Abstract
This study aimed to evaluate the potential of the marine microalgae Nannochloropsis oceanica as a sustainable source of n-3 polyunsaturated fatty acids (n-3 PUFA) for hen eggs enrichment. During 4 weeks, hens were fed with 3% (w/w) of Nannochloropsis oceanica supplemented diet. Throughout the assay, eggs were analyzed according to several nutritional and physical parameters, namely: (i) protein, fat, and ash content; (ii) fatty acid profile; (iii) thickness and colour of the shell; (iv) total egg weight; (v) protein quality (HU) and (vi) yolk colour. A remarkable increase in eicosapentaenoic (EPA), from 2.1 ± 0.1 to 5.2 ± 1.2 mg/100 g, and docosahexaenoic (DHA), from 50.3 ± 4.0 to 105 ± 18 mg/100 g, fatty acids was observed. Yolk colour also changed significantly according to the La Roche scale, from 9.6 ± 0.8 to 11.4 ± 0.8 (more orange). Feed supplementation did not lead to changes in the remaining analyzed parameters. A shelf life study, carried out for 28 days at room temperature, showed a decrease in eggs protein quality. In conclusion, eggs from hens fed with Nannochloropsis oceanica had a yolk colour more appealing to consumers and higher levels of EPA and DHA, allowing its classification as high in n-3 PUFA (CE nº 1924/2006).
1. Introduction
The consumption of n-3 polyunsaturated fatty acids, namely of docosahexaenoic (DHA, C22:6 n-3) and eicosapentaenoic (EPA, C20:5 n-3) fatty acids, provides several benefits for cardiovascular health by reducing arrhythmia risk, decreasing blood pressure, improving the atherogenic lipid profile and reducing platelet aggregation [1,2]. Moreover, protective effects of these fatty acids against metabolic syndrome [3], obesity [4], type 2 diabetes mellitus [5], depression [6], and eye degenerative disorders [7] have been reported. Despite the health-benefits of EPA and DHA, its intake is low in most Western countries, far below the recommended daily dosage of 140 to 600 mg/day [8]. These levels may be achieved by consuming fatty fish on a regular basis or, alternatively, by taking supplements rich in EPA and DHA [9]. Nevertheless, these sources of n-3 are expensive which limits its consumption. In this regard, eggs stand out as an interesting food product to enrich with n-3 PUFA, mostly due to their wide intake and high-nutritional value as a source of protein, essential fatty acids, vitamins, and minerals [10,11]. Also, feeding hens with n-3 PUFA enriched diet is a feasible way of modifying the fatty acid profile of eggs, providing higher levels of EPA, DHA and α-linolenic acid (ALA, C18:3 n-3). Several sources of n-3 PUFA have been used for eggs supplementation, namely (i) flaxseed, highly rich in ALA; (ii) fatty fish oils, with abundant levels of EPA and DHA and, more recently (iii) autotrophic microalgae rich in EPA and DHA [11,12]. Flaxseed has been extensively studied as a concentrated source of the essential fatty acid ALA for hen diets, resulting in eggs enriched with ALA and, to a much lesser extent, with DHA [11,12,13]. In humans, the metabolic pathway for n-3 PUFA synthesis comprises the conversion of ALA firstly into EPA and, at a later stage, to DHA, catalyzed by desaturases and elongases. Unfortunately, limited yields of EPA and DHA are obtained through their in vivo synthesis from ALA, making indispensable their consumption to meet the recommend dietary intakes [12]. Therefore, adding EPA and DHA to hen diet is a much more profitable way to enrich eggs in n-3 PUFA in comparison with flaxseed supplementation. In this sense, fatty fish oil, namely from sardine and menhaden, has been used as DHA and EPA sources for eggs enrichment [14,15,16]. Several studies reported higher levels of DHA in eggs from hens fed with fish oil, suggesting a large conversion of EPA into DHA [14,16,17,18,19]. One of the main constraints to the supplementation of the hen diet with fish oils is the appearance of off-flavors, identified as “fishy taste”, at fish oil concentration of about 1.5%, which results in yolk DHA levels below 100 mg/egg [17,20]. Higher levels of n-3 PUFA have been reached with microalgal feed supplementation. Microalgae are unicellular organisms, mainly found in oceans and in fresh or brackish water, that can be cultured either autotrophically or heterotrophically [21]. Autotrophic microalgae stand out as a more sustainable source of n-3 PUFA, since the heterotrophic production requires the intake of organic carbon sources provided from terrestrial crops [21,22]. Moreover, microalgae are an interesting source of bioactive compounds, such as polyphenols and carotenoids, for food and feed purposes [23]. Nannochloropsis is a marine microalgae genus highly efficient in the conversion of carbon dioxide into neutral lipids, with high content of EPA ranging from 1.1% to 12% DW across species [24]. EPA content of ca 3 to 5.5. (% DW) was reported for Nannochloropsis oceanica [25,26,27].
In this study, N. oceanica was used as source of n-3 PUFA for hen feed supplementation during 4 weeks. Several parameters were analyzed aiming at evaluating the nutritional profile (protein, lipids, ash, and n-3 PUFA content), physical characteristics (yolk colour, shell thickness and shell colour, egg protein quality (HU) and egg total weight) and shelf life for 28 days at room temperature.
