and adequacy of data results |
| Predicting biomarkers type of reviews (non-interventional) | Dal Prá et al. (2017) [43]; Lorenzo-pouso et al. (2019) [50] | Serious concerns regarding methodological limitations in one study and moderate concerns regarding methodological limitations in one study | Minor concerns about relevance | Minor concerns about coherence in both studies | Serious concerns regarding adequacy in both studies | Very low confidence | This finding was graded as: minor concerns for relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding methodological limitations (one study) and adequacy of data results |
| Dental rehabilitation (interventional) | Gelazius et al. (2018) [45]; Madrid & Sanz (2009) [51] | Serious concerns regarding methodological limitations in one study and moderate concerns regarding methodological limitations in one study | Minor concerns regarding relevance | Moderate concerns regarding coherence in both studies | Serious concerns regarding adequacy in both studies | Very low confidence | This finding was graded as: minor concerns for relevance, moderate concerns regarding methodological limitations and coherence, and serious concerns regarding adequacy of data results |
| Preventive strategy type of review (interventional) | Ottesen et al. (2020) [55] | Moderate concerns regarding methodological limitations | Minor concerns regarding relevance | Minor concerns regarding coherence | Serious concerns regarding adequacy | Very low confidence | This finding was graded as: minor concerns for relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding adequacy of data results |
| Preventive strategy type of review (non-interventional) | Cabras et al. (2021) [42] | Serious concerns regarding methodological | Minor concerns regarding relevance | Minor concerns regarding coherence | Serious concerns regarding Adequacy | Very low confidence | This finding was graded as: minor concerns for relevance, moderate concerns regarding methodological limitations and coherence, and serious concerns regarding adequacy of data results |
| Diagnostic type of review (non-interventional) | Querrer et al. (2021) [56] | Moderate concerns regarding methodological limitations | Minor concerns regarding relevance | Minor concerns regarding coherence | Serious concerns regarding adequacy | Very low confidence | This finding was graded as: minor concerns for relevance and coherence, moderate concerns regarding methodological limitations and serious concerns regarding adequacy of data results |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, R.; Calasans-Maia, M.D.; Woolley, J.; Akintola, O.; de Almeida Barros Mourão, C.F.; Moraschini, V.; Kushnerev, E.; Acocella, A.; Obisesan, O.; Yates, J. 18 Years of Medication-Related Osteonecrosis of the Jaw (MRONJ) Research: Where Are We Now?—An Umbrella Review. Appl. Sci. 2021, 11, 8818. https://doi.org/10.3390/app11198818
Sacco R, Calasans-Maia MD, Woolley J, Akintola O, de Almeida Barros Mourão CF, Moraschini V, Kushnerev E, Acocella A, Obisesan O, Yates J. 18 Years of Medication-Related Osteonecrosis of the Jaw (MRONJ) Research: Where Are We Now?—An Umbrella Review. Applied Sciences. 2021; 11(19):8818. https://doi.org/10.3390/app11198818
Chicago/Turabian StyleSacco, Roberto, Monica Diuana Calasans-Maia, Julian Woolley, Oladapo Akintola, Carlos Fernando de Almeida Barros Mourão, Vittorio Moraschini, Evgeny Kushnerev, Alessandro Acocella, Olamide Obisesan, and Julian Yates. 2021. "18 Years of Medication-Related Osteonecrosis of the Jaw (MRONJ) Research: Where Are We Now?—An Umbrella Review" Applied Sciences 11, no. 19: 8818. https://doi.org/10.3390/app11198818
APA StyleSacco, R., Calasans-Maia, M. D., Woolley, J., Akintola, O., de Almeida Barros Mourão, C. F., Moraschini, V., Kushnerev, E., Acocella, A., Obisesan, O., & Yates, J. (2021). 18 Years of Medication-Related Osteonecrosis of the Jaw (MRONJ) Research: Where Are We Now?—An Umbrella Review. Applied Sciences, 11(19), 8818. https://doi.org/10.3390/app11198818