2. Materials and Methods
2.1. Chemicals
Kjeldahl Catalyst with Se was obtained from PanReac AppliChem, Barcelona, Spain; Supelco 37 Component FAME Mix, was acquired from Sigma-Aldrich (St. Louis, MO, USA). Other reagents used were p.a. and obtained from various sources.
2.2. Diet Formulation and Experimental Design
Powder biomass of the autotrophic cultured microalgae Nannochloropsis oceanica was supplied by Allmicroalgae, Natural Products S.A., Pataias, Portugal. A supplemented diet for hens was prepared by adding spray-dried Nannochloropsis oceanica, in a proportion of 3% (w/w), to a standard feed provided by Alimave, S.A, Leiria, Portugal. The composition of standard and supplemented feeds as well as of the microalgae biomass was characterized concerning fatty acid profile, total protein, ash, and lipid content.
During 28 days (4 weeks), hens (about 3000 birds) from an outdoor farming system (Gameirovo, Caldas da Rainha, Portugal) were fed ad libitum with diet supplemented with N. oceanica. A group with the same size (3000 hens), fed with a standard diet, was used as control. The zootechnical performance of hens was monitored based on feed intake and eggs production. In each week (identified as W1, W2, W3, and W4) eggs were randomly collected and analyzed. In the harvest day, eggs (N = 12) were evaluated for (i) shell thickness and colour; (ii) total weight; (iii) egg quality and (iv) yolk colour. The eggs (N = 5) for chemical analysis (protein, lipids, ash, moisture, and fatty acid profile) were shelled, freeze-dried, ground to powder, and preserved at 4 °C until use. The weight ratio between fresh and freeze-dried eggs was used to express the parameters values as fresh weight. Eggs collected at day zero were used as control.
To evaluate the shelf life during 28 days of storage (legal expiration time), control eggs and eggs from hens fed with supplemented diet for 4 weeks (W4), were stored in paper boxes, protected from heat and light. Every week, eggs were evaluated for the parameters previously mentioned.
2.3. Nutritional Composition and Fatty Acid Profile of N. oceanica, Hen Diets, and Eggs
2.3.1. Total Protein
Crude protein was quantified by the Kjeldahl method [28] using the value 6.25 as nitrogen-protein conversion factor. Briefly, samples (0.25 g) were digested (Digestor 2006, Foss, Hillerød, Denmark), at 400 °C for 90 min, with 25 mL of 95% sulfuric acid and one catalyst tablet. Digested samples were then distillated (Kjeltec 2100, Foss, Hillerød, Denmark) under alkaline conditions and collected in a 4% boric acid solution. Finally, the resulting solution was titrated with standard chloride acid 0.1 mol/L. A blank assay was similarly prepared, without sample addition. Protein content was expressed as a percentage of fresh weight (% FW).
2.3.2. Total Lipids
The quantification of total lipids was based on the Folch method [29]. Samples (1 g) were hydrated with 0.8 mL of distilled water and homogenized with 10 mL of a methanol-chloroform (1:2) solution, under vortex stirring for 5 min. After addition of 1.2 mL of 0.8% NaCl solution, the mixture was vortexed (2 min) and centrifuged (Eppendorf, Centrifuge 5810 R, Hamburg, Germany) at 11,200× g during 5 min. The lower organic phase was filtered through a column of anhydrous sodium sulphate and collected to a round-bottom flask previously weighed. The extraction procedure was repeated with 5 mL of chloroform. Finally, solvent was removed in a rotary evaporator (Heidolph 2, LAB1ST, Shanghai, China) and the lipid extract was dried at 40 °C until constant weight. Total lipid content was expressed as % FW.
2.3.3. Ash Content
Ash quantification was performed by samples (1 g) incineration in an oven (Nabertherm, Liliemthal/Bermen, Germany) at 500 °C for 24 h. Results were expressed as % FW.
2.3.4. Fatty Acid Profile
Fatty acid methyl esters (FAME) of eggs, microalgae, standard and supplemented feeds were obtained by direct acid transmethylation of samples [30]. Briefly, samples (50 mg) were mixed with 2 mL of H2S04 (2%, v/v) in methanol and heated during 2 h at 80 °C. After the addition of 1 mL of MilliQ water and 2 mL of hexane, mixtures were vortex (1 min) and centrifuged (Eppendorf, Centrifuge 5810 R) at 123× g during 5 min. Finally, the hexane layer was collected and analyzed by gas chromatography (GC). GC analysis were carried out in a Finnigan Ultra Trace gas chromatograph, equipped with a Thermo Tr-FAME capillary column (60 m × 0.25 mm ID, 0.25 µm film thickness); an auto sampler AS 3000 from Thermo Electron Corporation and a flame ionization detector (FID). The temperatures of the detector and injector (splitless) were 280 °C and 250 °C, respectively. Oven temperature was set at 100 °C for 1 min, followed by an increase at 10 °C/min to 160 °C (maintaining for 10 min) and a second increase at 4 °C/min to 235 °C (during 10 min). Helium was used as carrier gas at a constant flow of 1.2 mL/min. Air and hydrogen were supplied to the detector at flow rates of 350 mL/min and 35 mL/min, respectively.
Fatty acids were identified by comparison of its retention time with those of Supelco 37 standard mixture. Eicosapentaenoic (EPA), alpha-linolenic (ALA) and docosahexaenoic (DHA) fatty acids were quantified by the external standard method, using a calibration curve (linear regression) constructed with a serial dilution of Supelco 37 mix: Area = 4 × 108 × [EPA] (µg/mL)–8 × 108, R = 0.990; Area = 2 × 108 × [ALA] (µg/mL)– 7 × 108, R = 0.989; Area = 4 × 108 × [DHA] (µg/mL)– 9 × 108, R = 0.981. Results were expressed as mg of FA per 100 g of sample (mg/100 g), taking into account the FAME concentration, the volume of hexane used to extract the FAME and the samples mass.
Aiming to evaluate if eggs could be labeled as a source of n-3 (40 mg EPA + DHA per 100 Kcal) or as high in n-3 (80 mg EPA + DHA per 100 Kcal) (CE nº 1924/2006), Equation (1) was applied, based in the amount of Kcal supplied per gram of protein, lipids, and carbohydrates. The maximum value of carbohydrate content (1%) was considered for the calculation [31].
where EPA + DHA is expressed as mg/100 g; protein, lipids and carbohydrates content expressed as %.
The efficiency of n-3 PUFA incorporation in eggs, was estimated by Equation (2) as described by [32]:
where fatty acids content is expressed as mg/100 g; Wx—eggs from hens fed with supplemented diet for x weeks; C—control eggs; SDF—standard feed; SPF—supplemented feed.
2.4. Physical Parameters of Eggs
All the instruments used to measure the physical parameters were connected to a QCM microprocessor unit equipped with a Eggware software [33].
Yolk colour was measured with a QCC yolk colourimeter. Results were expressed as values of the Roche scale from 1 (light yellow) to 15 (dark orange). The determination of shell colour was performed with a QCR—shell colour reflectometer. Results were expressed as percentage (0%—black and 100%—pure white). Shell thickness (µm) was measured with a QCT—shell thickness micrometres (TSS-York, 2018). The albumen height was measured with a QCH albumen height gauge micrometre. Egg protein quality was expressed as Haugh units (HU), and was calculated from the values of albumen height (H, mm) and egg weight (W, g), by applying Equation (3):
2.5. Statistical Analysis
In order to compare nutritional profile and fatty acids content between eggs from hens fed with standard and supplemented diet, a t-test was conducted [34]. All requirements for its execution (namely, normality of data and homogeneity of variances) were validated. Additionally, in order to compare physical parameters, nutritional profile and EPA, DHA and ALA content between control eggs and eggs from hens fed with supplemented diet with 3% of Nannochloropsis oceanica, during 4 weeks of trial, a one-way analysis of variance (ANOVA) was performed. Also, the same analysis was used to compare physical parameters, nutritional profile and fatty acids contents between control eggs and eggs from hens fed with supplemented diet (W4), at zero (Sl-0) and 28 days (Sl-28) of storage. Whenever the assumptions for performing the ANOVA were not met (namely, normality of data and homogeneity of variances), the non-parametric Kruskal–Wallis test was performed [34]. Whenever applicable, multiple comparisons between samples and the control group were performed using the Dunnett test [34]. For the remaining comparisons, Tukey’s honestly significant difference (HSD) or least significant difference (LSD) and Games–Howell multiple comparison tests were performed, according to the fulfillment (or not) of the ANOVA requirements (respectively) [34]. The results were considered statistically significant with a significance level of 5%. Where applicable, all results are presented as mean ± standard deviation (SD). Finally, principal components analysis (PCA), based on correlation matrices, was applied to identify the main associations among physical parameters, nutritional profile, and fatty acids of egg samples along the shelf life. The PCA provides information on the most meaningful parameters, which describe a whole data set, affording data reduction with minimum loss of original information. Although the results concerning the first two components (PC1 and PC2) were presented, the others were also analyzed. When applicable, data were standardized, and log (x + 1) transformed [35]. All calculations were performed with the CANOCO version 4.5 package.
3. Results and Discussion
3.1. Nutritional Characterization of N. oceanica, Standard and Supplemented Diets
The nutritional composition of microalgae and diets comprised the evaluation of total protein, lipids, and ash, as well as the fatty acid profile (Table 1). N. oceanica showed high protein (44.0 ± 0.7%) and lipids (20.9 ± 2.6%) content, being palmitoleic (C16:1 n-7), palmitic (16:0), and eicosapentaenoic (EPA, C20:5 n-3) the major fatty acids.

Table 1.
Nutritional composition and fatty acid profile of hen feed (standard and supplemented) and of the microalgae N. oceanica.
The amount of EPA (21.40 ± 0.64 ug/mg) attained for N. oceanica biomass is in agreement with the value reported by [36]. Although the nutritional profile of N. oceanica depends on the strain and growth conditions [37], these results are in line with other studies attesting that this microalga is a rich source of lipids, particularly of EPA [38,39,40].
Concerning feed composition, no statistically significant differences (p > 0.05) in the amounts of protein (from 15% to 17%), lipids (around 4%) and ash (around 11%) were observed for both diets. However, a distinct fatty acid profile was observed, noticing the presence of palmitoleic (1.67 ± 0.32) and EPA (0.95 ± 0.21 µg/mg) fatty acids only in the supplemented feed. Linoleic (C18:2 n-6) and oleic (C18:1 n-9) were the major fatty acids, regardless of diet, with higher values in the standard (29.2 ± 1.1 and 11.99 ± 0.65 µg/mg, respectively). The amounts of palmitic acid and alfa-linolenic (C18:3 n-3) were similar in both feeds, whereas stearic (C18:0) acid was more abundant in the standard. In a broadly sense, the supplemented diet provides more 89 mg/100 g of n-3 PUFA in comparison with the standard.
3.2. Influence of Hen Diet on Nutritional Profile and Physical Characteristics of Eggs
Hens fed with supplemented diet adapted well to the new feed, as proved by the zootechnical performance evaluation. Eggs were analyzed every week along the 28 days trial and compared with control samples (eggs from hens fed with standard diet). No significant differences were observed in the nutritional composition between samples throughout the assay (p > 0.05), with about 12% of protein, 10% of lipids and less than 1% of ash (Table 2), in agreement with the composition usually reported for whole eggs [31].

Table 2.
Physical parameters and nutritional profile of eggs from hens fed with supplemented diet with 3% of Nannochloropsis oceanica during 4 weeks of trial.
However, a remarkable increase in DHA and EPA content was observed at the end of week 1, reaching values two-fold higher than the control, which allowed eggs classification as high in n-3 PUFA (Figure 1). These values remained constant throughout the experiment, attaining values of 5.2 ± 1.2 for EPA and 105 ± 18 mg/100 g for DHA at the end of week 4. Conversely, no significant differences were observed in ALA content (p > 0.05) which was slightly higher than 50 mg/100 g in all samples.
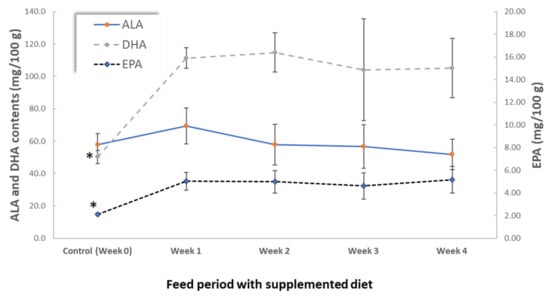
Figure 1.
ALA, EPA and DHA contents (mg/100 g) in eggs from hens fed with Nannochloropsis oceanica supplemented diet during the 4 weeks of trial. For the same FA, * means statistically significant differences (p < 0.05) (N = 5).
A similar accumulation pattern was described in other studies that used microalgae as a source of n-3 PUFA for eggs enrichment. Bruneel et al. [41] reported an increase in the values of EPA (to 2.3 ± 0.6) and DHA (to 44.9 ± 6.6 mg/egg) while ALA content remained constant (15.9 ± 2.0 mg/egg) after a 28-day trial, using 5.0% of N. gaditana in the supplemented feed. Moreover, the use of twice the concentration of microalgae in feed did not result in a proportional increase of n-3 PUFA in eggs. Fredriksson et al. [42] also observed a consistently increase in EPA and DHA when 20% of N. oculata was added to different standard diets. Feed supplemented with 5% N. oceanica had high efficiency for n-3 PUFA eggs enrichment, ranging from 85% to 60% at the end of week 1 and week 4, respectively (Figure 2).
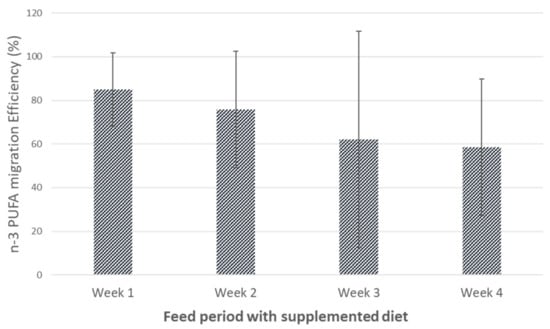
Figure 2.
Efficiency of n-3 polyunsaturated fatty acid (PUFA) migration from Nannochloropsis oceanica supplemented feed to hen eggs, along the feeding period.
Lemahieu et al. [32] compared four distinct microalgae and observed an increase in DHA and EPA, regardless of the species supplied to hen feed. Higher efficiency of n-3 PUFA enrichment was attained with Phaeodactylum tricornutum and Isochrysis galbana in comparison to Nannochloropsis oculata. An increase in ALA content was only observed with Chlorella fusca supplemented feed, which reflects the high amount of this fatty acid in its composition. Another study focused on the supplementation of hens feed with the microalga Isochrysis galbana in comparison with other sources of n-3 PUFA (flaxseed, fish oil, and DHA gold) for eggs enrichment. This microalga shown to be more efficient in the increase of EPA and DHA levels than flaxseed, which was mainly responsible for ALA improvement. Comparatively to fish oil and DHA Gold, I. galbana was less efficient for eggs supplementation, due to differences in both bio-accessibility and n-3 PUFA profile [43]. Concerning the physical parameters, statistically significant differences were observed in yolk colour that became more orange right at the end of week 1 (ANOVA, Dunnett’s test, F(4.55) = 6.237, p-value < 0.001; Figure 3). The shift towards higher values of Roche scale, as result of microalgae feed supplementation, was also reported by other authors [32,41,42]. The yolk colour intensification is associated to carotenes and xanthophyll migration from feed to eggs [10,32,42,44]. Nannochloropsis species are rich in carotenoids, flavonoids, and other phenolic compounds which have well-documented antioxidant activity [23,45]. The migration of these compounds from supplemented feed also improves the nutritional composition of eggs and its oxidative stability [10,46].
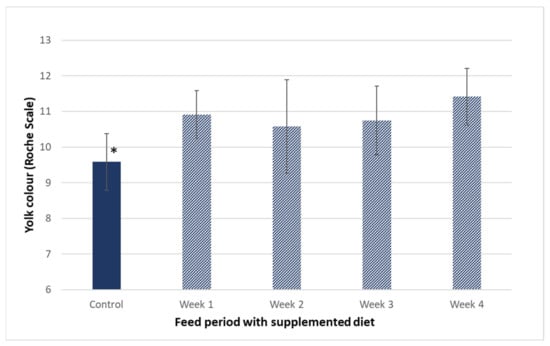
Figure 3.
Yolk colour of eggs from hens fed with 3% of Nannochloropsis oceanica supplemented diet during the 4 weeks of the trial. * means statistically significant differences (p < 0.05) (N = 12).
Regarding egg protein quality (HU), a slight increase was observed along feed supplementation, reaching a maximum value of 88.8 ± 5.5 at the end of week 3 (Table 2). Conversely, [46] did not observe changes in HU values when marigold extracts were used for hens feed supplementation. No significant differences were observed in the remaining physical parameters. At the end of the trial, egg weight, shell colour, and shell thickness achieved values of 63.17 ± 5.5 g, 31.7 ± 4.4% and 371 ± 27 µm, respectively. The shell thickness compromises egg integrity while its colour influences consumer choices. Weaker shelled eggs are more susceptible to cracks and more prone to microbial contamination, with a consequent decrease in sales [47]. Brown eggs (with values of 25–40%) are perceived by consumers as being more natural and healthier than white counterparts. However, no relation between shell colour and nutritional content of the egg has been proved [48].
3.3. Assessment of Shelf Life of Eggs from Hens Fed with Supplemented Diet
Control eggs and those from hens fed with a supplemented diet for four weeks (W4) were kept in paper boxes along 28 days, at room temperature, aiming at mimicking the storage conditions in the supermarket. Every 7 days, samples were collected and analyzed as previously described (cf 2.2). Egg protein quality (HU) was the only parameter that significantly decreased along time for both control and W4 eggs, although in a more pronounced way in control eggs (Figure 4). Nevertheless, no statistically significant differences (p > 0.05) were observed for control and supplemented eggs with the same storage time. Several authors state that albumen height, a parameter used for HU calculation (cf 2.4), decreases with storage time as pH of egg white increases [46,48].
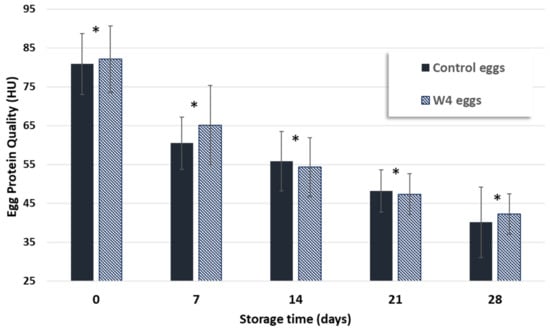
Figure 4.
Comparison of protein quality (Haugh units, HU) of control eggs and W4 eggs (from hens fed with 3% of Nannochloropsis oceanica supplemented diet during the 4 weeks) along the storage time. * means statistically significant differences (p < 0.05) between samples with different storage time (N = 12).
The remaining physical parameters were constant during storage. Also, the amount of the fatty acids ALA, EPA and DHA did not significantly change during the storage period (p > 0.05), demonstrating that eggs from hens fed with supplemented diet still preserve high levels of n-3 PUFA after 28 days (Table 3). The same pattern was observed for protein and lipids content conversely to the amount of ash that slightly increased throughout the storage.

Table 3.
Comparison of fatty acids contents, nutritional profile and physical parameters of control eggs those from hens fed with a supplemented diet (W4), at zero (0) and 28 days of storage.
Several studies concerning the evaluation of eggs shelf life highlight distinct observations in nutritional and quality parameters. Grčević et al. [46] reported an increase in shell thickness, yolk colour intensity as well as in lipid oxidation, assessed by thiobarbituric acid reactive substance (TBARS) assay, in control and supplemented eggs with marigold extract. A decrease of carotenoids content in yolk eggs throughout storage time, both at room temperature and under refrigeration, was observed by Barbosa et al. [49]. Another study showed a decrease of egg weight, HU value, and yolk colour in eggs stored for 3 weeks in refrigerator [50]. According to these authors, eggshell firmness does not change with storage period but depends on factors such as hen’s age, genotype, and diet.
In this study, a PCA was carried out aiming at providing an integrative approach of the influence of storage time on the nutritional composition and physical parameters of the control and of eggs from hens fed with the supplemented diet for 4 weeks (W4) (Figure 5).
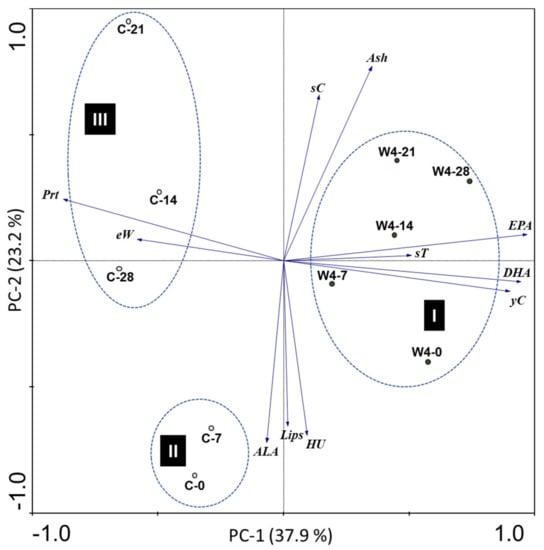
Figure 5.
Principal component analysis (PCA) biplot of egg samples along the shelf-life, concerning 11 parameters: amounts of the fatty acids EPA, DHA and ALA; protein (Prt), lipids (Lips) and ash (Ash); Yolk colour (yC); Shell colour (sC); shell thickness (sT); egg weight (eW) and egg protein quality (HU). C-0; C-7; C-14; C-21 and C-28—Eggs from hens fed with standard diet, after zero (0), 7, 14; 21 and 28 days of storage. W4-0; W4-7; W4-14; W4-21 and W4-28—eggs from hens fed with N. oceanica supplemented diet, after 0, 7, 14; 21 and 28 days of storage. PC-1 and PC-2 explain, respectively, 37.9% and 23.2% of data variability.
The first principal component PC1, representing 37.9% of sample variability, is mainly defined by the values of EPA, DHA, yolk colour and protein. Also representative of the variance explained by PC1 appears the total weight and the shell thickness, although with less weight to explain the observed pattern (shorter vectors). The second principal component PC2 explains 23.2% of the total variability and is more related to the egg protein quality (HU), lipids, ALA, ash contents and shell colour. The results obtained by the PCA allowed three groups to be distinguished (identified as I, II and III). Group I comprised W4 eggs and was mainly characterized by the higher levels of EPA, DHA and yolk colour. Comparing with control samples (groups II and III), W4 eggs were more homogeneous, suggesting higher stability of these samples during shelf life. Control samples distinguished from W4, by higher values of protein and of egg weight. Group II corresponded to 0 and 7 days of storage, whereas group III (shifted towards lower values of HU) contained the eggs with higher self-life (from 14 to 28 days). It should be noted that eggs with higher shelf life were shifted towards lower values of egg protein quality, although this trend was more pronounced in control samples.
4. Conclusions
An efficient and sustainable approach for increasing n-3 PUFA content in eggs was presented in this study. A robust sample of about 3000 hens were fed with a 3% N. oceanica supplemented diet. After 1 week of the trial, a remarkable increase in DHA and EPA fatty acid was observed, allowing eggs classification as high in n-3 (CE nº 1924/2006). It is worth mentioning that the supplemented diet gave rise to yolks with more intense orange colour. This characteristic is highly appreciated by consumers, who associate orange yolk with healthy and nutrient-rich eggs. In conclusion, N. oceanica was shown to be a reliable source of n-3 PUFA for eggs enrichment, while preserving its quality and nutritional patterns during storage time.
Author Contributions
Conceptualization: J.L.S., S.M., M.M.G. and C.T.; methodology: M.N., S.M., M.M.G. and C.T.; software: M.N., A.F., M.A., S.M., M.M.G. and C.T.; validation: M.N., A.F., M.A., S.M., M.M.G. and C.T.; formal analysis: M.N., A.F. and M.A.; investigation: M.N., A.F., M.A., S.M., M.M.G. and C.T.; resources: M.N., A.F., M.A., S.M., M.M.G. and C.T.; data curation: M.N., A.F., M.A., S.M., M.M.G. and C.T.; writing—original draft preparation: M.N., M.A., S.M., and C.T.; writing—review and editing: M.N., M.A., J.L.S., S.M., M.M.G. and C.T.; visualization: M.N., A.F., M.A., S.M., M.M.G. and C.T.; Supervision: M.N., S.M., M.M.G. and C.T.; Project administration: M.N., S.M., M.M.G. and C.T.; Funding acquisition: S.M., M.M.G. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Portuguese Foundation for Science and Technology (FCT) to the research units of MARE (UIDB/04292/2020) and by the Integrated Programme of SR&TD “Smart Valorization of Endogenous Marine Biological Resources Under a Changing Climate” (reference Centro-01-0145-FEDER-000018), co-funded by Centro 2020 program, Portugal 2020, European Union, through the European Regional Development Fund.
Acknowledgments
The authors are grateful to Allmicroalgae—Natural Products S.A., Portugal for supplying Nannochloropsis oceanica and to Alimave, S.A, Portugal and Gameirovo, Caldas da Rainha, Portugal, for creating the conditions to carry out the assays.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Watanabe, Y.; Tatsuno, I. Omega-3 Polyunsaturated Fatty Acids for Cardiovascular Diseases: Present, Past and Future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Park, K. Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 Fatty Acids in Obesity and Metabolic Syndrome: A Mechanistic Update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Katz, R. N-3 Long-Chain Polyunsaturated Fatty Acids in Type 2 Diabetes: A Review. J. Am. Diet. Assoc. 2005, 105, 428–440. [Google Scholar] [CrossRef]
- Ciappolino, V.; Delvecchio, G.; Agostoni, C.; Mazzocchi, A.; Altamura, A.C.; Brambilla, P. The Role of N-3 Polyunsaturated Fatty Acids (n-3PUFAs) in Affective Disorders. J. Affect. Disord. 2017, 224, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.; Cutolo, C.; Ferrari, D.; Corazza, P.; Traverso, C. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef] [Green Version]
- Molendi-Coste, O.; Legry, V.; Leclercq, I.A. Why and How Meet N-3 PUFA Dietary Recommendations? Gastroenterol. Res. Pract. 2011, 2011, 364040. [Google Scholar] [CrossRef] [Green Version]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 Fatty Acids: A Comprehensive Review of Their Role in Health and Disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef]
- Saleh, A.A.; Gawish, E.; Mahmoud, S.F.; Amber, K.; Awad, W.; Alzawqari, M.H.; Shukry, M.; Abdel-Moneim, A.M.E. Effect of Natural and Chemical Colourant Supplementation on Performance, Egg-Quality Characteristics, Yolk Fatty-Acid Profile, and Blood Constituents in Laying Hens. Sustainability 2021, 13, 4503. [Google Scholar] [CrossRef]
- Lemahieu, C.; Bruneel, C.; Muylaert, K.; Buyse, J.; Foubert, I. Microalgal Feed Supplementation to Enrich Eggs with Omega-3 Fatty Acids. In Egg Innovations and Strategies for Improvements; Academic Press: Cambridge, MA, USA, 2017; pp. 383–391. ISBN 9780128011515. [Google Scholar]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary Enrichment of Eggs with Omega-3 Fatty Acids: A Review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards Establishing Dietary Reference Intakes for Eicosapentaenoic and Docosahexaenoic Acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar] [CrossRef] [Green Version]
- Baucells, M.D.; Crespo, N.; Barroeta, A.C.; López-Ferrer, S.; Grashorn, M.A. Incorporation of Different Polyunsaturated Fatty Acids into Eggs. Poult. Sci. 2000, 79, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cachaldora, P.; García-Rebollar, P.; Alvarez, C.; de Blas, J.C.; Méndez, J. Effect of Type and Level of Fish Oil Supplementation on Yolk Fat Composition and N-3 Fatty Acids Retention Efficiency in Laying Hens. Br. Poult. Sci. 2006, 47, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bovet, P.; Faeh, D.; Madeleine, G.; Viswanathan, B.; Paccaud, F. Decrease in Blood Triglycerides Associated with the Consumption of Eggs of Hens Fed with Food Supplemented with Fish Oil. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Elswyk, M.E.; Dawson, P.L.; Sams, A. Dietary Menhaden Oil Influences Sensory Characteristics and Headspace Volatiles of Shell Eggs. J. Food Sci. 1995, 60, 85–89. [Google Scholar] [CrossRef]
- Farrell, D.J. Enrichment of Hen Eggs with N-3 Long-Chain Fatty Acids and Evaluation of Enriched Eggs in Humans. Am. J. Clin. Nutr. 1998, 68, 538–544. [Google Scholar] [CrossRef]
- Cachaldora, P.; García-Rebollar, P.; Alvarez, C.; de Blas, J.C.; Méndez, J. Effect of Type and Level of Basal Fat and Level of Fish Oil Supplementation on Yolk Fat Composition and N-3 Fatty Acids Deposition Efficiency in Laying Hens. Anim. Feed Sci. Technol. 2008, 141, 104–114. [Google Scholar] [CrossRef]
- Gonzalez-Esquerra, R.; Leeson, S. Effect of Feeding Hens Regular or Deodorized Menhaden Oil on Production Parameters, Yolk Fatty Acid Profile, and Sensory Quality of Eggs. Poult. Sci. 2000, 79, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Microalgae in Human and Animal Nutrition. In Handbook of Microalgal Culture; Richmond, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2003; ISBN 9780470995280. [Google Scholar]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, Photobioreactor Design and Harvesting of Microalgae for Biodiesel Production: A Critical Review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; el Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Huang, C.-C.; Lee, W.-L.; Chang, J.-S. Engineering Strategies for Enhancing the Production of Eicosapentaenoic Acid (EPA) from an Isolated Microalga Nannochloropsis Oceanica CY2. Bioresour. Technol. 2013, 147, 160–167. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Ho, S.-H.; Chang, J.-S. Enhancing the Production of Eicosapentaenoic Acid (EPA) from Nannochloropsis Oceanica CY2 Using Innovative Photobioreactors with Optimal Light Source Arrangements. Bioresour. Technol. 2015, 191, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Jiang, J.; Wang, H.; Cao, X.; Xue, S.; Yang, Q.; Wang, W. The Characteristics of TAG and EPA Accumulation in Nannochloropsis Oceanica IMET1 under Different Nitrogen Supply Regimes. Bioresour. Technol. 2015, 179, 483–489. [Google Scholar] [CrossRef] [PubMed]
- AOAC. AOAC: Official Methods of Analysis, 15th ed.; AOAC: Rockville, MD, USA, 1990; Volume 1. [Google Scholar]
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch Methods for Total Lipid Determination in a Broad Range of Marine Tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef]
- Fernández, A.; Grienke, U.; Soler-vila, A.; Guihéneuf, F.; Stengel, D.B.; Tasdemir, D. Seasonal and Geographical Variations in the Biochemical Composition of the Blue Mussel (Mytilus Edulis L.) from Ireland. Food Chem. 2015, 177, 43–52. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Kim, H.-O. Structure and Chemical Compositions of Eggs. In Egg Bioscience and Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–97. [Google Scholar]
- Lemahieu, C.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of Feed Supplementation with Different Omega-3 Rich Microalgae Species on Enrichment of Eggs of Laying Hens. Food Chem. 2013, 141, 4051–4059. [Google Scholar] [CrossRef]
- TSS-York QCM+. Available online: http://www.tss-york.com/products/qcmrange#QCC (accessed on 19 November 2018).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Hoboken, NJ, USA, 2010. [Google Scholar]
- Legendre, L.; Legendre, P. Écologie Numérique. Tome 1: Le Traitement Multiple Des Données Écologiques. Collection d’Écologie. Hydrobiology 1981, 66, 775–776. [Google Scholar]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty Acid Composition of 12 Microalgae for Possible Use in Aquaculture Feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Ashour, M.; Elshobary, M.E.; El-Shenody, R.; Kamil, A.W.; Abomohra, A.E.F. Evaluation of a Native Oleaginous Marine Microalga Nannochloropsis Oceanica for Dual Use in Biodiesel Production and Aquaculture Feed. Biomass Bioenergy 2019, 120, 439–447. [Google Scholar] [CrossRef]
- Guerra, I.; Pereira, H.; Costa, M.; Silva, J.T.; Santos, T.; Varela, J.; Mateus, M.; Silva, J. Operation Regimes: A Comparison Based on Nannochloropsis Oceanica Biomass and Lipid Productivity. Energies 2021, 14, 1542. [Google Scholar] [CrossRef]
- Alves, S.P.; Mendonça, S.H.; Silva, J.L.; Bessa, R.J.B. Nannochloropsis Oceanica, a Novel Natural Source of Rumen-Protected Eicosapentaenoic Acid (EPA) for Ruminants. Sci. Rep. 2018, 8, 2–11. [Google Scholar] [CrossRef]
- Sukenik, A.; Beardall, J.; Kromkamp, J.C.; Kopecký, J.; Masojídek, J.; van Bergeijk, S.; Gabai, S.; Shaham, E.; Yamshon, A. Photosynthetic Performance of Outdoor Nannochloropsis Mass Cultures under a Wide Range of Environmental Conditions. Aquat. Microb. Ecol. 2009, 56, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Bruneel, C.; Lemahieu, C.; Fraeye, I.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of Microalgal Feed Supplementation on Omega-3 Fatty Acid Enrichment of Hen Eggs. J. Funct. Foods 2013, 5, 897–904. [Google Scholar] [CrossRef]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty Acid and Carotenoid Composition of Egg Yolk as an Effect of Microalgae Addition to Feed Formula for Laying Hens. Food Chem. 2006, 99, 530–537. [Google Scholar] [CrossRef]
- Lemahieu, C.; Bruneel, C.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of Different Omega-3 Polyunsaturated Fatty Acid (n-3 PUFA) Sources (Flaxseed, Isochrysis Galbana, Fish Oil and DHA Gold) on n-3 LC-PUFA Enrichment (Efficiency) in the Egg Yolk. J. Funct. Foods 2015, 19, 821–827. [Google Scholar] [CrossRef]
- Li, I.C.; Wu, S.Y.; Liou, J.F.; Liu, H.H.; Chen, J.H.; Chen, C.C. Physiology and Reproduction: Effects of Deinococcus Spp. Supplement on Egg Quality Traits in Laying Hens. Poult. Sci. 2018, 97, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-Del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as Source of Commercially Valuable Pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Grčević, M.; Kralik, Z.; Kralik, G.; Galović, D.; Radišić, Ž.; Hanžek, D. Quality and Oxidative Stability of Eggs Laid by Hens Fed Marigold Extract Supplemented Diet. Poult. Sci. 2019, 98, 3338–3344. [Google Scholar] [CrossRef]
- Ahmadi, F.; Rahimi, F. Factors Affecting Quality and Quantity of Egg Production in Laying Hens: A Review. World Appl. Sci. J. 2011, 12, 372–384. [Google Scholar]
- Scott, T.A.; Silversides, F.G. The Effect of Storage and Strain of Hen on Egg Quality. Poult. Sci. 2000, 79, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.C.; Gaspar, A.; Calixto, L.F.L.; Agostinho, T.S.P. Stability of the Pigmentation of Egg Yolks Enriched with Omega-3 and Carophyll Stored at Room Temperature and under Refrigeration. Rev. Bras. De Zootec. 2011, 40, 1540–1544. [Google Scholar] [CrossRef] [Green Version]
- Perić, L.; Đukić Stojčić, M.; Bjedov, S. The Effect of Storage and Age of Hens on the Quality of Table Eggs. Adv. Res. Life Sci. 2017, 1, 64–67. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).